New Potential Antitumor Pyrazole Derivatives: Synthesis and Cytotoxic Evaluation
Abstract
:1. Introduction
2. Results and Discussion
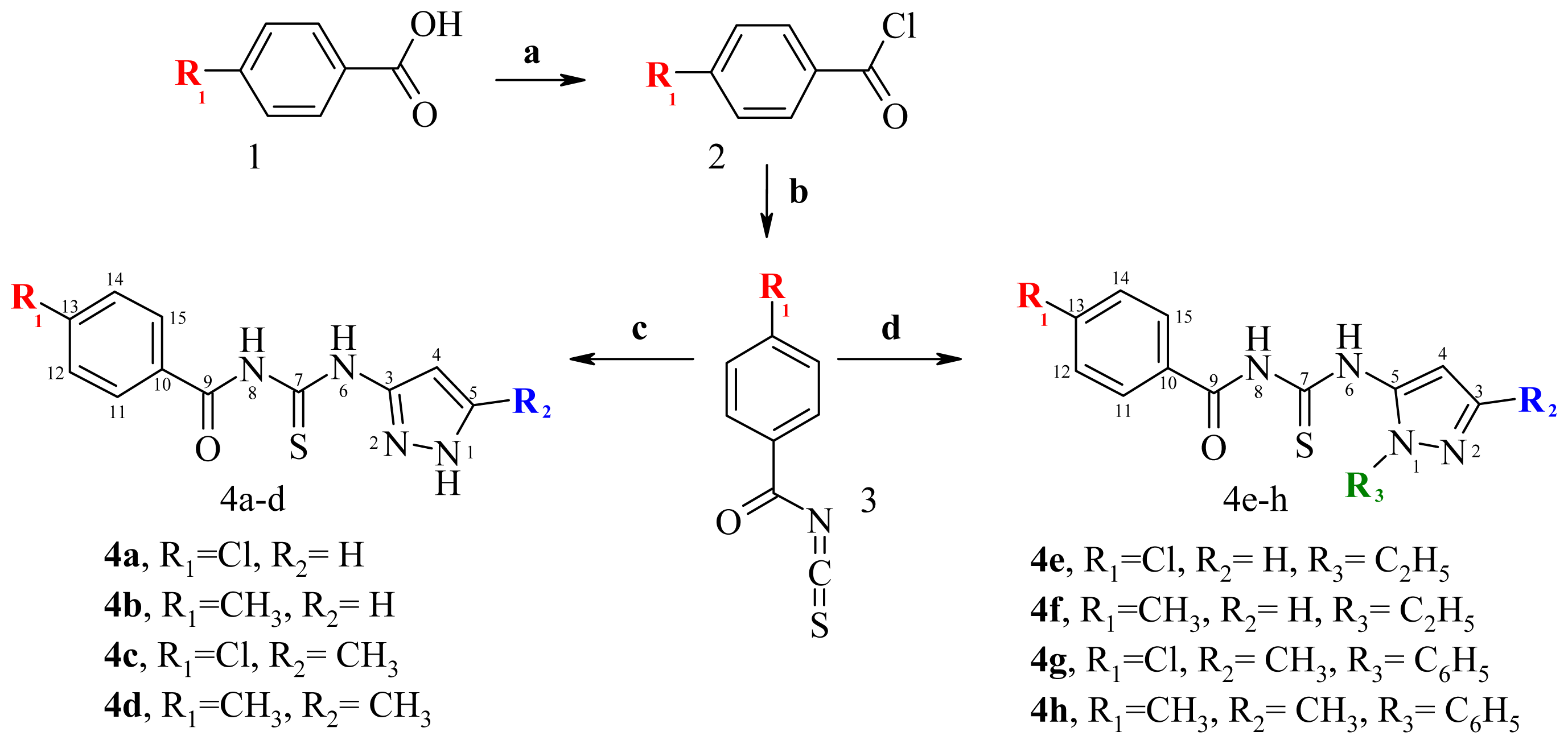
2.1. Chemistry
2.2. Biological Screening
2.2.1. Acute Toxicity Assay against Artemia salina
2.2.2. Acute Toxicity Assay against Daphnia magna
2.2.3. Cytotoxicity Assay against Triticum aestivum
2.3. Data Analysis
3. Experimental Section
3.1. Chemistry
3.1.1. General
3.1.2. Synthetic Procedures
N-(4-chlorobenzoyl)-N′-(1H-pyrazol-3-yl)-thiourea (4a)
N-(1H-pyrazol-3-yl)-N′-(4-methylbenzoyl)-thiourea (4b)
N-(4-chlorobenzoyl)-N′-(5-methyl-1H-pyrazol-3-yl)-thiourea (4c)
N-(5-methyl-1H-pyrazol-3-yl)-N′-(4-methylbenzoyl)-thiourea (4d)
N-(4-chlorobenzoyl)-N′-(1-ethyl-1H-pyrazol-5-yl)-thiourea (4e)
N-(1-ethyl-1H-pyrazol-5-yl)-N′-(4-methylbenzoyl)-thiourea (4f)
N-(4-chlorobenzoyl)-N′-(3-methyl-1-phenyl-1H-pyrazol-5-yl)-thiourea (4g)
N-(3-methyl-1-phenyl-1H-pyrazol-5-yl)-N′-(4-methylbenzoyl)-thiourea (4h)
3.2. Biological Screening
3.2.1. Acute Toxicity Assay against Artemia salina
3.2.2. Acute Toxicity Assay against Daphnia magna
3.2.3. Acute Toxicity Assay against Triticum aestivum
3.3. Data Analysis
4. Conclusions

| Compound | LC50 (μmol/L) | LC50 95% confidence interval (μmol/L) | Goodness of fit (r2) |
|---|---|---|---|
| 4a | 4.68 | – a | 0.7103 |
| 4b | 4.61 | 0.87–24.43 | 0.9057 |
| 4c | 8.15 | 2.37–27.99 | 0.9110 |
| 4d | 7.85 | –a | 0.6080 |
| 4e | 3.84 | –a | 0.6175 |
| 4f | 8.83 | –a | 0.7304 |
| 4g | 3.05 | 0.35–26.18 | 0.8336 |
| 4h | 11.09 | –a | 0.7348 |
| COL | 2.17 | 0.64–7.38 | 0.9490 |
| PHZ | 6.64 | –a | 0.7518 |
| Compound | LC50 (μmol/L) | LC50 95% confidence interval (μmol/L) | Goodness of fit (r2) |
|---|---|---|---|
| 4a | 9.44 | 8.59–10.38 | 0.9839 |
| 4b | 15.14 | –a | 0.9973 |
| 4c | 12.16 | 11.35–13.06 | 0.9452 |
| 4d | 13.65 | 12.82–14.49 | 0.9904 |
| 4e | 12.91 | 12.59–13.21 | 0.9858 |
| 4f | 13.34 | 12.68–14.03 | 0.9483 |
| 4g | 15.07 | –a | 0.9459 |
| 4h | 13.40 | 12.74–14.13 | 0.9634 |
| COL | 13.90 | 13.37–14.45 | 0.9702 |
| PHZ | 15.24 | 14.42–16.07 | 0.9442 |
| Compound | IC50 (μmol/L) | IC50 95% confidence interval (μmol/L) | Goodness of fit (r2) |
|---|---|---|---|
| 4a | 9.82 | 5.70–16.87 | 0.9793 |
| 4b | 2.91 | 0.16–53.58 | 0.7685 |
| 4c | 13.61 | 12.39–14.96 | 0.9980 |
| 4d | 11.75 | 7.52–18.32 | 0.9596 |
| 4e | 14.35 | 10.54–19.54 | 0.9921 |
| 4f | 11.19 | 9.20–13.61 | 0.9921 |
| 4g | 13.46 | 10.64–17.06 | 0.9694 |
| 4h | 10.79 | 10.21–11.40 | 0.9995 |
| COL | 0.95 | 0.12–7.31 | 0.9020 |
| PHZ | 13.24 | 8.83–19.86 | 0.9482 |
Acknowledgments
Conflicts of Interest
References
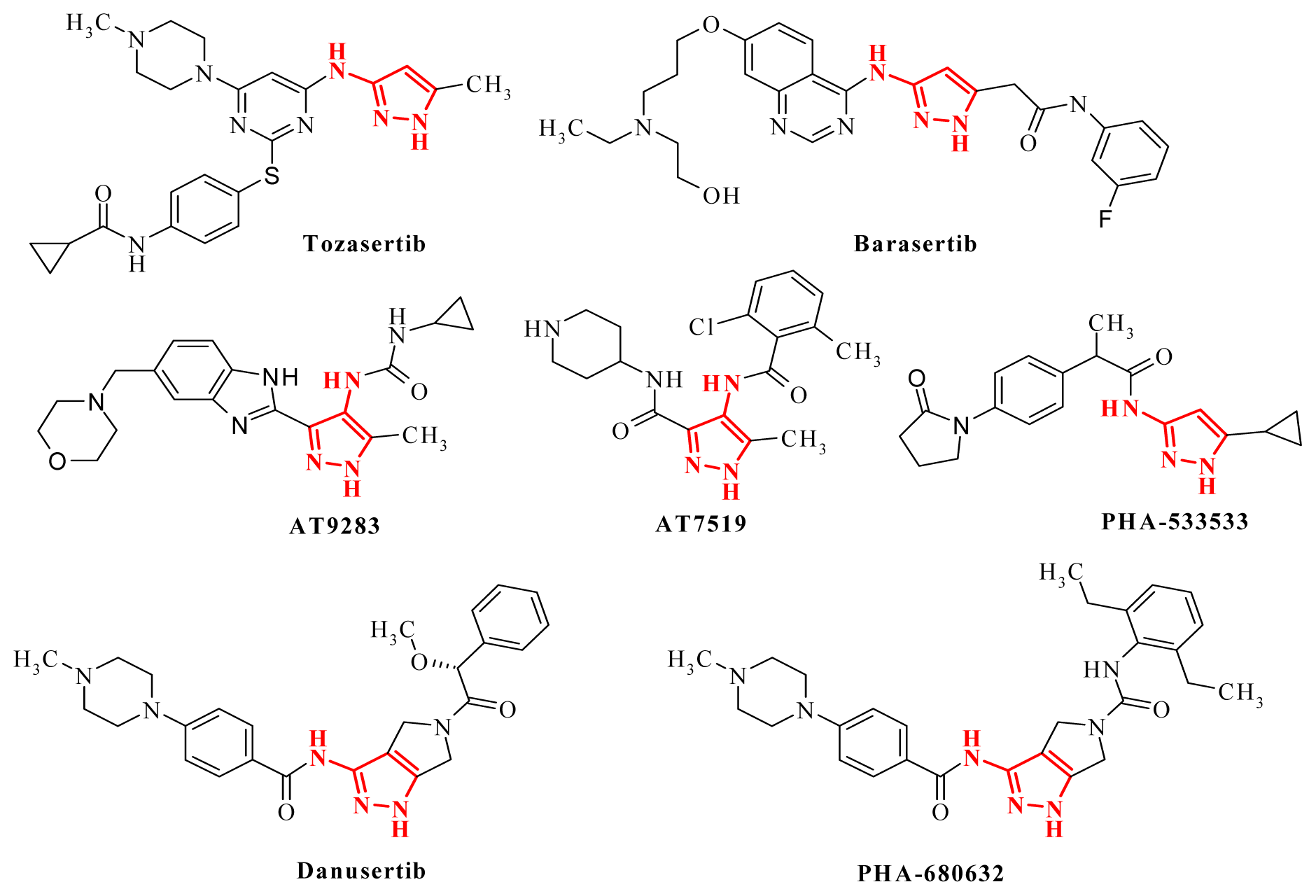
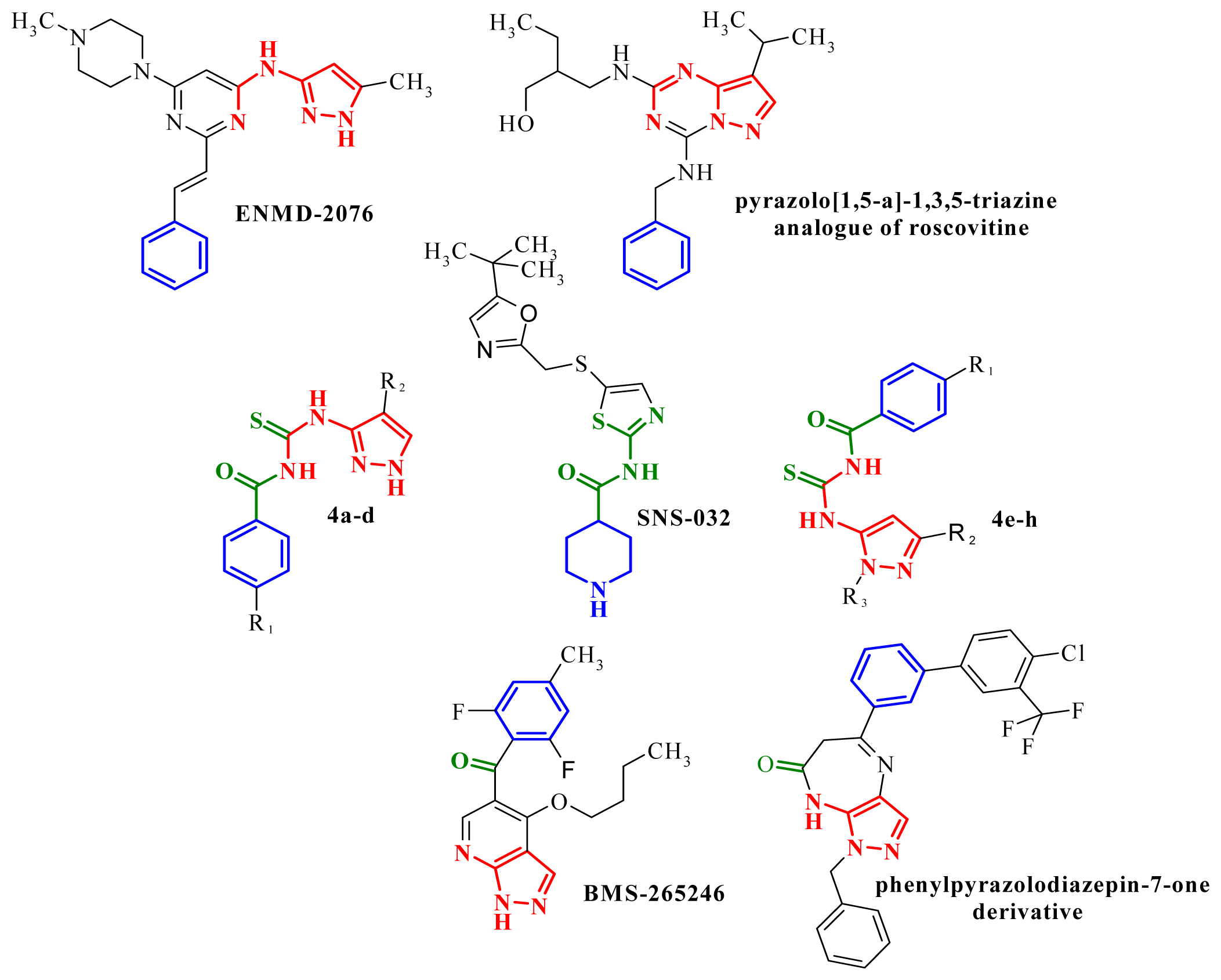
- Chahrour, O.; Cairns, D.; Omran, Z. Small molecule kinase inhibitors as anti-cancer therapeutics. Mini-Rev. Med. Chem 2012, 5, 399–411. [Google Scholar]
- Bebbington, D.; Binch, H.; Charrier, J.-D.; Everitt, S.; Fraysse, D.; Golec, J.; Kay, D.; Knegtel, R.; Mak, C.; Mazzei, F.; et al. The discovery of the potent Aurora inhibitor MK-0457 (VX-680). Bioorg. Med. Chem. Lett 2009, 19, 3586–3592. [Google Scholar]
- Fletcher, G.C.; Brokx, R.D.; Denny, T.A.; Hembrough, T.A.; Plum, S.M.; Fogler, W.E.; Sidor, C.F.; Bray, M.R. ENMD-2076 is an orally active kinase inhibitor with antiangiogenic and antiproliferative mechanisms of action. Mol. Cancer Ther 2011, 1, 126–137. [Google Scholar]
- Mortlock, A.A.; Foote, K.M.; Heron, N.M.; Jung, F.H.; Pasquet, G.; Lohmann, J.J.; Warin, N.; Renaud, F.; de Savi, C.; Roberts, N.J.; et al. Discovery, synthesis, and in vivo activity of a new class of pyrazoloquinazolines as selective inhibitors of aurora B kinase. J. Med. Chem 2007, 9, 2213–2224. [Google Scholar]
- Howard, S.; Berdini, V.; Boulstridge, J.A.; Carr, M.G.; Cross, D.M.; Curry, J.; Devine, L.A.; Early, T.R.; Fazal, L.; Gill, A.L.; et al. Fragment-based discovery of the pyrazol-4-yl urea (AT9283), a multitargeted kinase inhibitor with potent aurora kinase activity. J. Med. Chem 2009, 2, 379–388. [Google Scholar]
- Wyatt, P.G.; Woodhead, A.J.; Berdini, V.; Boulstridge, J.A.; Carr, M.G.; Cross, D.M.; Davis, D.J.; Devine, L.A.; Early, T.R.; Feltell, R.E.; et al. Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H-pyrazole-3-carboxamide (AT7519), a novel cyclin dependent kinase inhibitor using fragment-based X-ray crystallography and structure based drug design. J. Med. Chem 2008, 16, 4986–4999. [Google Scholar]
- Pevarello, P.; Brasca, M.G.; Orsini, P.; Traquandi, G.; Longo, A.; Nesi, M.; Orzi, F.; Piutti, C.; Sansonna, P.; Varasi, M.; et al. 3-Aminopyrazole inhibitors of CDK2/cyclin A as antitumor agents. 2. Lead optimization. J. Med. Chem 2005, 8, 2944–2956. [Google Scholar]
- Meulenbeld, H.J.; Mathijssen, R.H.; Verweij, J.; de Wit, R.; de Jonge, M.J. Danusertib, an aurora kinase inhibitor. Expert Opin. Invest. Drug 2012, 3, 383–393. [Google Scholar]
- Soncini, C.; Carpinelli, P.; Gianellini, L.; Fancelli, D.; Vianello, P.; Rusconi, L.; Storici, P.; Zugnoni, P.; Pesenti, E.; Croci, V.; et al. PHA-680632, a novel Aurora kinase inhibitor with potent antitumoral activity. Clin. Cancer Res 2006, 13, 4080–4089. [Google Scholar]
- Bindi, S.; Fancelli, D.; Alli, C.; Berta, D.; Bertrand, J.A.; Cameron, A.D.; Cappella, P.; Carpinelli, P.; Cervi, G.; Croci, V.; et al. Thieno[3,2-c]pyrazoles: A novel class of Aurora inhibitors with favorable antitumor activity. Bioorg. Med. Chem 2010, 19, 7113–7120. [Google Scholar]
- Kim, H.; Kim, M.; Lee, J.; Yu, H.; Hah, J.M. Syntheses of phenylpyrazolodiazepin-7-ones as conformationally rigid analogs of aminopyrazole amide scaffold and their antiproliferative effects on cancer cells. Bioorg. Med. Chem 2011, 22, 6760–6767. [Google Scholar]
- Popowycz, F.; Fournet, G.; Schneider, C.; Bettayeb, K.; Ferandin, Y.; Lamigeon, C.; Tirado, O.M.; Mateo-Lozano, S.; Notario, V.; Colas, P. Pyrazolo[1,5-a]-1,3,5-triazine as a purine bioisostere: Access to potent cyclin-dependent kinase inhibitor (R)-roscovitine analogue. J. Med. Chem 2009, 52, 655–663. [Google Scholar]
- Jorda, R.; Havlícek, L.; McNae, I.W.; Walkinshaw, M.D.; Voller, J.; Sturc, A.; Navrátilová, J.; Kuzma, M.; Mistrík, M.; Bártek, J.; et al. Pyrazolo[4,3-d]pyrimidine bioisostere of roscovitine: Evaluation of a novel selective inhibitor of cyclin-dependent kinases with antiproliferative activity. J. Med. Chem 2011, 54, 2980–2993. [Google Scholar]
- Nitulescu, G.M.; Draghici, C.; Missir, A.V. Synthesis of new pyrazole derivatives and their anticancer evaluation. Eur. J. Med. Chem 2010, 45, 4914–4919. [Google Scholar]
- Nitulescu, G.M.; Draghici, C.; Chifiriuc, M.-C.; Missir, A.V. Synthesis of isomeric N-(1-methyl-1-H-pyrazole-4-carbonyl)-N′-(xylyl)-thiourea and their antimicrobial evaluation. Farmacia 2009, 57, 527–533. [Google Scholar]
- Choudhary, A.; Raines, R.T. An evaluation of peptide-bond isosteres. Chembiochem 2011, 12, 1801–1807. [Google Scholar]
- Misra, R.N.; Xiao, H.Y.; Kim, K.S.; Lu, S.; Han, W.C.; Barbosa, S.A.; Hunt, J.T.; Rawlins, D.B.; Shan, W.; Ahmed, S.Z.; et al. N-(cycloalkylamino)acyl-2-aminothiazole inhibitors of cyclin-dependent kinase 2. N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-piperidinecarboxamide (BMS-387032), a highly efficacious and selective antitumor agent. J. Med. Chem 2004, 7, 1719–1728. [Google Scholar]
- Anwar, H.F.; Elnagdi, M.H. Recent developments in aminopyrazole chemistry. Arkivoc 2009, 1, 198–250. [Google Scholar]
- Aggarwal, R.; Kumar, V.; Kumar, R.; Singh, S.P. Approaches towards the synthesis of 5-aminopyrazoles. Beilstein J. Org. Chem 2011, 7, 179–197. [Google Scholar]
- Misra, R.N.; Xiao, H.Y.; Rawlins, D.B.; Shan, W.; Kellar, K.A.; Mulheron, J.G.; Sack, J.S.; Tokarski, J.S.; Kimball, S.D.; Webster, K.R. 1H-Pyrazolo[3,4-b]pyridine inhibitors of cyclin-dependent kinases: Highly potent 2,6-difluorophenacyl analogues. Bioorg. Med. Chem. Lett 2003, 14, 2405–2408. [Google Scholar]
- Nunes, B.S.; Carvalho, F.D.; Guilhermino, L.M.; van Stappen, G. Use of the genus Artemia in ecotoxicity testing. Environ. Pollut 2006, 2, 453–462. [Google Scholar]
- Kahru, A.; Dubourguier, H.-C.; Blinova, I.; Ivask, A.; Kasemets, K. Biotests and biosensors for ecotoxicology of metal oxide nanoparticles: A minireview. Sensors 2008, 8, 5153–5170. [Google Scholar]
- Tan, D.C.; Flematti, G.R.; Ghisalberti, E.L.; Sivasithamparam, K.; Barbetti, M.J. Toxigenicity of enniatins from Western Australian Fusarium species to brine shrimp (Artemia franciscana). Toxicon 2011, 57, 817–825. [Google Scholar]
- Rajeh, M.A.B.; Zuraini, Z.; Sasidharan, S.; Latha, L.Y.; Amutha, S. Assessment of Euphorbia hirta L. leaf, flower, stem and root extracts for their antibacterial and antifungal activity and brine shrimp lethality. Molecules 2010, 15, 6008–6018. [Google Scholar]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett 2003, 3, 185–194. [Google Scholar]
- El-Gohary, N.S.; Shaaban, M.I. Synthesis, antimicrobial, antiquorum-sensing, antitumor and cytotoxic activities of new series of fused [1,3,4] thiadiazoles. Eur. J. Med. Chem 2013, 63, 185–195. [Google Scholar]
- Sanchez-Fortun, S.; Barahona, M.V. Toxicity and characterization of cholinesterase-inhibition induced by diisopropyl fluorophosphate in Artemia salina larvae. Ecotoxicol. Environ. Safe 2009, 72, 775–780. [Google Scholar]
- Mansour, S.A.; Gad, M.F. Risk assessment of pesticides and heavy metals contaminants in vegetables: A novel bioassay method using Daphnia magna Straus. Food Chem. Toxicol 2010, 48, 377–389. [Google Scholar]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol 2006, 76, 122–159. [Google Scholar]
- Yuet Ping, K.; Darah, I.; Yusuf, U.K.; Yeng, C.; Sasidharan, S. Genotoxicity of Euphorbia hirta: An Allium cepa Assay. Molecules 2012, 17, 7782–7791. [Google Scholar]
- Crielaard, B.J.; van der Wal, S.; Le, H.T.; Bode, A.T.L.; Lammers, T.; Hennink, W.E.; Schiffelers, R.M.; Fens, M.H.; Storm, G. Liposomes as carriers for colchicine-derived prodrugs: Vascular disrupting nanomedicines with tailorable drug release kinetics. Eur. J. Pharm. Sci 2012, 45, 428–435. [Google Scholar]
- Rosu, T.; Pasculescu, S.; Lazar, V.; Chifiriuc, C.; Cernat, R. Copper(II) complexes with ligands derived from 4-amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one: Synthesis and biological activity. Molecules 2006, 11, 904–914. [Google Scholar]
- Issam, E.G.; Sanaa, S.; Paulo, C.A.; Youness, O.; Francisca, D.C.F.; Brahim, O.; Vitor, V. Effect of different microcystin profiles on toxin bioaccumulation in commoncarp (Cyprinus carpio) larvae via Artemia nauplii. Ecotoxicol. Environ. Safe 2010, 73, 762–770. [Google Scholar]
- Fan, W.; Cui, M.; Liu, H.; Wang, C.; Shi, Z.; Tan, C.; Yang, X. Nano-TiO2 enhances the toxicity of copper in natural water to Daphnia magna. Environ. Pollut 2011, 159, 729–734. [Google Scholar]
- Olaru, O.T.; Anghel, A.I.; Istudor, V.; Ancuceanu, R.V.; Dinu, M. Contributions to the pharmacognostical study of Fallopia aubertii (L. Henry) Holub. (Polygonaceae). Farmacia 2013, 61, 991–999. [Google Scholar]
- Xie, X.; Zhou, Q.; Lin, D.; Guo, J.; Bao, Y. Toxic effect of tetracycline exposure on growth, antioxidative and genetic indices of wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res 2011, 18, 566–575. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nitulescu, G.M.; Draghici, C.; Olaru, O.T. New Potential Antitumor Pyrazole Derivatives: Synthesis and Cytotoxic Evaluation. Int. J. Mol. Sci. 2013, 14, 21805-21818. https://doi.org/10.3390/ijms141121805
Nitulescu GM, Draghici C, Olaru OT. New Potential Antitumor Pyrazole Derivatives: Synthesis and Cytotoxic Evaluation. International Journal of Molecular Sciences. 2013; 14(11):21805-21818. https://doi.org/10.3390/ijms141121805
Chicago/Turabian StyleNitulescu, George Mihai, Constantin Draghici, and Octavian Tudorel Olaru. 2013. "New Potential Antitumor Pyrazole Derivatives: Synthesis and Cytotoxic Evaluation" International Journal of Molecular Sciences 14, no. 11: 21805-21818. https://doi.org/10.3390/ijms141121805





