1. Introduction
The detachment of single cells or aggregates of cells from biofilms is increasingly recognized as an intrinsic part of this surface-associated mode of bacterial existence. The detachment or dispersal of cells from the biofilm is typically viewed as the “final” step in biofilm development, as reviewed by [
1], which enables a fraction of the biofilm-associated cells to escape unfavorable conditions within the biofilm and initiate the establishment of a new biofilm elsewhere. While this conceptual view of biofilm development as a linear process, consisting of five consecutive phases [
2], can be beneficial in elucidating the various biotic and abiotic factors that influence biofilm formation, it can also obscure the fact that detachment does occur during earlier stages of the biofilm life-cycle and is not restricted to “mature” biofilm microcolonies only. For example it was estimated that up to 44% of
Pseudomonas aeruginosa PA01 cells emigrated within the first 35 h of attachment [
3].
Different detachment mechanisms have been identified and are generally classified as either passive or active based on whether the removal of attached biomass is due to factors such as human intervention (i.e., antimicrobial treatment), the result of abiotic forces (i.e., increase shear or particle abrasion), or an active microbial response to environmental cues that requires genetic regulation.
The rapid dissolution of biofilms of various Pseudomonad species in response to changes in carbon and nitrogen availability has been reported [
4–
6]. Large numbers of planktonic cells were observed to be swimming away from a previously stable
P. putida biofilm within minutes of the removal of carbon from the growth medium, or a cessation in the liquid flow [
5]. A decrease in the amount of intracellular secondary signaling molecule cyclic-di-GMP was found to induce biofilm dispersal through the induction of the LapG cysteine proteinase, which in turn modified the adhesion protein LapA, thereby dissolving the biofilm [
7]. This observation indicates that individual cells have the ability to dissociate themselves from their neighbors and/or the biofilm matrix.
“Seeding dispersal”, describes a process where large (>80 μm diameter)
P. aeruginosa PAO1 microcolonies break open to release a subpopulation of planktonic cells into the bulk-liquid [
8]. It is as yet unclear how widespread this phenomenon is among different bacterial species or whether the above-mentioned global dispersion-response to changes in the environment occurs by the same mechanism(s).
From a mass balance point of view, biofilm development is the net result of biomass accumulation and biomass detachment or decay. Indeed, biofilm biomass does not accumulate indefinitely, but instead a
pseudo steady state is reached, where further growth is balanced by detachment or decay. Passive biofilm detachment mechanisms have been defined, somewhat arbitrarily, as erosion or sloughing, based on the detachment frequency and size of the detached particle, in addition to biomass removal due to abrasion, predator grazing or human intervention strategies [
9]. The term “erosion” is frequently used to describe one of the most common, and likely underestimated, biofilm detachment processes. While erosion is defined in the Merriam-Webster Learner’s Dictionary as “the gradual destruction of something by natural forces”, or “the process by which something is worn away”, in biofilm-related literature the term “erosion” has been defined as the “continuous removal of small particles of biofilm” and is “presumed to be the result of shear forces exerted by moving fluid in contact with the biofilm surface” [
10]. In order to sustain a continuous detachment rate while maintaining a
pseudo steady state biofilm structure, continuous growth by the attached biomass is required. In fact, from a theoretical examination of a biofilm mass balance, it is evident that the maximum sustainable detachment rate is governed by the biomass growth rate [
10].
Previous work by Rittman in 1982 [
11] proposed a model wherein the rate of biofilm erosion due to liquid shear stress was dependent on the amount of attached biofilm, based on the analysis of a subset of empirical data produced by Trulear and Characklis (1982) [
12] for aerobic, multispecies biofilms cultivated in an annular reactor and subjected to different shear forces. Subsequent experimentation by Peyton and Characklis (1993) [
10] investigated the response of multispecies and pure culture
Pseudomonas aeruginosa biofilms to variations in shear stress (1.44, 2.20, or 2.97 Pa) and substrate availability (0.8, 4.0, or 7.2 grams glucose carbon m
−3). Biofilm substrate utilization rates and erosion rates were shown to increase with an increase in biofilm biomass, as proposed by the previous model [
11]. However, contrary to this model, the erosion rates were not directly related to the applied liquid shear stress, but instead to the substrate loading rates; where an increase in carbon availability led to an increase in the detachment rate. The authors furthermore re-analyzed the full data set previously published [
12] and found no indication of a linear relationship between biofilm erosion rates and the applied shear stress (
r2 = 0.00038).
Stewart (1993) [
13] further reinforced the link between a metabolically active biofilm region, responsible for carbon utilization, and biofilm detachment. Several mathematical expressions were derived to explain biofilm detachment, one of which explicitly accounted for spatial variation of growth rates within the biofilm. This expression provided a good qualitative prediction of detachment rates for the empirical steady state data from Trulear and Characklis (1982) [
12].
Despite the existence of these publications, the presence of single cells in the effluent of continuous-flow biofilm cultivation systems is still often ascribed to the erosion of the biofilm surface solely due to liquid shear stress [
14–
16].
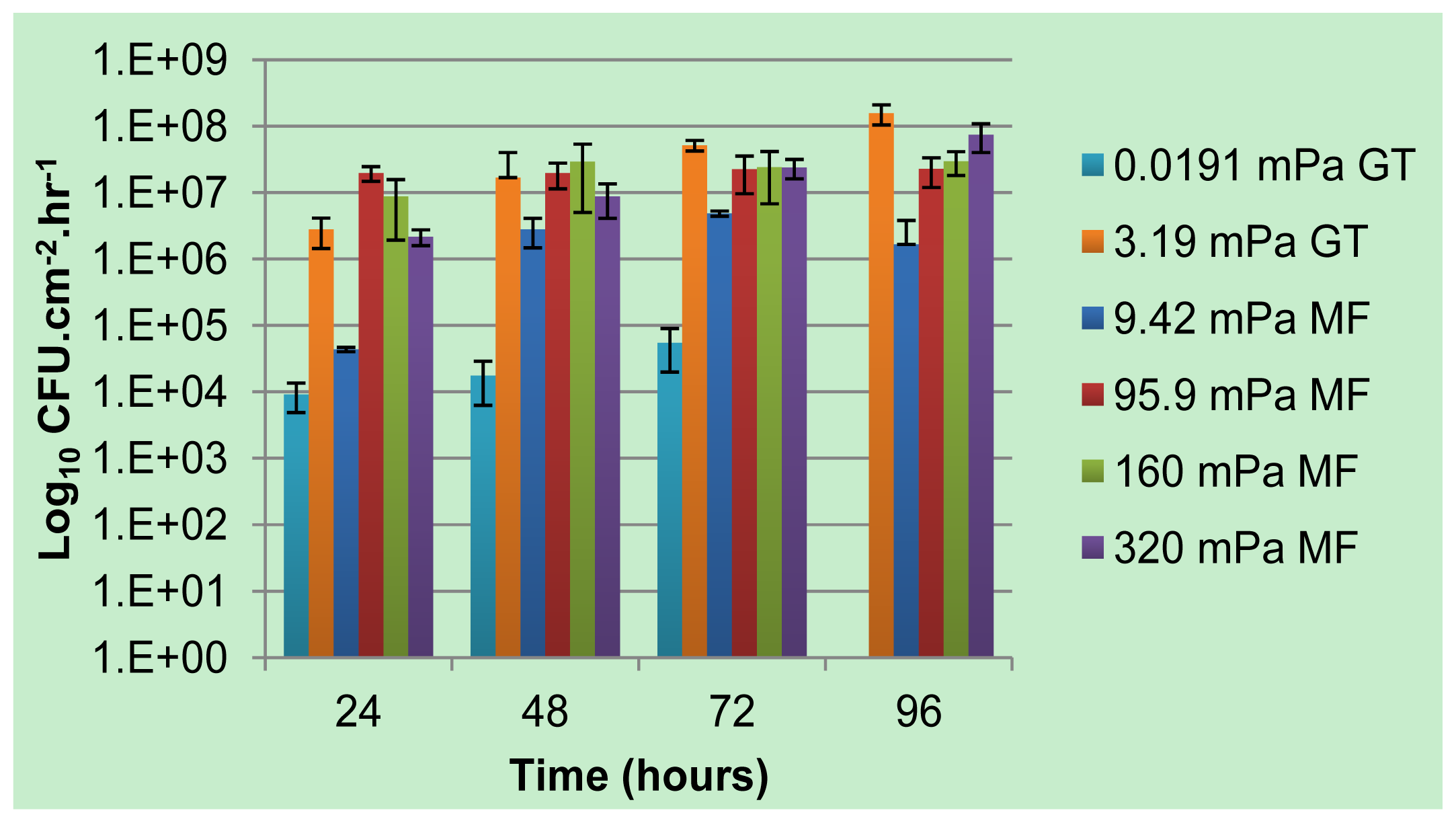
Previous work by our group has detailed the extent to which single and multi-species biofilms cultivated under continuous flow conditions in glass tubes produced and released planktonic cells to the bulk-liquid. Contrary to the accepted 5-stage model of biofilm development where dispersion of single cells from the biofilm only takes place upon maturation of a microcolony, an increase in planktonic cell numbers was evident as early as 6 hours after reactor inoculation [
17,
18]. The planktonic cell numbers in the effluent continued to increase during biofilm development and generally reached a plateau after 3 to 4 days, likely once the biofilm biomass reached a
pseudo steady state. Variation in the bulk-liquid flow rates, leading to average shear rates ranging from 19.1 × 10
−3 mPa to 93.9 mPa, did not result in a linear response in the magnitude of planktonic cells enumerated from the effluent, as would be expected if shear erosion was the dominant determinant of detachment (use of the term “average” to describe shear rates is due to the variation in shear rates along the cross section of a channel; lower shear rates will be present near the walls, with high shear rates in the center). Instead, the impact of reduced flow rates on the availability of nutrients and/or oxygen on biofilm development was found to be a greater determinant of planktonic cell numbers [
18]. The removal of the sole carbon source, while maintaining a constant average shear stress, was shown to not only reduce the metabolic activity of a biofilm to below detection limits, but was accompanied by a 1 to 2 order of magnitude reduction in the number of viable planktonic cells produced by the biofilm [
19]. Upon the re-introduction of carbon after eight days of starvation, the pre-disturbance levels of planktonic cell yield and CO
2 respiration rates were re-established within 24 h.
To distinguish these observations from the widely held erosion-mediated cell removal and to emphasize the role played by microbial physiology, we proposed the use of the term “biofilm-derived planktonic cell yield” to describe this process.
The use of square glass tubes with a comparatively large cross sectional area (0.04 cm
2), restricted the period of time during which biofilms could be subjected to high shear rates due to the large volumes of sterile growth media required. For example, a continuous flow rate of 450 mL h
−1, which results in an average shear stress of 93.9 mPa in an uncolonized glass tube, could only be maintained for 12 h in a typical laboratory continuous flow system [
18]. Using microfluidic flowcells, we quantify various biofilm parameters as well as biofilm-derived planktonic cell yield under higher average shear stresses than those applied previously to determine whether
Pseudomonas sp. strain CT07
gfp biofilms can maintain their physical structure (biomass) as well as the continuous yield of planktonic cells if subjected to significant removal forces.
3. Experimental Section
3.1. Strain and Culture Conditions
An environmental pseudomonad, isolated from a cooling tower and designated as
Pseudomonas sp. strain CT07 (GenBank Accession number DQ 777633) was used for all of the experimentation [
17]. A gene sequence encoding for the constitutive expression of a green fluorescent protein (GFP) was inserted into the bacterial chromosome using a mini-Tn7 system as previously described [
18].
Cultivation took place at room temperature (24 ± 2 °C) in modified AB defined medium (final concentration of 1.51 mmol/L (NH
4)
2SO
4, 3.37 mmol/L Na
2HPO
4, 2.20 mmol/L KH
2PO
4, 179 mmol/L NaCl, 0.1 mmol/L MgCl
2·6H
2O, 0.01 mmol/L CaCl
2·2H
2O and 0.001 mmol/L FeCl
3) [
31] with 1 mmol/L Na-Citrate·6H
2O as the sole carbon source. Pre-cultures were incubated in batch with aeration for 16–18 h prior to the inoculation of microfluidic channels.
3.2. Microfluidic Device Fabrication
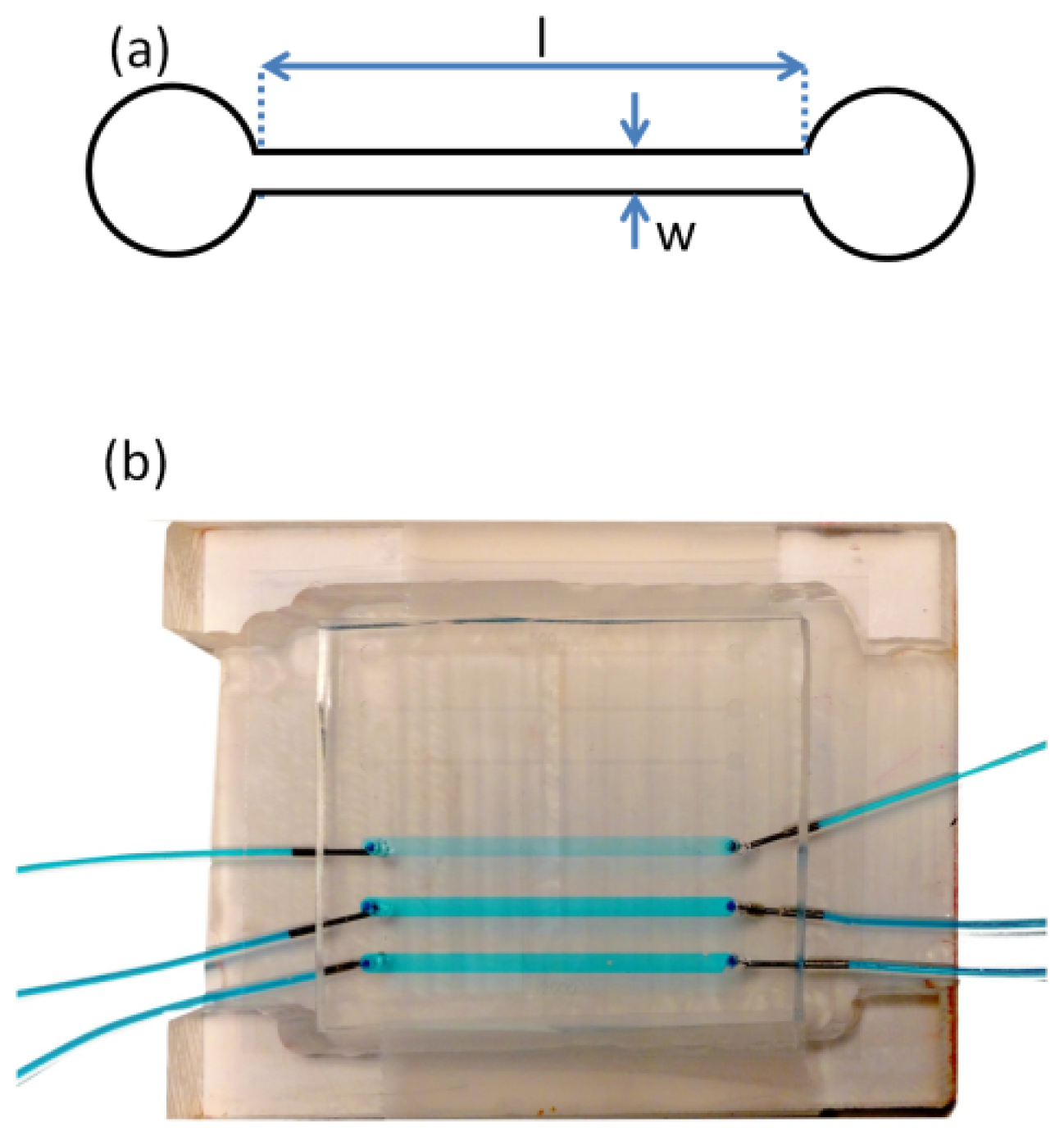
A silicon template containing microfeatures from a photoresist (SU-8 50, Microchem Inc., Newton, MA, USA) was prepared via photolithography, based on a photo mask that was produced using computer aided design software (AutoCAD). The height of all features on the silicon template was 130 μm as defined by the spin coating process. After photolithography, the silicon template contained the inverse features required for the microfluidic device. These consisted of two sets of channels in triplicate, for statistical purposes. The first set of channels had a width (
w) of 2 mm and the second had a width of 300 μm. All 6 channels had a length (
l) of 40 mm. In this study, only the 300 μm-wide channels were used in order to achieve the desired range of average shear rates.
Figure 7a shows a schematic of one
w = 300 μm channel. The microfluidic devices were prepared by casting uncured poly dimethyl siloxane (PDMS) (Ellsworth Adhesives Canada, Syligard 184) against the silicon template and heating to 70 °C overnight [
32]. After the cured PDMS was demoulded from the silicon template the micro fabrication contained the features of the microfluidic channel and inlet and outlet access holes were punched at each end of the channel. Finally, a glass cover slip (VWR, catalogue number CA48404-143) was bonded to the PDMS after exposure to air plasma (HARRICK PLASMA-PCD-001) for 90 s, thereby sealing the device. The coverslip thickness was 170 μm, which matched working distance requirements for the confocal microscope objectives. Liquid delivery tubing was connected to the device inlets and outlets via metal elbow capillaries and glued in place using multi-purpose silicone sealant. The entire device was placed in a custom polycarbonate holder, which positioned the device with the glass side up for inspection by the upright confocal imaging system (
Figure 7).
3.3. Continuous-Flow Cultivation of Biofilms
Biofilms were cultivated in microfluidic channels for up to 4 days. Thick-walled silicone tubing (Cole Parmer, 0.89 mm inner diameter, catalogue number 07 625-26) connected the inlet of each microfluidic channel to a multi-channel syringe pump (Model NE-1600, New Era Pump Systems Inc., Wantagh, NY, USA), while Tygon tubing transferred the effluent to a waste container.
The connective tubing and channels were disinfected with a once-through flow of ethanol for 1.5 h, followed by sterile distilled water for a minimum of 24 h. The rinsing water was replaced with sterile growth medium and any air bubbles were flushed out prior to stopping the flow for inoculation. A sterile needle and syringe were used to inject 0.05 mL of the pre-culture containing on average 107 CFU mL−1 into each channel, by puncturing a hole through the upstream silicone tubing. The hole was sealed immediately afterwards with all-purpose silicone sealant, and liquid flow was initiated after 10 min at different flow velocities with the syringe pump. The sterile distilled water and growth medium were placed in an ultrasonic cleaner and sonicated at 40 kHz for 20–40 min to remove excess dissolved gases prior to aspirating the liquid into sterile syringes and connecting the syringes to the inlet tubing.
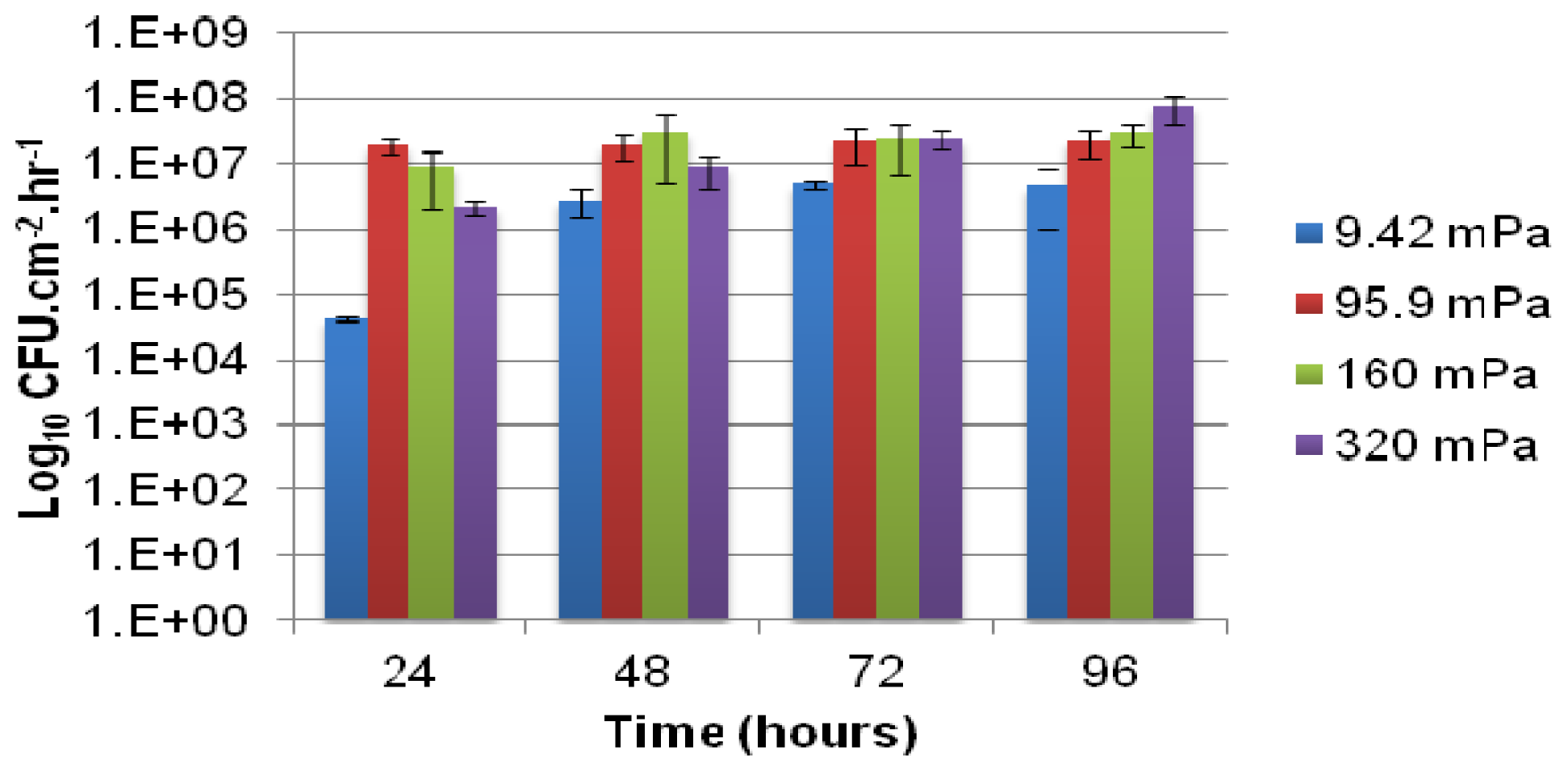
Biofilm development and the associated planktonic cell yield were determined at four different growth medium flow velocities (28.6 × 10
−3, 29.1 × 10
−2, 48.5 × 10
−2, 97.0 × 10
−2 mL h
−1) resulting in laminar flow in the channels. The corresponding average shear stresses were calculated by
Equation (1) below, and are noted in
Table 1 together with Reynolds number for each of the four flow rates.
where u is the dynamic viscosity (taken as 0.001002 Pa.s for water at 20 °C), Q is the volumetric flow rate (m3 s−1), w is the width of the channel (m) and h is the height of the channel (m).
3.4. Biofilm-Derived Planktonic Cell Yield
3.4.1. Viable Cell Counts
The number of viable cells that were released from the biofilm and became entrained in the bulk-liquid phase was determined using serial dilution in sterile saline (0.9% NaCl) and drop plating onto agar-solidified-modified AB medium, containing 10 mmol L−1 citrate. Connectors were emplaced near the outlet side of each channel from which effluent was collected at 24-h intervals after inoculation. The number of colony forming units per milliliter (CFU mL−1) of effluent was enumerated after 4 to 6 days of incubation at room temperature and normalized with respect to the growth medium flow velocity (mL h−1) and the total internal surface area of the channel consisting of both PDMS and glass (cm2) to compare the rate of cell yield (CFU cm−2 h−1) among biofilms subjected different shear rates.
3.4.2. Qualitative Assessment of Biomass Size Distribution
In addition to daily sampling the effluent for viable cell counts, additional effluent was collected periodically. These samples were incubated with formaldehyde (final concentration 3.8% v/v) to preserve cell integrity and prevent growth and cell division, at 4 °C prior to dilution of a subsample (if necessary) and incubation with the fluorescent nucleic acid stain 4′,6-diamidino-2-phenylindole (DAPI) at a final concentration of 20 μg mL−1 for 20 min in the absence of light. Each sample was filtered onto a black polycarbonate filter (0.2 μm pore-size, 25 mm diameter, Nucleopore, Whatman), followed by placing the filter on a microscope slide with drop of Citifluor antifade mounting medium (AF2, Electron Microscopy Sciences, Hatfield, PA, USA) and a coverslip. Several microscope fields were investigated at random from each filter (60× oil immersion objective, Nikon 90i epifluorescent microscope, Mississauga, ON, Canada) to qualitatively evaluate the size distribution of detached biomass (single cells vs. large biomass aggregates).
3.5. Confocal Scanning Laser Microscopy and COMSTAT Image Analysis
The extent of biofilm development in duplicate microfluidic channels was examined at 24 hour-intervals with confocal scanning laser microscopy (CLSM, Nikon Eclipse 90i, Mississauga, ON, Canada), using a 40×/0.75 Plan-Fluor objective with excitation of the green fluorescent protein at 488 nm and detection of emission with a band pass 515/30 filter. Ten microscope fields, each with an area of 101,761 μm
2 (318 μm × 318 μm), were chosen at random along a central transect starting from the channel inlet, and a stack of images was captured in the
z-direction at 0.60 μm intervals and stored for subsequent analysis with COMSTAT [
33].
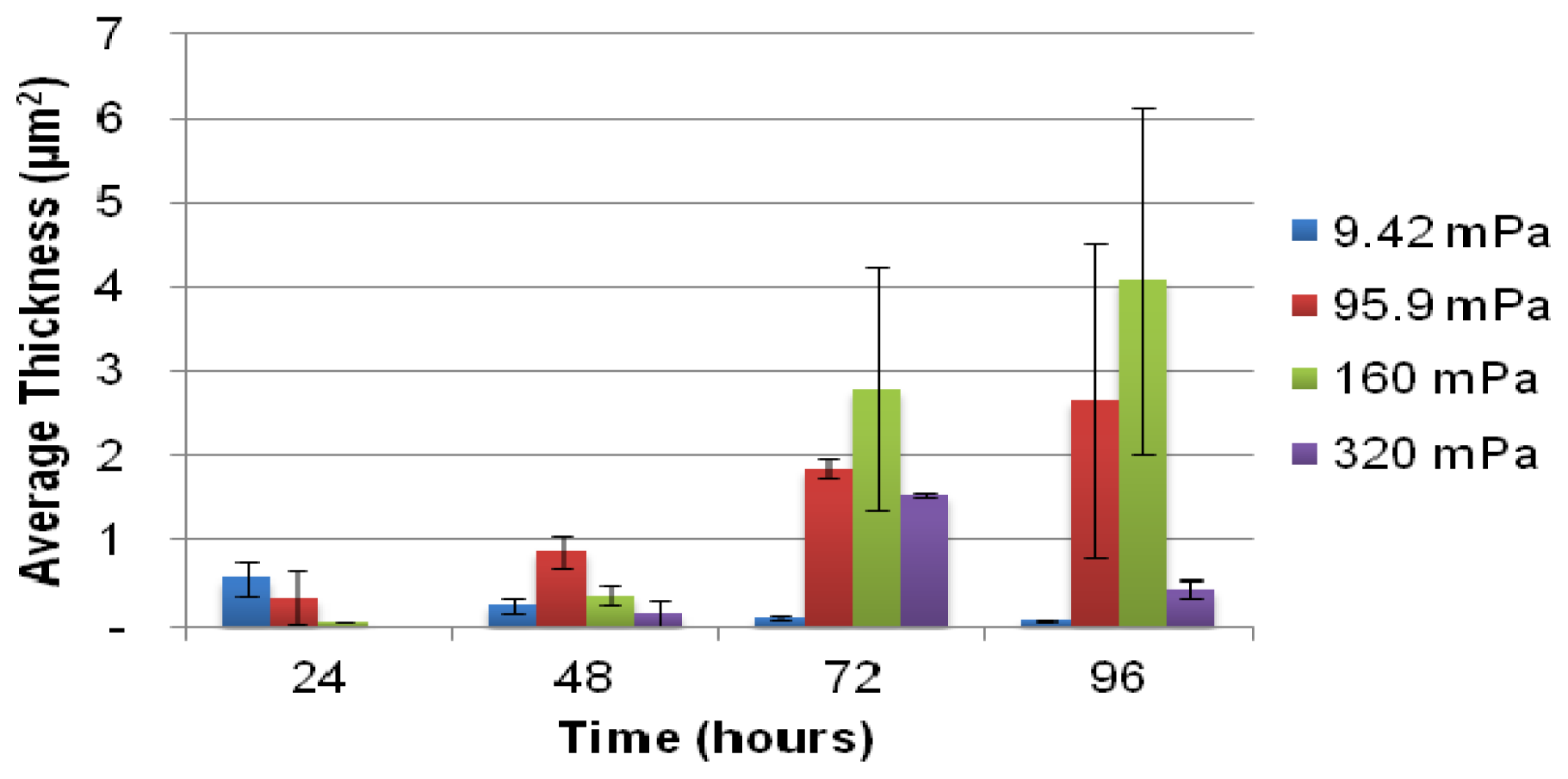
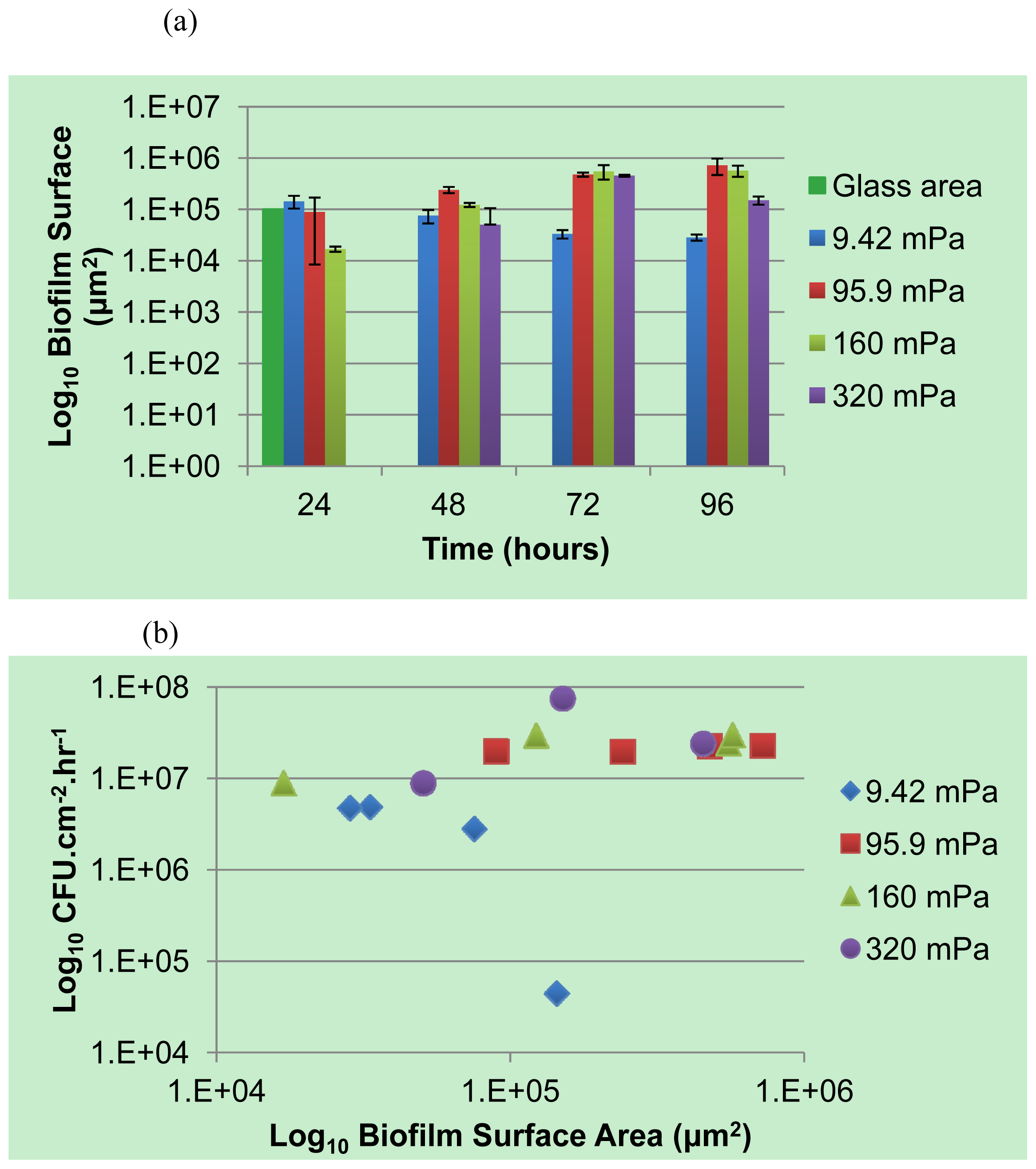
A selected number of the COMSTAT functions were used for the analysis of the biofilm: the biovolume of each image stack (μm3 μm−2), the mean thickness of the biofilm (μm) and the biofilm surface area-to-volume ratio (μm2 μm−3). The biofilm surface area (um2) (i.e., the area of the biofilm exposed to the bulk-liquid) was calculated using the results of COMSTAT analysis, by multiplying the biofilm biovolume (μm2 μm−3) by the xy attachment area (101,761 μm2), and the surface area-to-biovolume ratio (μm2 μm−3) for each Z-stack of images. The corresponding values obtained correlated well with the degree of biofilm development, as seen in the extent of substratum coverage and average biofilm thickness. The values were primarily utilized for qualitative purposes to identify potential relationships between the planktonic cell yield from biofilms and the different shear forces that they are subjected to.
3.6. Statistical Analysis
The extent of biofilm-derived planktonic cell yield for each of the four growth medium flow velocities was evaluated in 2 independent experimental rounds. Each round consisted of biofilm development in duplicate microfluidic channels. The planktonic cell yield data was analyzed using two-factor Analysis of variance (ANOVA, with replication) at a significance level of 0.05, followed by pair-wise comparisons with Tukey’s Post Hoc test.
3.7. Comsol Simulations
Shear stress simulations were conducted using Comsol MultiPhysics (version 3.5) on a PC system featuring an Intel Core i5-2400 (3.10 GHz) processor running Windows 7 with 64-bit precision. Course, physics-controlled mesh was verified to produce rapid results without sacrificing accuracy. A segment of the channel was created with dimensions 300 μm × 130 μm with channel length between the inlet and measurement point being chosen as 1 mm. Flow was directed along the axis connecting the inlet and outlet with the inlet velocity calculated based on the flow rate and the channel dimensions. The wall shear stress was measured along the long edge of the channel cross-section (300 μm) for each flow rate and a smoothing algorithm was used to remove small digitization effects related from the course mesh.
4. Conclusions
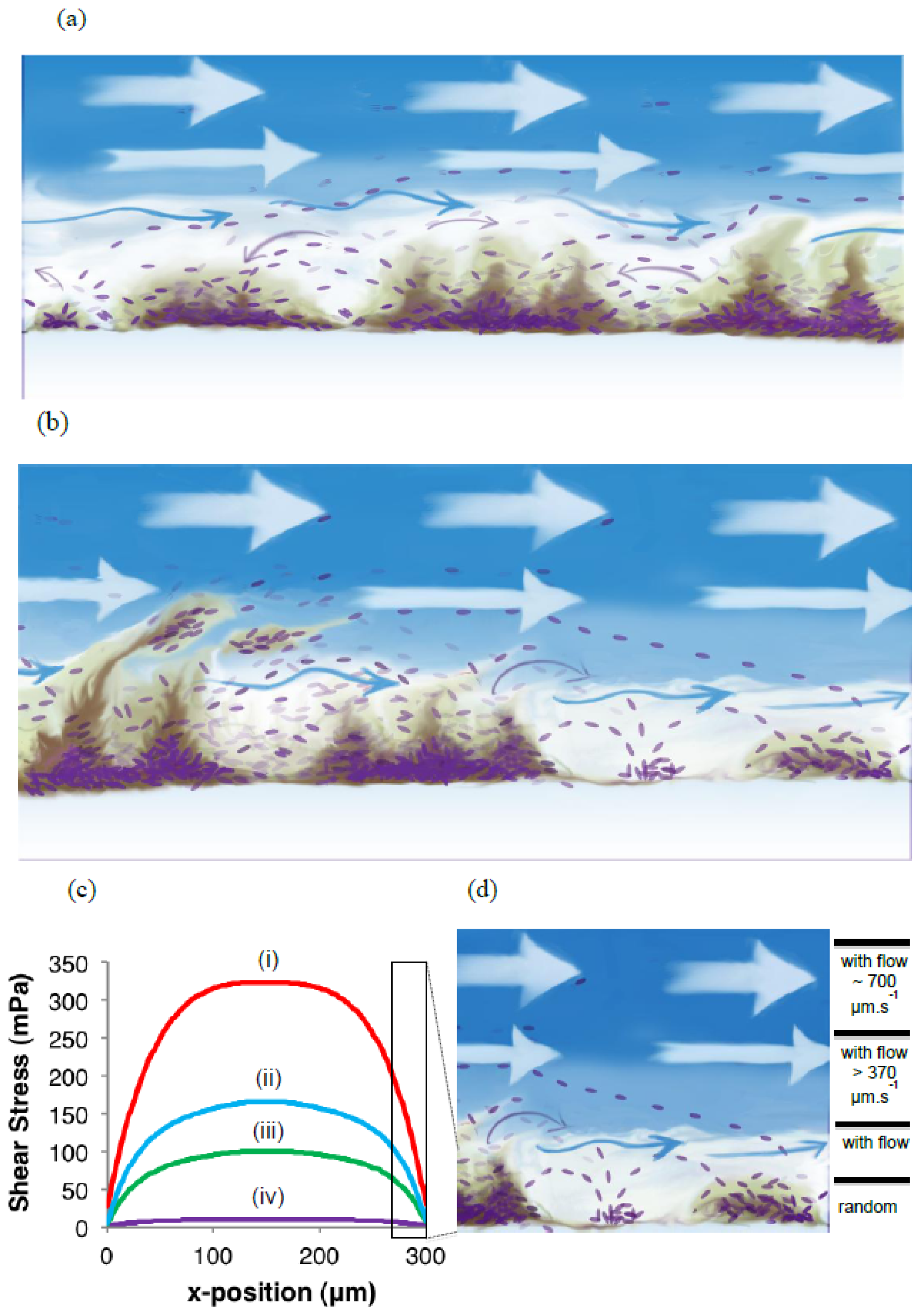
The role of biofilms as a microbial survival strategy is often recognized, such as the continued survival of EPS-encased microbes despite multiple antimicrobial challenges. This study demonstrates that in addition to the prevention of cell washout at high liquid shear rates, biofilms also fulfill a proliferation function with continuous cell release into the bulk-liquid, and that shear rates have relatively little impact on such biofilm-to-planktonic cell yield. Instead, biofilms utilize shear to maintain optimum thickness and metabolic activity, which differs from the traditional view that biofilms primarily consist of slow-growing cells.
As a complement to the conventional conceptual biofilm models, we propose the existence of a biofilm surface-associated zone of planktonic cell proliferation. The results suggest the existence of a zone at the biofilm-liquid interface with high metabolic activity where cells are neither irreversibly attached nor imbedded in the EPS matrix, and where shear rates are too low to cause erosion. Such a surface-associated zone with non-attached cells greatly extends biofilm function and probably consists mostly of cells that do not have a typical biofilm phenotype. This phenomenon emphasizes the need to develop improved techniques to better define the EPS boundaries within which cells are immobilized. It appears possible that the EPS in this region is highly responsive to the prevailing environmental conditions, with microbes having the ability to modify EPS rigidity in order to restrict cell movement and thereby maintaining biofilm biomass under conditions that do not support active metabolism and cell replication, or under conditions conducive to microbial activity to allow cells to move away in order to maintain optimum gradients of nutrients and metabolites.
The production and release of significant numbers of planktonic cells by biofilms has long been disregarded. However, results indicate that the traditional perception of biofilms as promoting microbial survival should be expanded to include the potential to contribute to microbial proliferation.