The Protective Effect of Bcl-xl Overexpression against Oxidative Stress-Induced Vascular Endothelial Cell Injury and the Role of the Akt/eNOS Pathway
Abstract
:1. Introduction
2. Results and Discussion
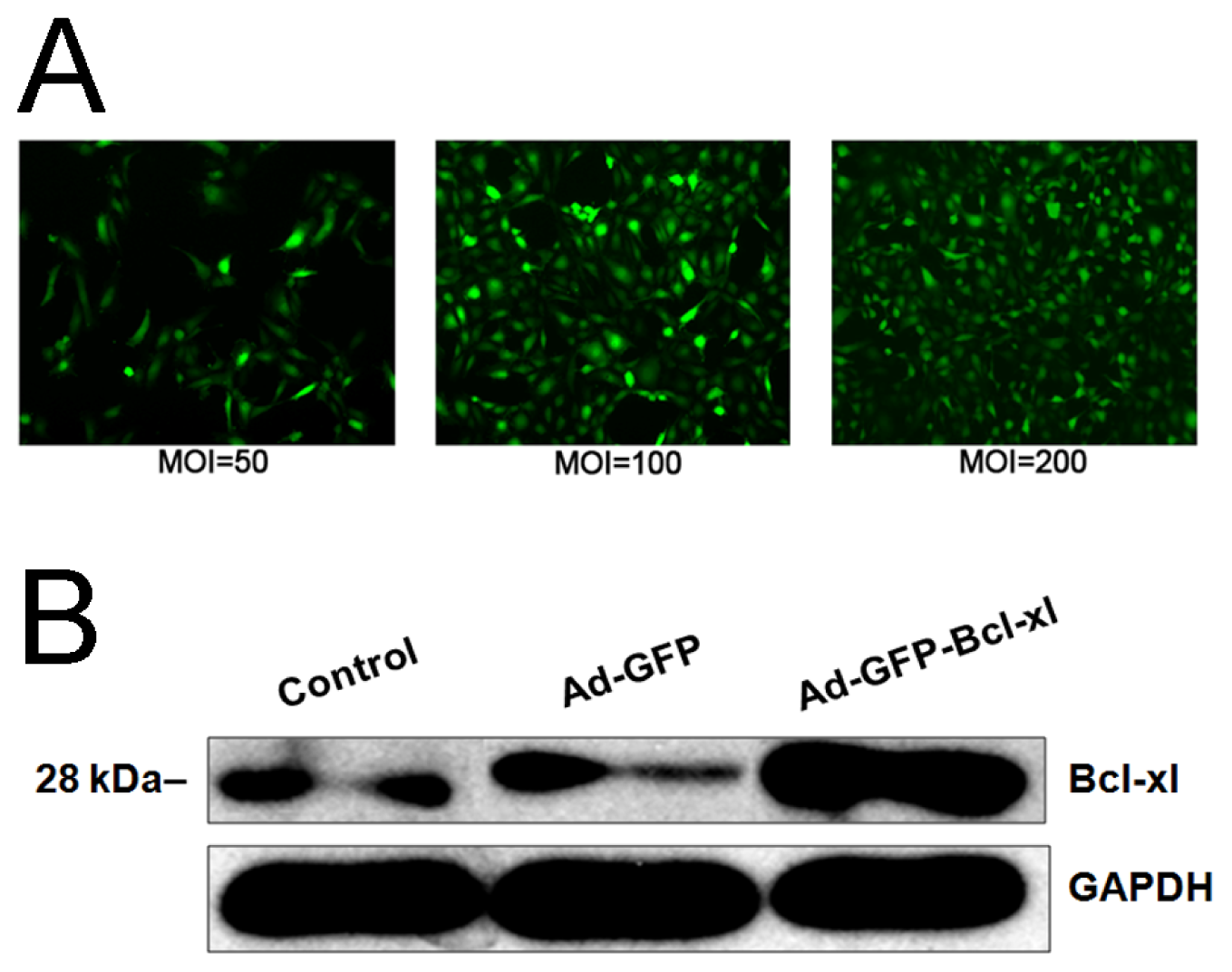
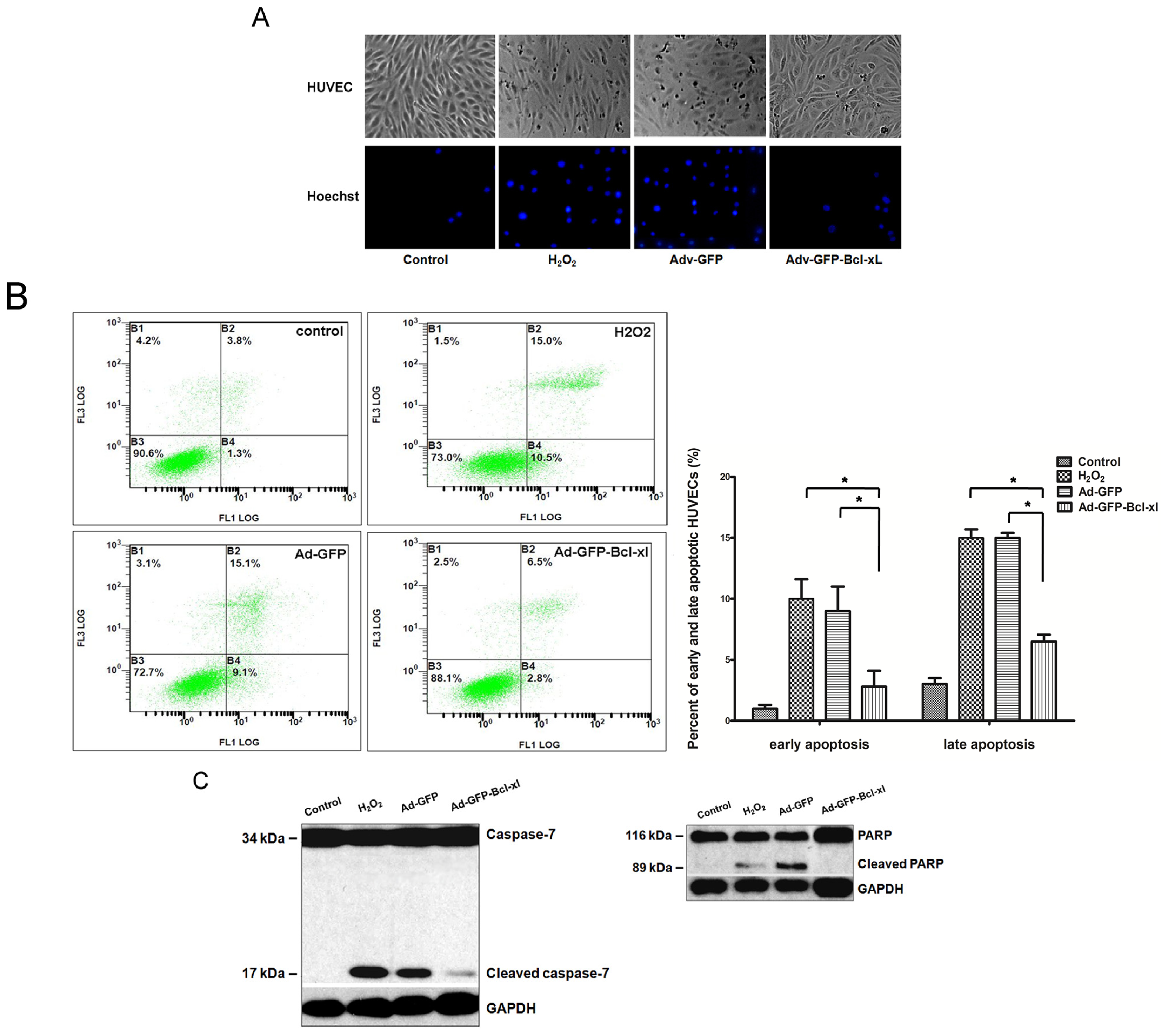
2.1. Bcl-xl Overexpression Attenuates Oxidative Injury-Induced Apoptosis in HUVECs
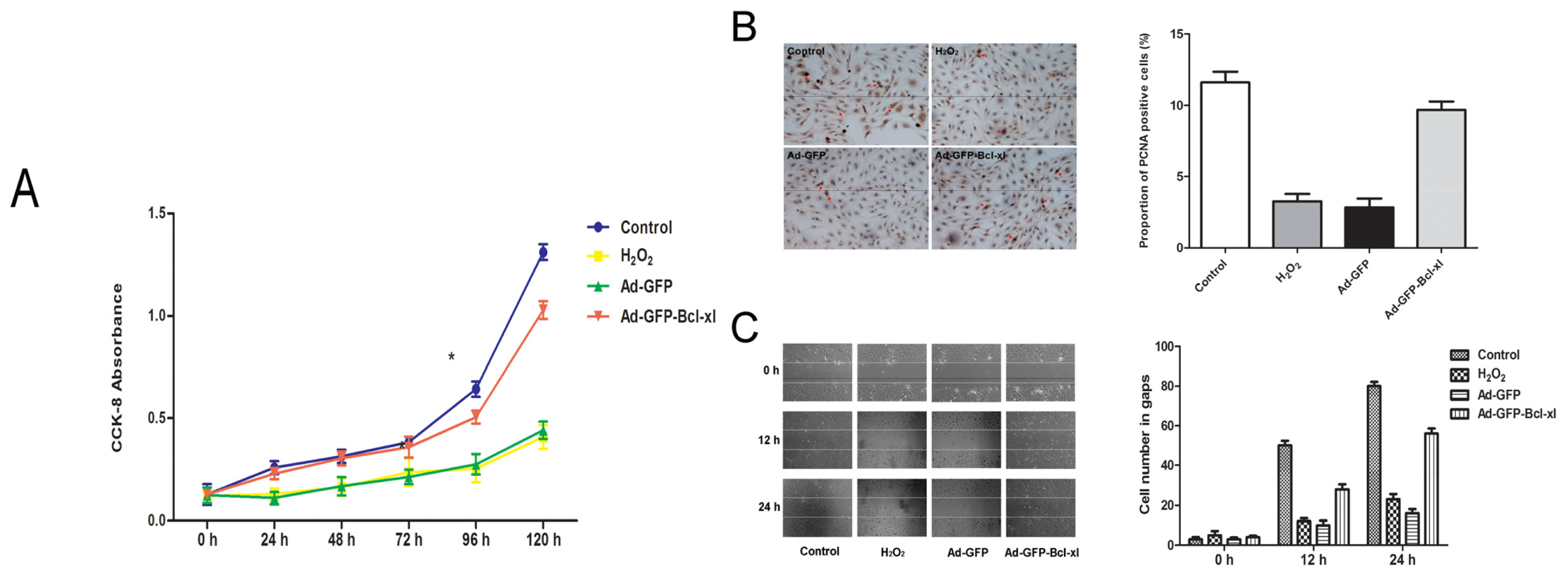
2.2. Protective Effect of Bcl-xl Overexpression on Maintaining the Viability of HUVECs
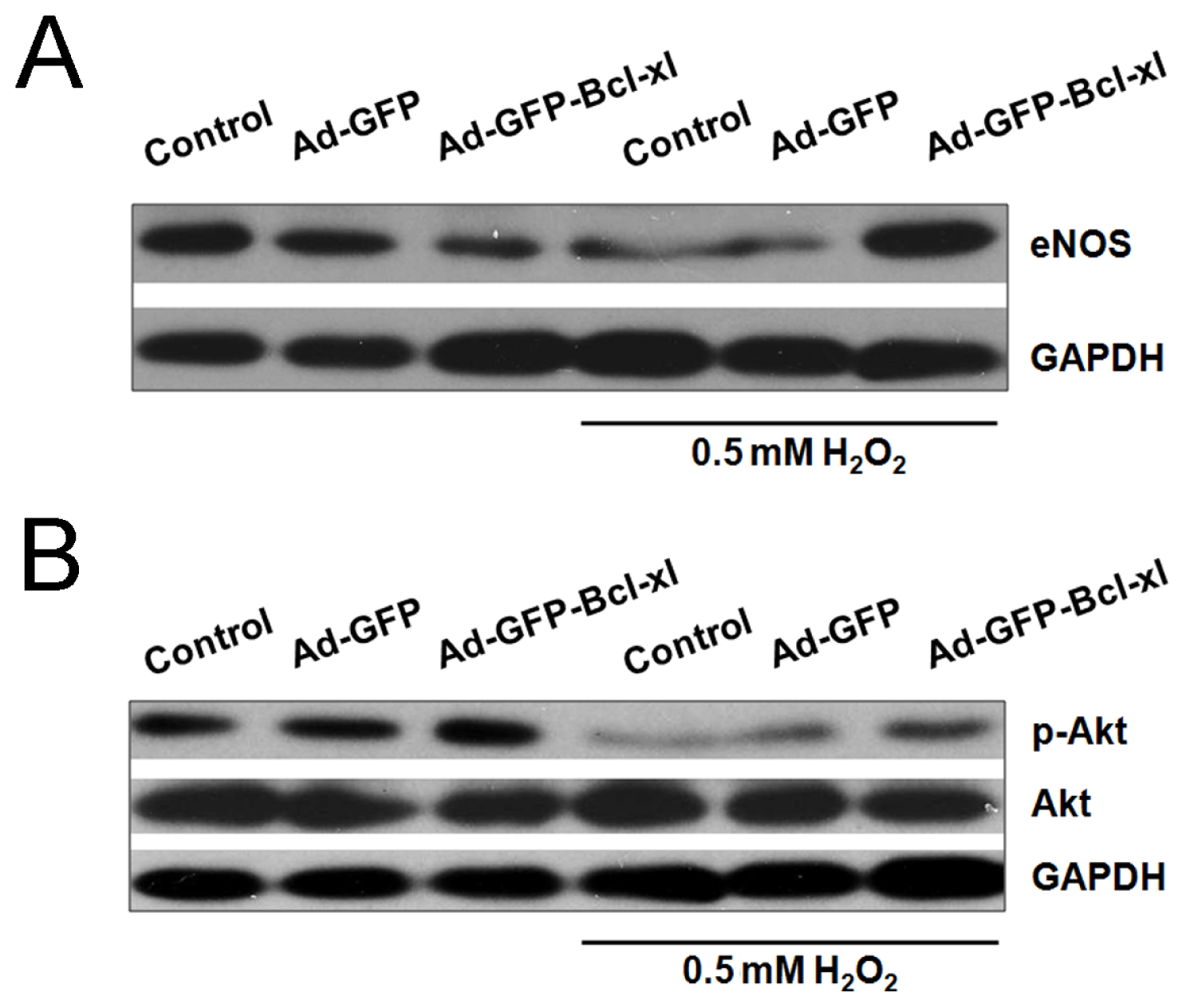
2.3. Involvement of the Akt/eNOS Pathway in Bcl-xl gene Transfected H2O2-Stimulated HUVECs
2.4. Discussion
3. Experimental Section
3.1. Culturing of HUVECs
3.2. Generation of Recombinant Adenovirus Vectors
3.3. HUVECs Infection with Recombinant Adenovirus
3.4. Treatment of HUVECs with Hydrogen Peroxide
3.5. Analysis of Apoptosis by Hoechst 33,258 and Annexin V/PI Staining
3.6. Analysis of Protein Levels by Western Blotting
3.7. Cell Viability Assay
3.8. Immunocytochemical Detection
3.9. Scratching Assay
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Guan, H.; Li, Y.J.; Xu, Z.R.; Li, G.W.; Guo, X.H.; Liu, Z.M.; Zou, D.J.; Xing, H.L.; Liu, W.; Sheng, Z.Y.; et al. Prevalence and risk factors of peripheral arterial disease in diabetic patients over 50 years old in China. Chin. Med. Sci. J 2007, 22, 83–88. [Google Scholar]
- Rosenbaum, M.A.; Miyazaki, K.; Colles, S.M.; Graham, L.M. Antioxidant therapy reverses impaired graft healing in hypercholesterolemic rabbits. J. Vasc. Surg 2010, 51, 184–193. [Google Scholar]
- Kipshidze, N.; Dangas, G.; Tsapenko, M.; Moses, J.; Leon, M.B.; Kutryk, M.; Serruys, P. Role of the endothelium in modulating neointimal formation: Vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J. Am. Coll. Cardiol 2004, 44, 733–739. [Google Scholar]
- Rudijanto, A. The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med. Indones 2007, 39, 86–93. [Google Scholar]
- Durand, E.; Scoazec, A.; Lafont, A.; Boddaert, J.; Al Hajzen, A.; Addad, F.; Mirshahi, M.; Desnos, M.; Tedgui, A.; Mallat, Z. In vivo induction of endothelial apoptosis leads to vessel thrombosis and endothelial denudation: A clue to the understanding of the mechanisms of thrombotic plaque erosion. Circulation 2004, 109, 2503–2506. [Google Scholar]
- Zhao, J.; Bolton, E.M.; Bradley, J.A.; Lever, A.M. Lentiviral-mediated overexpression of Bcl-xl protects primary endothelial cells from ischemia/reperfusion injury-induced apoptosis. J. Heart Lung Transplant 2009, 28, 936–943. [Google Scholar]
- Huang, J.; Ito, Y.; Morikawa, M.; Uchida, H.; Kobune, M.; Sasaki, K.; Abe, T.; Hamada, H. Bcl-xl gene transfer protects the heart against ischemia/reperfusion injury. Biochem. Biophys. Res. Commun 2003, 311, 64–70. [Google Scholar]
- Clowes, A.W.; Reidy, M.A. Prevention of stenosis after vascular reconstruction: Pharmacologic control of intimal hyperplasia—A review. J. Vasc. Surg 1991, 13, 885–891. [Google Scholar]
- Aoki, M.; Nata, T.; Morishita, R.; Matsushita, H.; Nakagami, H.; Yamamoto, K.; Yamazaki, K.; Nakabayashi, M.; Ogihara, T.; Kaneda, Y. Endothelial apoptosis induced by oxidative stress through activation of NF-kappaB: Antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension 2001, 38, 48–55. [Google Scholar]
- Cines, D.B.; Pollak, E.S.; Buck, C.A.; Loscalzo, J.; Zimmerman, G.A.; McEver, R.P.; Pober, J.S.; Wick, T.M.; Konkle, B.A.; Schwartz, B.S.; et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91, 3527–3561. [Google Scholar]
- Zhang, J.; Tan, Z.; Tran, N.D. Chemical hypoxia-ischemia induces apoptosis in cerebromicrovascular endothelial cells. Brain Res 2000, 877, 134–140. [Google Scholar]
- Bombeli, T.; Schwartz, B.R.; Harlan, J.M. Endothelial cells undergoing apoptosis become proadhesive for nonactivated platelets. Blood 1999, 93, 3831–3838. [Google Scholar]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol 2008, 585, 325–337. [Google Scholar]
- Yao, E.H.; Yu, Y.; Fukuda, N. Oxidative stress on progenitor and stem cells in cardiovascular diseases. Curr. Pharm. Biotechnol 2006, 7, 101–108. [Google Scholar]
- Kayanoki, Y.; Higashiyama, S.; Suzuki, K.; Asahi, M.; Kawata, S.; Matsuzawa, Y.; Taniguchi, N. The requirement of both intracellular reactive oxygen species and intracellular calcium elevation for the induction of heparin-binding EGF-like growth factor in vascular endothelial cells and smooth muscle cells. Biochem. Biophys. Res. Commun 1999, 259, 50–55. [Google Scholar]
- DeForge, L.E.; Preston, A.M.; Takeuchi, E.; Kenney, J.; Boxer, L.A.; Remick, D.G. Regulation of interleukin 8 gene expression by oxidant stress. J. Biol. Chem 1993, 268, 25568–25576. [Google Scholar]
- Wung, B.S.; Cheng, J.J.; Hsieh, H.J.; Shyy, Y.J.; Wang, D.L. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ. Res 1997, 81, 1–7. [Google Scholar]
- Cai, H. Hydrogen peroxide regulation of endothelial function: Origins, mechanisms, and consequences. Cardiovasc. Res 2005, 68, 26–36. [Google Scholar]
- Ma, J.; Lau, C.K.; Obed, A.; Dada, A.; Doenecke, A.; Fan, S.T.; Schlitt, H.J.; Tsui, T.Y. A cell penetrating heme oxygenase protein protects heart graft against ischemia/reperfusion injury. Gene Ther 2009, 16, 320–328. [Google Scholar]
- Qian, J.; Jiang, F.; Wang, B.; Yu, Y.; Zhang, X.; Yin, Z.; Liu, C. Ophiopogonin D prevents H2O2-induced injury in primary human umbilical vein endothelial cells. J. Ethnopharmacol 2010, 128, 438–445. [Google Scholar]
- Gopaul, N.K.; Anggard, E.E.; Mallet, A.I.; Betteridge, D.J.; Wolff, S.P.; Nourooz-Zadeh, J. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett 1995, 368, 225–229. [Google Scholar]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res 2000, 86, 494–501. [Google Scholar]
- Morrow, J.D.; Frei, B.; Longmire, A.W.; Gaziano, J.M.; Lynch, S.M.; Shyr, Y.; Strauss, W.E.; Oates, J.A.; Roberts, L.J., II. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N. Engl. J. Med 1995, 332, 1198–1203. [Google Scholar]
- Ahanchi, S.S.; Tsihlis, N.D.; Kibbe, M.R. The role of nitric oxide in the pathophysiology of intimal hyperplasia. J. Vasc. Surg 2007, 45, A64–A73. [Google Scholar]
- Shen, B.; Gao, L.; Hsu, Y.T.; Bledsoe, G.; Hagiwara, M.; Chao, L.; Chao, J. Kallistatin attenuates endothelial apoptosis through inhibition of oxidative stress and activation of Akt-eNOS signaling. Am. J. Physiol. Heart Circ. Physiol 2010, 299, H1419–H1427. [Google Scholar]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol 2012, 86, 1649–1665. [Google Scholar]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Natural antioxidants: Therapeutic prospects for cancer and neurological diseases. Mini Rev. Med. Chem 2009, 9, 1202–1214. [Google Scholar]
- Wang, B.; Luo, T.; Chen, D.; Ansley, D.M. Propofol reduces apoptosis and up-regulates endothelial nitric oxide synthase protein expression in hydrogen peroxide-stimulated human umbilical vein endothelial cells. Anesth. Analg 2007, 105, 1027–1033. [Google Scholar]
- Herdeg, C.; Oberhoff, M.; Baumbach, A.; Blattner, A.; Axel, D.I.; Schroder, S.; Heinle, H.; Karsch, K.R. Local paclitaxel delivery for the prevention of restenosis: Biological effects and efficacy in vivo. J. Am. Coll. Cardiol. 2000, 35, 1969–1976. [Google Scholar]
- Shibata, R.; Kai, H.; Seki, Y.; Kusaba, K.; Takemiya, K.; Koga, M.; Jalalidin, A.; Tokuda, K.; Tahara, N.; Niiyama, H.; et al. Rho-kinase inhibition reduces neointima formation after vascular injury by enhancing Bax expression and apoptosis. J. Cardiovasc. Pharmacol 2003, 42, S43–S47. [Google Scholar]
- Tang, X.; Yang, X.; Peng, Y.; Lin, J. Protective effects of lycopene against H2O2-induced oxidative injury and apoptosis in human endothelial cells. Cardiovasc. Drugs Ther 2009, 23, 439–448. [Google Scholar]
- Li, Y.; Liu, J.; Zhan, X. Tyrosine phosphorylation of cortactin is required for H2O2-mediated injury of human endothelial cells. J. Biol. Chem 2000, 275, 37187–37193. [Google Scholar]
- Igase, M.; Okura, T.; Kitami, Y.; Hiwada, K. Apoptosis and Bcl-xs in the intimal thickening of balloon-injured carotid arteries. Clin. Sci 1999, 96, 605–612. [Google Scholar]
- Asada, S.; Fukuda, K.; Nishisaka, F.; Matsukawa, M.; Hamanisi, C. Hydrogen peroxide induces apoptosis of chondrocytes; involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm. Res 2001, 50, 19–23. [Google Scholar]
- Asahara, T.; Chen, D.; Tsurumi, Y.; Kearney, M.; Rossow, S.; Passeri, J.; Symes, J.F.; Isner, J.M. Accelerated restitution of endothelial integrity and endothelium-dependent function after phVEGF165 gene transfer. Circulation 1996, 94, 3291–3302. [Google Scholar]
- Dimmeler, S.; Zeiher, A.M. Nitric oxide-an endothelial cell survival factor. Cell. Death Differ 1999, 6, 964–968. [Google Scholar]
- Cooney, R.; Hynes, S.O.; Sharif, F.; Howard, L.; O’Brien, T. Effect of gene delivery of NOS isoforms on intimal hyperplasia and endothelial regeneration after balloon injury. Gene Ther 2007, 14, 396–404. [Google Scholar]
- Chavakis, E.; Dernbach, E.; Hermann, C.; Mondorf, U.F.; Zeiher, A.M.; Dimmeler, S. Oxidized LDL inhibits vascular endothelial growth factor-induced endothelial cell migration by an inhibitory effect on the Akt/endothelial nitric oxide synthase pathway. Circulation 2001, 103, 2102–2107. [Google Scholar]
- Zhang, L.N.; Wilson, D.W.; da Cunha, V.; Sullivan, M.E.; Vergona, R.; Rutledge, J.C.; Wang, Y.X. Endothelial NO synthase deficiency promotes smooth muscle progenitor cells in association with upregulation of stromal cell-derived factor-1alpha in a mouse model of carotid artery ligation. Arterioscler. Thromb. Vasc. Biol 2006, 26, 765–772. [Google Scholar]
- Kapadia, M.R.; Chow, L.W.; Tsihlis, N.D.; Ahanchi, S.S.; Eng, J.W.; Murar, J.; Martinez, J.; Popowich, D.A.; Jiang, Q.; Hrabie, J.A.; et al. Nitric oxide and nanotechnology: A novel approach to inhibit neointimal hyperplasia. J. Vasc. Surg 2008, 47, 173–182. [Google Scholar]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest 1973, 52, 2745–2756. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ni, L.; Li, T.; Liu, B.; Song, X.; Yang, G.; Wang, L.; Miao, S.; Liu, C. The Protective Effect of Bcl-xl Overexpression against Oxidative Stress-Induced Vascular Endothelial Cell Injury and the Role of the Akt/eNOS Pathway. Int. J. Mol. Sci. 2013, 14, 22149-22162. https://doi.org/10.3390/ijms141122149
Ni L, Li T, Liu B, Song X, Yang G, Wang L, Miao S, Liu C. The Protective Effect of Bcl-xl Overexpression against Oxidative Stress-Induced Vascular Endothelial Cell Injury and the Role of the Akt/eNOS Pathway. International Journal of Molecular Sciences. 2013; 14(11):22149-22162. https://doi.org/10.3390/ijms141122149
Chicago/Turabian StyleNi, Leng, Tianjia Li, Bao Liu, Xitao Song, Genhuan Yang, Linfang Wang, Shiying Miao, and Changwei Liu. 2013. "The Protective Effect of Bcl-xl Overexpression against Oxidative Stress-Induced Vascular Endothelial Cell Injury and the Role of the Akt/eNOS Pathway" International Journal of Molecular Sciences 14, no. 11: 22149-22162. https://doi.org/10.3390/ijms141122149



