Synthetic Resveratrol Analogue, 3,3',4,4',5,5'-Hexahydroxy-trans-Stilbene, Accelerates Senescence in Peritoneal Mesothelium and Promotes Senescence-Dependent Growth of Gastrointestinal Cancers
Abstract
:1. Introduction
2. Results and Discussions
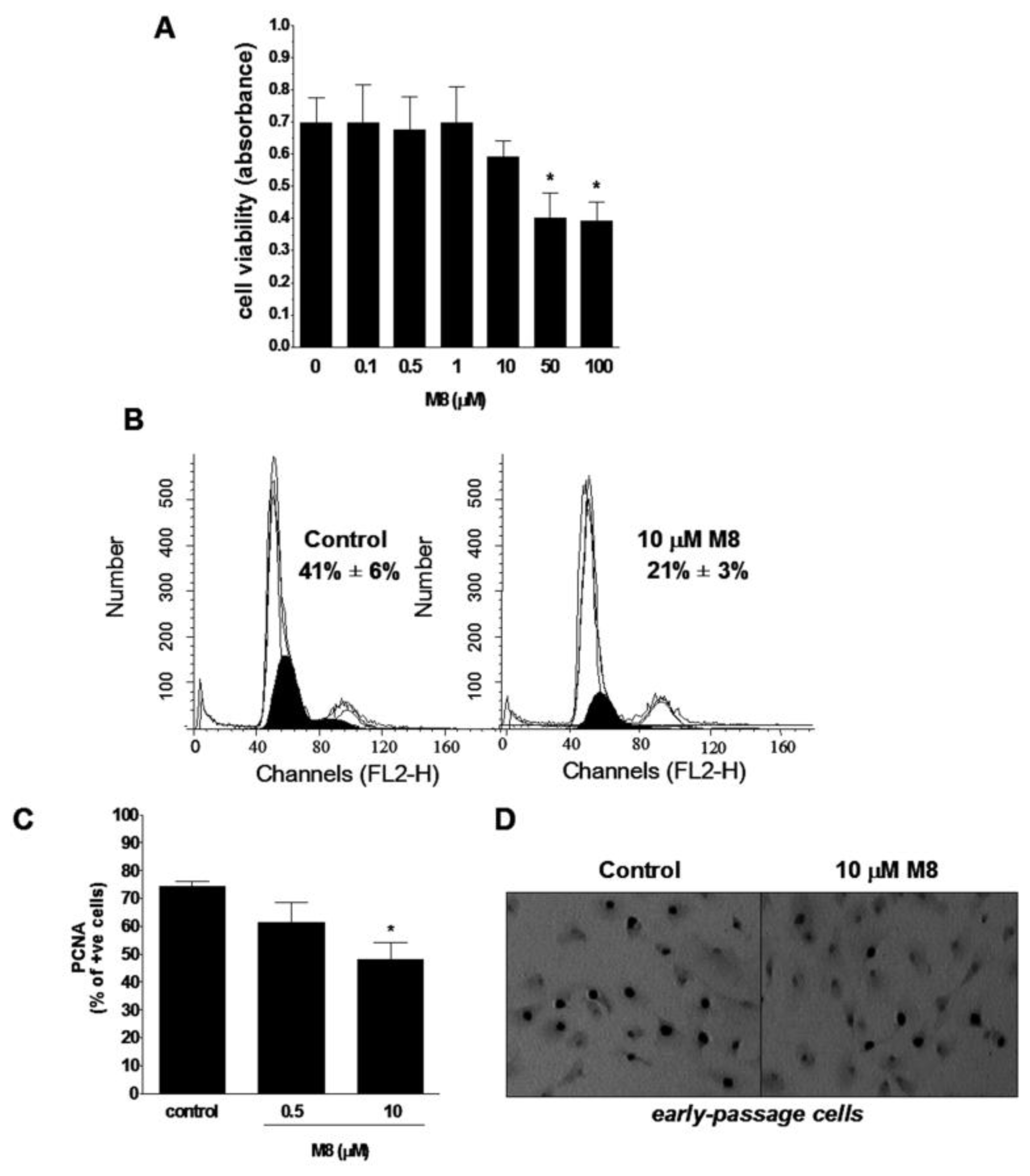
2.1. Stilbene M8 (3,3′,4,4′,5,5′-Hexahydroxy-trans-Stilbene) Impairs Viability of HPMCs (Human Peritoneal Mesothelial Cells) at the Doses Higher than 10 μM
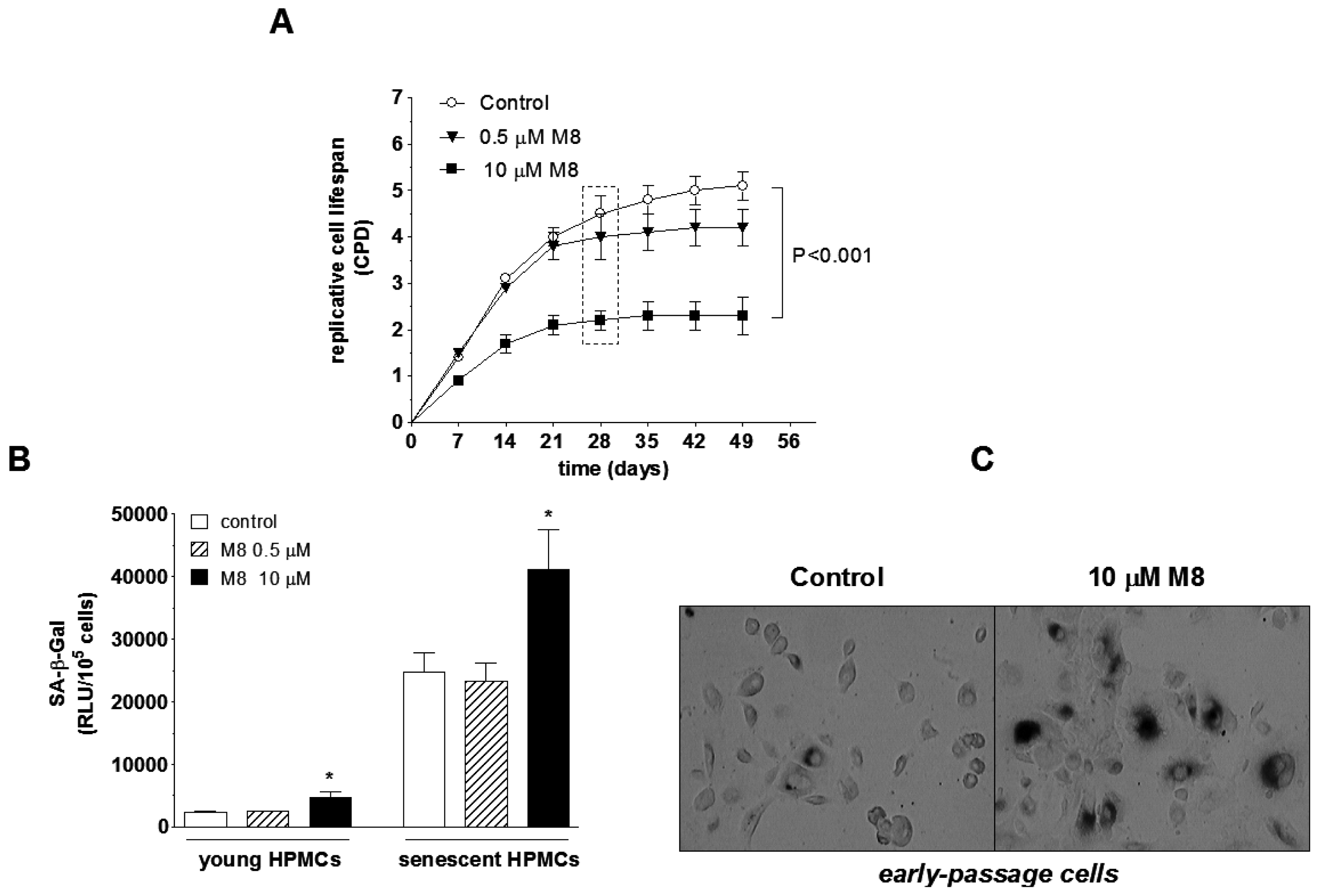
2.2. Stilbene M8 Inhibits Proliferation of Early-Passage HPMCs
2.3. Stilbene M8 Accelerates Senescence of HPMCs
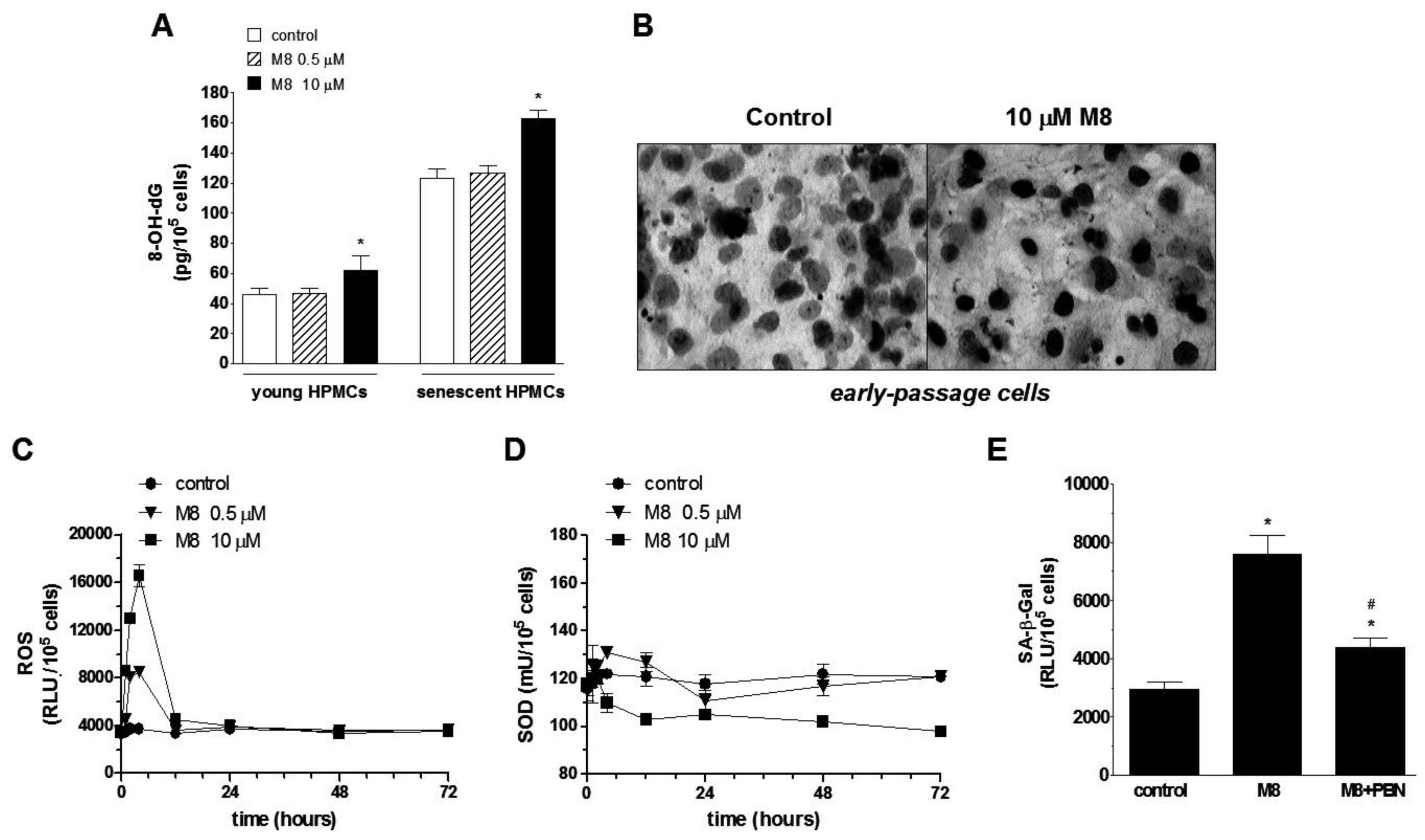
2.4. Stilbene M8 Induces Oxidative Stress in Early-Passage and Senescent HPMCs
2.5. Stilbene M8 Promotes Senescent HPMC-Dependent Growth of Cancer Cells
2.6. Discussion
3. Experimental Section
3.1. Chemicals
3.2. Cell Cultures
3.3. Determination of Cell Viability
3.4. Cell Cycle Analysis
3.5. Immunocytochemistry for Proliferating Cell Nuclear Antigen (PCNA)
3.6. Detection of Senescence-Associated β-Galactosidase (SA-β-Gal)
3.7. Measurements of Oxidative Stress-Related Parameters
3.8. HPMC-Derived Conditioned Medium
3.9. Cancer Cell Proliferation Measurements
3.10. Western Blotting for Cell Cycle Regulators
3.11. Statistics
4. Conclusions


Acknowledgments
Conflicts of Interest
References
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar]
- Campisi, J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell 2005, 120, 513–522. [Google Scholar]
- Giovannelli, L.; Pitozzi, V.; Jacomelli, M.; Mulinacci, N.; Laurenzana, A.; Dolara, P.; Mocali, A. Protective effects of resveratrol against senescence-associated changes in cultured human fibroblasts. J. Gerontol. A Biol. Sci. Med. Sci 2011, 66, 9–18. [Google Scholar]
- Mikula-Pietrasik, J.; Kuczmarska, A.; Rubis, B.; Filas, V.; Murias, M.; Zielinski, P.; Piwocka, K.; Ksiazek, K. Resveratrol delays replicative senescence of human mesothelial cells via mobilization of antioxidative and DNA repair mechanisms. Free Radic. Biol. Med 2012, 52, 2234–2245. [Google Scholar]
- Marques, F.Z.; Markus, M.A.; Morris, B.J. Resveratrol: Cellular actions of a potent natural chemical that confers a diversity of health benefits. Int. J. Biochem. Cell Biol 2009, 41, 2125–2128. [Google Scholar]
- Smoliga, J.M.; Baur, J.A.; Hausenblas, H.A. Resveratrol and health—A comprehensive review of human clinical trials. Mol. Nutr. Food Res 2011, 55, 1129–1141. [Google Scholar]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res 2004, 24, 2783–2840. [Google Scholar]
- Belleri, M.; Ribatti, D.; Nicoli, S.; Cotelli, F.; Forti, L.; Vannini, V.; Stivala, L.A.; Presta, M. Antiangiogenic and vascular-targeting activity of the microtubule-destabilizing trans-resveratrol derivative 3,5,4′-trimethoxystilbene. Mol. Pharmacol 2005, 67, 1451–1459. [Google Scholar]
- Kimura, Y.; Sumiyoshi, M.; Baba, K. Antitumor activities of synthetic and natural stilbenes through antiangiogenic action. Cancer Sci 2008, 99, 2083–2096. [Google Scholar]
- Fulda, S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today 2010, 15, 757–765. [Google Scholar]
- Murias, M.; Luczak, M.W.; Niepsuj, A.; Krajka-Kuzniak, V.; Zielinska-Przyjemska, M.; Jagodzinski, P.P.; Jager, W.; Szekeres, T.; Jodynis-Liebert, J. Cytotoxic activity of 3,3′,4,4′,5,5′-hexahydroxystilbene against breast cancer cells is mediated by induction of p53 and downregulation of mitochondrial superoxide dismutase. Toxicol. In Vitro 2008, 22, 1361–1370. [Google Scholar]
- Saiko, P.; Horvath, Z.; Murias, M.; Handler, N.; Jaeger, W.; Erker, T.; Fritzer-Szekeres, M.; Szekeres, T. Antitumor effects of 3,3′,4,4′,5,5′-hexahydroxystilbene in HL-60 human promyelocytic leukemia cells. Nucleosides Nucleotides Nucleic Acids 2006, 25, 1013–1017. [Google Scholar]
- Ovesna, Z.; Kozics, K.; Bader, Y.; Saiko, P.; Handler, N.; Erker, T.; Szekeres, T. Antioxidant activity of resveratrol, piceatannol and 3,3′,4,4′,5,5′-hexahydroxy-trans-stilbene in three leukemia cell lines. Oncol. Rep 2006, 16, 617–624. [Google Scholar]
- Paulitschke, V.; Schicher, N.; Szekeres, T.; Jager, W.; Elbling, L.; Riemer, A.B.; Scheiner, O.; Trimurtulu, G.; Venkateswarlu, S.; Mikula, M.; et al. 3,3′,4,4′,5,5′-Hexahydroxystilbene impairs melanoma progression in a metastatic mouse model. J. Invest. Dermatol 2010, 130, 1668–1679. [Google Scholar]
- Ruweler, M.; Gulden, M.; Maser, E.; Murias, M.; Seibert, H. Cytotoxic, cytoprotective and antioxidant activities of resveratrol and analogues in C6 astroglioma cells in vitro. Chem. Biol. Interact. 2009, 182, 128–135. [Google Scholar]
- Horvath, Z.; Murias, M.; Saiko, P.; Erker, T.; Handler, N.; Madlener, S.; Jaeger, W.; Grusch, M.; Fritzer-Szekeres, M.; Krupitza, G.; et al. Cytotoxic and biochemical effects of 3,3′,4,4′,5,5′-hexahydroxystilbene, a novel resveratrol analog in HL-60 human promyelocytic leukemia cells. Exp. Hematol 2006, 34, 1377–1384. [Google Scholar]
- Horvath, Z.; Marihart-Fazekas, S.; Saiko, P.; Grusch, M.; Ozsuy, M.; Harik, M.; Handler, N.; Erker, T.; Jaeger, W.; Fritzer-Szekeres, M.; et al. Novel resveratrol derivatives induce apoptosis and cause cell cycle arrest in prostate cancer cell lines. Anticancer Res 2007, 27, 3459–3464. [Google Scholar]
- Murias, M.; Jager, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure-activity relationship. Biochem. Pharmacol 2005, 69, 903–912. [Google Scholar]
- Mikula-Pietrasik, J.; Kuczmarska, A.; Kucinska, M.; Murias, M.; Wierzchowski, M.; Winckiewicz, M.; Staniszewski, R.; Breborowicz, A.; Ksiazek, K. Resveratrol and its synthetic derivatives exert opposite effects on mesothelial cell-dependent angiogenesis via modulating secretion of VEGF and IL-8/CXCL8. Angiogenesis 2012, 15, 361–376. [Google Scholar]
- Low, R.N. MR imaging of the peritoneal spread of malignancy. Abdom. Imaging 2007, 32, 267–283. [Google Scholar]
- Jayne, D.G.; Fook, S.; Loi, C.; Seow-Choen, F. Peritoneal carcinomatosis from colorectal cancer. Br. J. Surg 2002, 89, 1545–1550. [Google Scholar]
- Sodek, K.L.; Murphy, K.J.; Brown, T.J.; Ringuette, M.J. Cell-cell and cell-matrix dynamics in intraperitoneal cancer metastasis. Cancer Metastasis Rev 2012, 31, 397–414. [Google Scholar]
- Ksiazek, K.; Mikula-Pietrasik, J.; Korybalska, K.; Dworacki, G.; Jorres, A.; Witowski, J. Senescent peritoneal mesothelial cells promote ovarian cancer cell adhesion: The role of oxidative stress-induced fibronectin. Am. J. Pathol 2009, 174, 1230–1240. [Google Scholar]
- Ksiazek, K.; Mikula-Pietrasik, J.; Catar, R.; Dworacki, G.; Winckiewicz, M.; Frydrychowicz, M.; Dragun, D.; Staniszewski, R.; Jorres, A.; Witowski, J. Oxidative stress-dependent increase in ICAM-1 expression promotes adhesion of colorectal and pancreatic cancers to the senescent peritoneal mesothelium. Int. J. Cancer 2010, 127, 293–303. [Google Scholar]
- Ksiazek, K.; Mikula-Pietrasik, J.; Jorres, A.; Witowski, J. Oxidative stress-mediated early senescence contributes to the short replicative life span of human peritoneal mesothelial cells. Free Radic. Biol. Med 2008, 45, 460–467. [Google Scholar]
- Saiko, P.; Pemberger, M.; Horvath, Z.; Savinc, I.; Grusch, M.; Handler, N.; Erker, T.; Jaeger, W.; Fritzer-Szekeres, M.; Szekeres, T. Novel resveratrol analogs induce apoptosis and cause cell cycle arrest in HT29 human colon cancer cells: Inhibition of ribonucleotide reductase activity. Oncol. Rep 2008, 19, 1621–1626. [Google Scholar]
- Klink, J.C.; Tewari, A.K.; Masko, E.M.; Antonelli, J.; Febbo, P.G.; Cohen, P.; Dewhirst, M.W.; Pizzo, S.V.; Freedland, S.J. Resveratrol worsens survival in SCID mice with prostate cancer xenografts in a cell-line specific manner, through paradoxical effects on oncogenic pathways. Prostate 2013, 73, 754–762. [Google Scholar]
- Ahmed, N.; Riley, C.; Rice, G.; Quinn, M. Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment. Clin. Exp. Metastasis 2005, 22, 391–402. [Google Scholar]
- Gardner, M.J.; Catterall, J.B.; Jones, L.M.; Turner, G.A. Human ovarian tumour cells can bind hyaluronic acid via membrane CD44: A possible step in peritoneal metastasis. Clin. Exp. Metastasis 1996, 14, 325–334. [Google Scholar]
- Lessan, K.; Aguiar, D.J.; Oegema, T.; Siebenson, L.; Skubitz, A.P. CD44 and β1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am. J. Pathol 1999, 154, 1525–1537. [Google Scholar]
- Arroyo, M.P.; Downey, K.M.; So, A.G.; Wang, T.S. Schizosaccharomyces pombe proliferating cell nuclear antigen mutations affect DNA polymerase delta processivity. J. Biol. Chem 1996, 271, 15971–15980. [Google Scholar]
- Ksiazek, K.; Passos, J.F.; Olijslagers, S.; Saretzki, G.; Martin-Ruiz, C.; von Zglinicki, T. Premature senescence of mesothelial cells is associated with non-telomeric DNA damage. Biochem. Biophys. Res. Commun 2007, 362, 707–711. [Google Scholar]
- Luppi, F.; Longo, A.M.; de Boer, W.I.; Rabe, K.F.; Hiemstra, P.S. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer 2007, 56, 25–33. [Google Scholar]
- Liang, Y.; Brekken, R.A.; Hyder, S.M. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr. Relat. Cancer 2006, 13, 905–919. [Google Scholar]
- Cannistra, S.A.; DeFranzo, B.; Niloff, J.; Ottensmeir, C. Functional heterogeneity of CD44 molecules in ovarian cancer cell lines. Clin. Cancer Res 1995, 1, 333–342. [Google Scholar]
- Murias, M.; Handler, N.; Erker, T.; Pleban, K.; Ecker, G.; Saiko, P.; Szekeres, T.; Jager, W. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: Synthesis and structure-activity relationship. Bioorg. Med. Chem 2004, 12, 5571–5578. [Google Scholar]
- Ksiazek, K.; Jorres, A.; Witowski, J. Senescence induces a proangiogenic switch in human peritoneal mesothelial cells. Rejuvenation Res 2008, 11, 681–683. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mikuła-Pietrasik, J.; Sosińska, P.; Wierzchowski, M.; Piwocka, K.; Książek, K. Synthetic Resveratrol Analogue, 3,3',4,4',5,5'-Hexahydroxy-trans-Stilbene, Accelerates Senescence in Peritoneal Mesothelium and Promotes Senescence-Dependent Growth of Gastrointestinal Cancers. Int. J. Mol. Sci. 2013, 14, 22483-22498. https://doi.org/10.3390/ijms141122483
Mikuła-Pietrasik J, Sosińska P, Wierzchowski M, Piwocka K, Książek K. Synthetic Resveratrol Analogue, 3,3',4,4',5,5'-Hexahydroxy-trans-Stilbene, Accelerates Senescence in Peritoneal Mesothelium and Promotes Senescence-Dependent Growth of Gastrointestinal Cancers. International Journal of Molecular Sciences. 2013; 14(11):22483-22498. https://doi.org/10.3390/ijms141122483
Chicago/Turabian StyleMikuła-Pietrasik, Justyna, Patrycja Sosińska, Marcin Wierzchowski, Katarzyna Piwocka, and Krzysztof Książek. 2013. "Synthetic Resveratrol Analogue, 3,3',4,4',5,5'-Hexahydroxy-trans-Stilbene, Accelerates Senescence in Peritoneal Mesothelium and Promotes Senescence-Dependent Growth of Gastrointestinal Cancers" International Journal of Molecular Sciences 14, no. 11: 22483-22498. https://doi.org/10.3390/ijms141122483




