Nox Gene Expression and Cytochemical Localization of Hydrogen Peroxide in Polyporus umbellatus Sclerotial Formation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
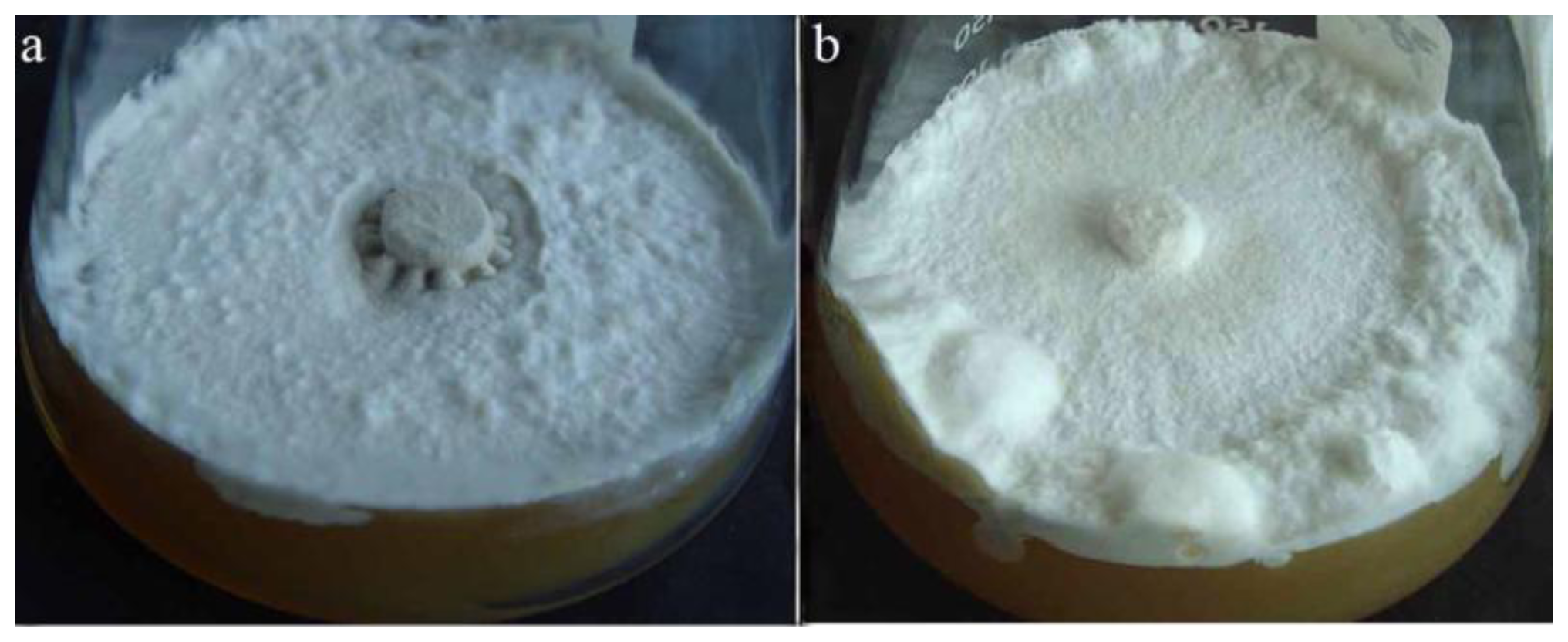
2.1.1. Polyporus umbellatus Sclerotial Formation by Temperature Shift Treatment
2.1.2. The Effect of NADPH Oxidase Inhibitor on Sclerotial Formation by Temperature Shift Treatment
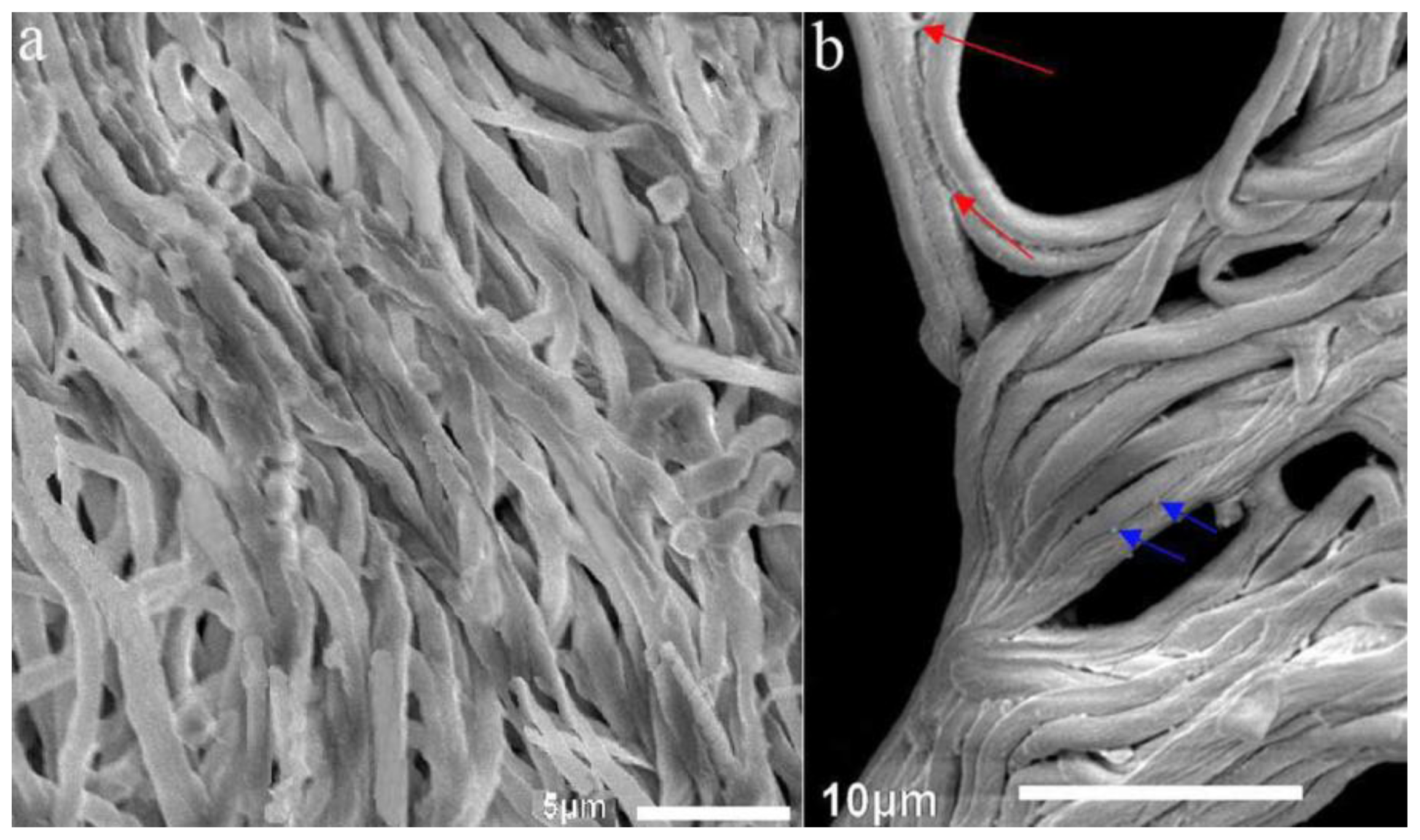
2.1.3. SEM Examination of Artificial Sclerotia in the Temperature Shift Group and Mycelia in the Control Group after 120 days of Cultivation
2.1.4. Cytochemical Localization of H2O2 Production Induced by Temperature Shift Treatment
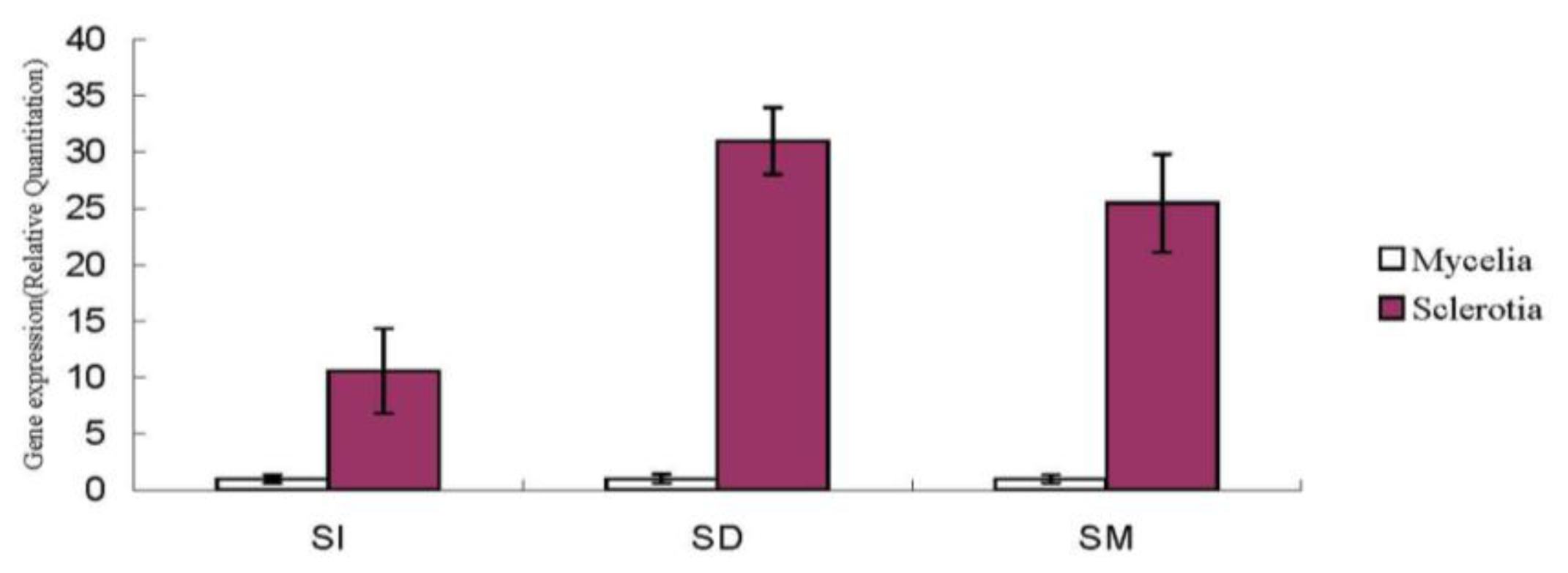
2.1.5. Nox Gene Expression in Sclerotial Formation
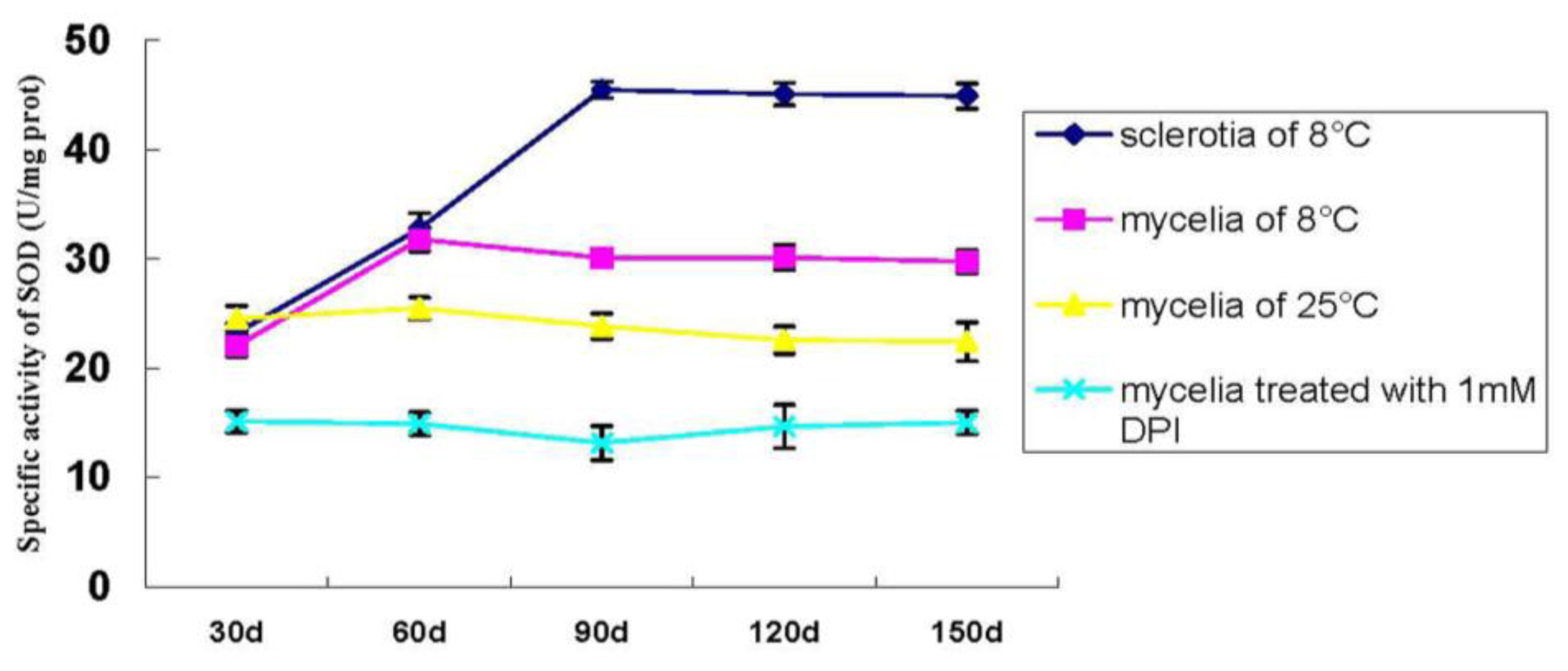
2.1.6. Specific Activity of SOD during P. umbellatus Sclerotial Formation
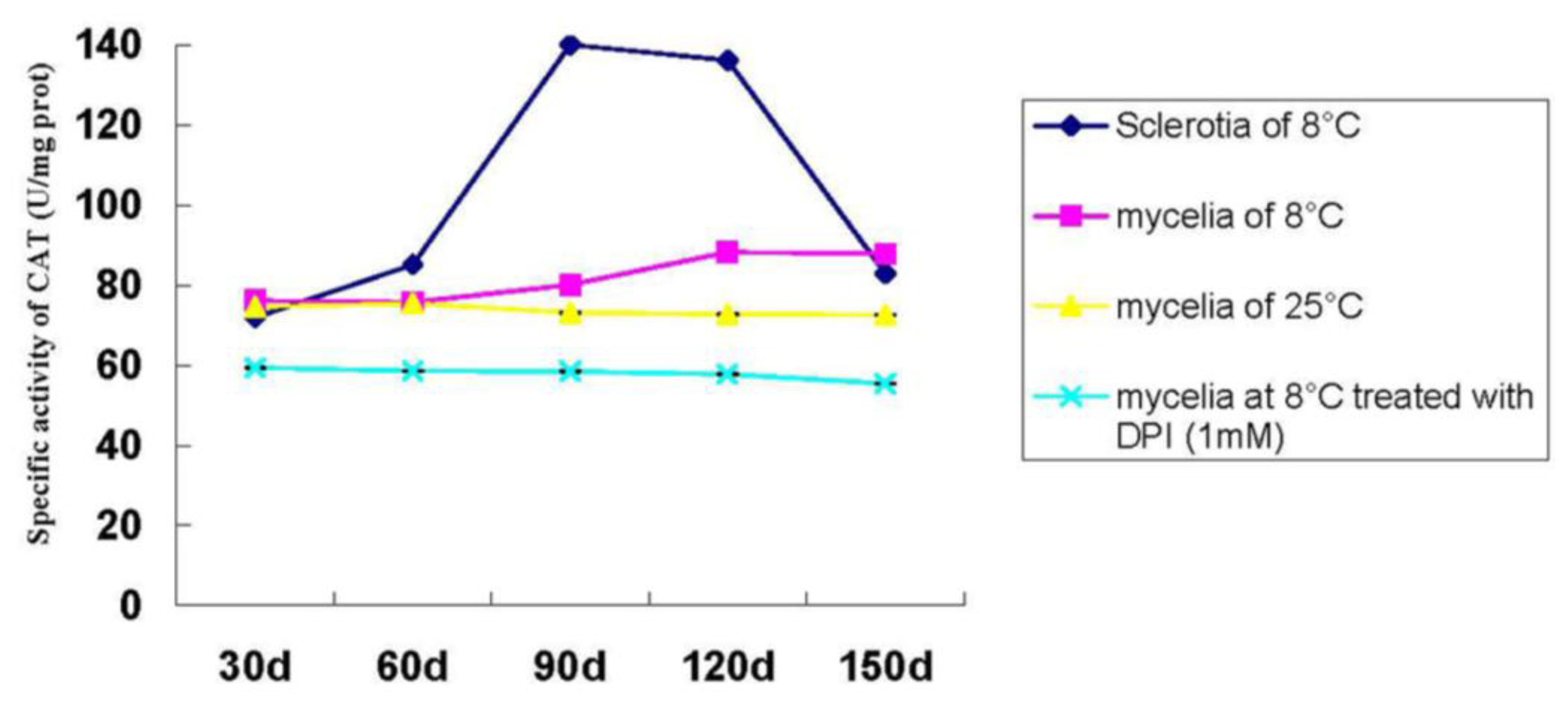
2.1.7. Catalase Activity during P. umbellatus Sclerotial Formation
2.2. Discussion
3. Experimental Section
3.1. Fungal Strain
3.2. Reagent
3.3. P. umbellatus Cultivation
3.4. The Effect of Temperature Shift on Sclerotial Formation in P. umbellatus
3.5. SEM Examination of the Sclerotia in the Temperature Shift Group and the Mycelia in the Control Group after Cultivation for 120 Days
3.6. Cytochemical Localization of H2O2
3.7. qRT-PCR Analysis of Nox Gene in Sclerotial Formation
3.8. The Effect of NADPH Oxidase Inhibitor on Sclerotial Formation by Temperature-Shift Treatment
3.9. Mycelial and Sclerotial Protein Extraction and Concentration Determination
3.10. SOD Detection during P. umbellatus Sclerotial Formation
3.11. CAT-Dependent H2O2 Consumption Assay during P. umbellatus Sclerotial Formation
3.12. Data Analysis
4. Conclusions



Acknowledgments
Conflicts of Interest
References
- Guo, W.J.; Xing, Y.M.; Chen, J.; Guo, S.X. Growth promoting effects of water extract of Armillaria mellea rhizomorph on mycelia ofPolyporus umbellatus. Crypto. Mycol 2011, 32, 171–176. [Google Scholar]
- Liu, Y.Y.; Guo, S.X. Involvement of Ca2+ channel signaling in sclerotial formation ofPolyporus umbellatus. Mycopathologia 2010, 169, 139–150. [Google Scholar]
- Xing, Y.M.; Chen, J.; Lv, Y.L.; Liang, H.Q.; Guo, S.X. Dertermination of optimal carbon source and pH value for sclerotial formation of Polyporus umbellatus under artificial conditions. Mycol. Prog 2011, 10, 121–125. [Google Scholar]
- Georgiou, C.D. Lipid peroxidation in Sclerotium rolfsii: A new look into the mechanism of sclerotial biogenesis in fungi. Mycol. Res 1997, 101, 460–464. [Google Scholar]
- Georgiou, C.D.; Zees, A. Lipofuscins and sclerotial differentiation in phytopathogenic fungi. Mycopathologia 2001, 153, 203–208. [Google Scholar]
- Georgiou, C.D.; Patsoukis, N.; Papapostolou, L.; Geroge, Z. Sclerotial metamorphosis in filamentous fungi is induced by oxidative stress. Integr. Com. Biol 2006, 46, 691–712. [Google Scholar]
- Kim, H.J.; Chen, C.B.; Kabbage, M.; Dickman, M.B. Identification and characterization of Sclerotinia sclerotium NADPH oxidases. Appl. Environ. Microb 2011, 77, 7721–7729. [Google Scholar]
- Segmüller, N.; Kokkelink, L.; Giesbert, S.; Odinius, D.; Kan, J.V.; Tudzynski, P. NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Mol. Plant Microbe Interact 2008, 21, 808–819. [Google Scholar]
- Siegmund, U.; Heller, J.; Kann, J.A.; Tudzynski, P. The NADPH oxidase complexed in Botrytis cinerea: Evidence for a clse association with the ER and the Tetraspanin Pls1. PLoS One 2013, 8, e55879. [Google Scholar]
- Papapostolou, I.; Georgiou, C.D. Superoxide radical is involved in the sclerotial differentiation of filamentous phytopathogenic fungi: Identification of a fungal xanthine oxidase. Fungal Biol 2010, 114, 387–395. [Google Scholar]
- Angelova, M.B.; Pashova, S.B.; Spasova, B.K.; Vassilev, S.V.; Slokoska, L.S. Oxidative stress response of filamentous fungi induced by hydrogen peroxide and paraquat. Mycol. Res 2005, 109, 150–158. [Google Scholar]
- Xing, Y.M.; Zhang, L.C.; Liang, H.Q.; Lv, J.; Song, C.; Guo, S.X.; Wang, C.L.; Lee, T.S.; Lee, M.W. Sclerotial formation of Polyporus umbellatus by low temperature treatment under artificial conditions. PLoS One 2013, 8, e56190. [Google Scholar]
- Zhang, G.; Zhao, M.M.; Song, C.; Luo, A.X.; Bai, J.F.; Guo, S.X. Characterization of reference genes for quantitative real-time PCR analysis in various tissues of Anoectochilus roxburghii. Mol. Biol. Rep 2012, 39, 5905–5912. [Google Scholar]
- Erental, A.; Dickman, M.B.; Yarden, O. Sclerotial development in Sclerotinia sclerotium: Awakening molecular analysis of a “Dormant” structure. Fungal Biol. Rev 2008, 22, 6–16. [Google Scholar]
- Willetts, H.J.; Bullock, S. Developmental biology of sclerotia. Mycol. Res 1992, 96, 801–816. [Google Scholar]
- Song, C. Differentially Expressed Genes and Clonal Analysis during Sclerotial Formation from Hyphae in Polyporus umbellatus. Ph.D. Thesis, Chinese Academy of Medical Sciences & Peking Union Medical College, June 2012. [Google Scholar]
- Halliwell, B.; Gutteridge, C.M.J. Free Radicals in Biology and Medicine, 4th ed; Oxford University Press: New York,NY, USA, 2007. [Google Scholar]
- Liu, N.; Lin, Z.F. Reactive oxygen species and relative enzyme activities in the development of aerial roots of Chines Banyan (Ficus microcarpa). J. Plant Growth Regul 2013. [Google Scholar] [CrossRef]
- Sideri, M.; Georgiou, C.D. Differentiation and hydrogen peroxide production in Sclerotium rolfsii are induced by the oxidizing growth factors, light and iron. Mycologia 2000, 92, 1033–1042. [Google Scholar]
- Gao, X.L.; Li, J.M.; Wang, Y.L.; Jiu, M.; Yan, G.H.; Liu, S.S.; Wang, X.W. Cloning, expression and characterization of mitochondrial manganese superoxide dismutase from the whitefly Bemisia tabaci. Int. J. Mol. Sci 2013, 14, 871–887. [Google Scholar]
- Asuman, C.; Hatice, G.; Atilla, E. The activities of catalase and ascorbate peroxidase in olive (Olea europaea L. cv. Gemlik) under low temperature stress. Hort. Enviorn. Biotechnol 2011, 52, 113–120. [Google Scholar]
- Hu, X.L.; Jing, M.Y.; Zhang, A.M.; Lun, J. Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves. Planta 2005, 223, 57–68. [Google Scholar]
- Song, C.; Guo, S.X. Molecular cloning and characterization of a hemolysin gene from medicinal fungus Polyporus umbellatus (in Chinese). Mycosystema 2013, 32, 690–697. [Google Scholar]
- Papapostolou, I.; Georgiou, C.D. Hydrogen peroxide is involved in the sclerotial differentiation of filamentous phytopathogenic fungi. J. Appl. Microbiol 2010, 109, 1929–1936. [Google Scholar]
- Bradley, J.O.; John, M. Assays for determination of protein concentration. Curr. Protoc. Pharmacol 2007, 48, 3.4.1–3.4.29. [Google Scholar]
- Lu, C.; Bucher, G.; Sander, W. Photoinduced interactions between oxidized and reduced lipoic acid and riboflavin (Vitamin B2). Chem. Phys. Chem 2004, 5, 47–56. [Google Scholar]
- Mirsa, P.H.; Fridovich, I. Superoxide dismutase: A photochemical augmentation assay. Arch. Biochem. Biophys 1977, 181, 308–312. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xing, Y.-M.; Chen, J.; Song, C.; Liu, Y.-Y.; Guo, S.-X.; Wang, C.-L. Nox Gene Expression and Cytochemical Localization of Hydrogen Peroxide in Polyporus umbellatus Sclerotial Formation. Int. J. Mol. Sci. 2013, 14, 22967-22981. https://doi.org/10.3390/ijms141122967
Xing Y-M, Chen J, Song C, Liu Y-Y, Guo S-X, Wang C-L. Nox Gene Expression and Cytochemical Localization of Hydrogen Peroxide in Polyporus umbellatus Sclerotial Formation. International Journal of Molecular Sciences. 2013; 14(11):22967-22981. https://doi.org/10.3390/ijms141122967
Chicago/Turabian StyleXing, Yong-Mei, Juan Chen, Chao Song, Ying-Ying Liu, Shun-Xing Guo, and Chun-Lan Wang. 2013. "Nox Gene Expression and Cytochemical Localization of Hydrogen Peroxide in Polyporus umbellatus Sclerotial Formation" International Journal of Molecular Sciences 14, no. 11: 22967-22981. https://doi.org/10.3390/ijms141122967



