Alteration of Tight Junction Gene Expression by Calcium- and Vitamin D-Deficient Diet in the Duodenum of Calbindin-Null Mice
Abstract
:1. Introduction
2. Results
2.1. Regulation of Serum Calcium Concentrations after the Consumption of Calcium-Deficient and Calcium/Vitamin D-Deficient Diets
2.2. Expression of Tight Junction Genes in the Duodenum
2.3. Regulation of Duodenal Tight Junction Protein Expression
2.4. Localization of Duodenal Tight Junction Proteins
3. Experimental Section
3.1. Animals
3.2. Experimental Treatments
3.3. Serum Calcium Concentration Analysis
3.4. Quantitative Real-Time PCR
3.5. Western Blot Analysis
3.6. Immunohistochemistry
3.7. Data Analysis
4. Conclusions
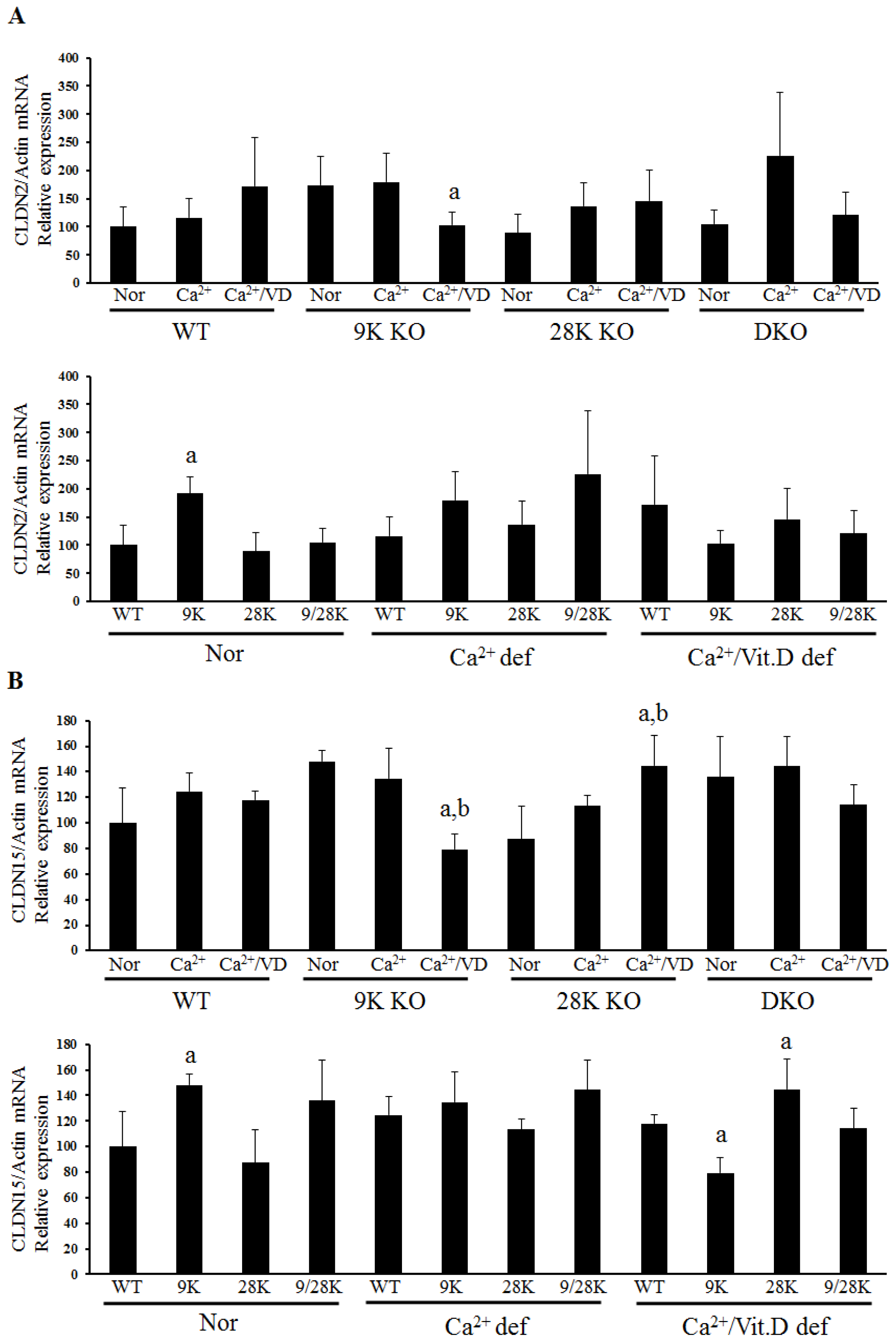
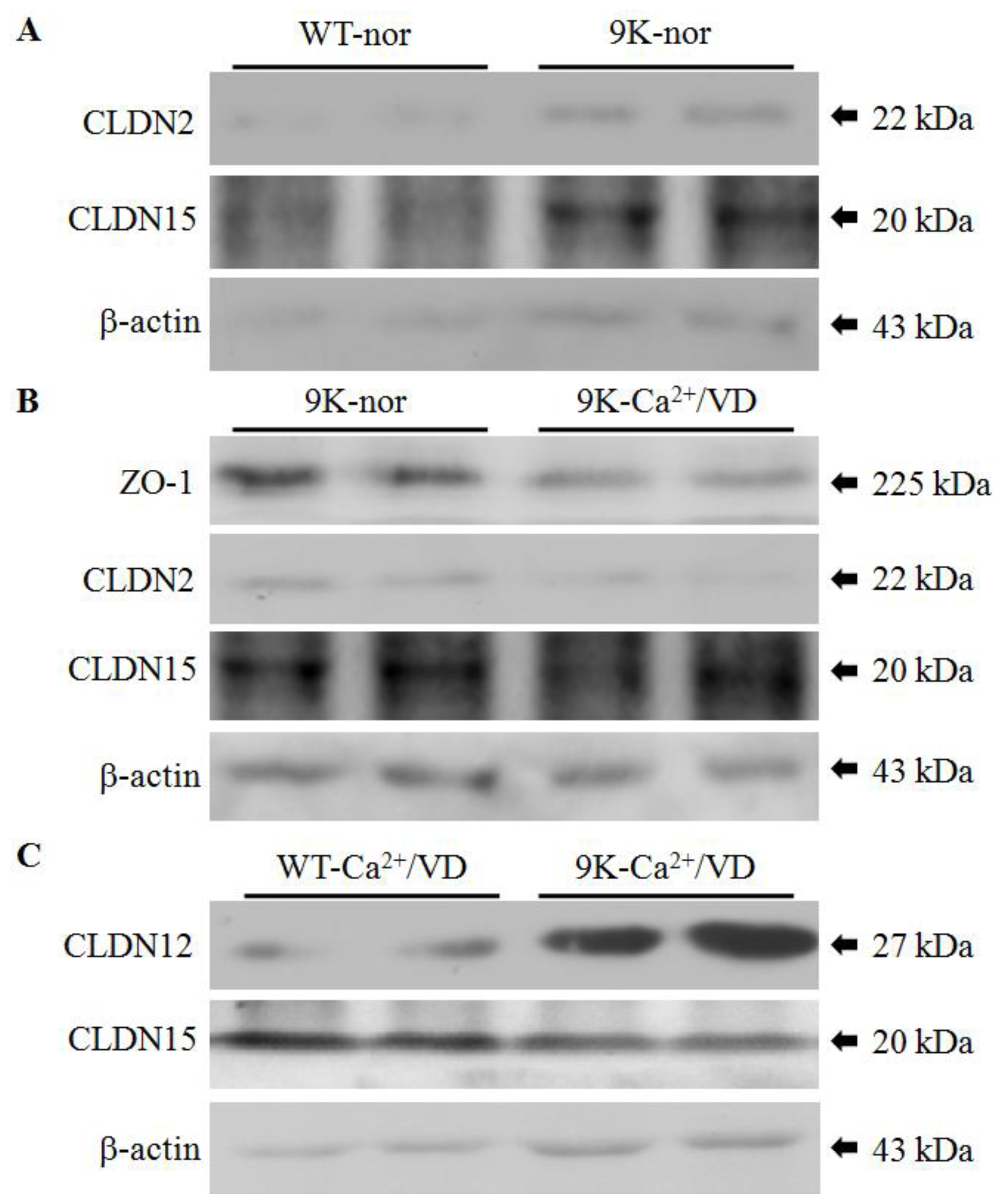

| Diet | WT | CaBP-9K KO | CaBP-28K KO | DKO |
|---|---|---|---|---|
| Normal | 9.6 ± 0.15 | 9.8 ± 0.12 | 9.2 ± 0.10 | 9.6 ± 0.10 |
| Ca2+ def | 9.4 ± 0.06 * | 9.4 ± 0.06 * | 9.2 ± 0.06 | 9.0 ± 0.10 * |
| Ca2+/VD def | 8.3 ± 0.15 ** | 9.4 ± 0.12 * | 8.2 ± 0.06 ** | 7.5 ± 0.06 ** |
| Diet | WT | CaBP-9K KO | CaBP-28K KO | DKO |
|---|---|---|---|---|
| Normal | - | OCLN↑, CLDN2↑, CLDN15↑ | CLDN10b↑ | CLDN10b↑ |
| Ca2+ def | - | CLDN10b↑ | - | - |
| Ca2+/VD def | - | OCLN↓, ZO-1↓, CLDN12↑ CLDN15↓ | CLDN15↑ | - |
| Gene | Forward | Reverse |
|---|---|---|
| β-actin | 5′-ACAGGCATTGTGATGGACTC-3′ | 5′-ATTTCCCTCTCAGCTGTGGT-3′ |
| OCLN | 5′-ACTGGGTCAGGGAATATCCA-3′ | 5′-TCAGCAGCAGCCATGTACTC-3′ |
| ZO-1 | 5′-ACTCCCACTTCCCCAAAAAC-3′ | 5′-CCACAGCTGAAGGACTCACA-3′ |
| CLDN2 | 5′-TGGTTCCTGACAGCATGAAA-3′ | 5′-CTTTGGGCTGTTGAGCAGAT-3′ |
| CLDN10b | 5′-TCGCCTTCGTAGTCTCCATC-3′ | 5′-TCTCCTTCTCCGCCTTGATAC-3′ |
| CLDN12 | 5′-AGGAAGTTTGAGCCGGTCTT-3′ | 5′-CGTGATGAATAGGGCTGTGA-3′ |
| CLDN15 | 5′-GCCTCTTTCTAGGCATGGTG-3′ | 5′-TCCAGCATACAGTGGGTTGA-3′ |
Acknowledgments
Conflicts of Interest
References
- Nellans, H.N.; Kimberg, D.V. Cellular and paracellular calcium transport in rat ileum: Effects of dietary calcium. Am. J. Physiol 1978, 235, 726–737. [Google Scholar]
- Bronner, F. Renal calcium transport: Mechanisms and regulation—An overview. Am. J. Physiol 1989, 257, 707–711. [Google Scholar]
- Hoenderop, J.G.; van der Kemp, A.W.; Hartog, A.; van de Graaf, S.F.; van Os, C.H.; Willems, P.H.; Bindels, R.J. Molecular identification of the apical Ca2+ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J. Biol. Chem 1999, 274, 8375–8378. [Google Scholar]
- Peng, J.B.; Chen, X.Z.; Berger, U.V.; Vassilev, P.M.; Tsukaguchi, H.; Brown, E.M.; Hediger, M.A. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J. Biol. Chem 1999, 274, 22739–22746. [Google Scholar]
- Montell, C.; Birnbaumer, L.; Flockerzi, V.; Bindels, R.J.; Bruford, E.A.; Caterina, M.J.; Clapham, D.E.; Harteneck, C.; Heller, S.; Julius, D.; et al. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 2002, 9, 229–231. [Google Scholar]
- DiPolo, R.; Beauge, L. Sodium/calcium exchanger: influence of metabolic regulation on ion carrier interactions. Physiol. Rev 2006, 86, 155–203. [Google Scholar]
- Yu, S.P.; Choi, D.W. Na+-Ca2+ exchange currents in cortical neurons: Concomitant forward and reverse operation and effect of glutamate. Eur. J. Neurosci 1997, 9, 1273–1281. [Google Scholar]
- Jensen, T.P.; Buckby, L.E.; Empson, R.M. Expression of plasma membrane Ca2+ ATPase family members and associated synaptic proteins in acute and cultured organotypic hippocampal slices from rat. Brain Res. Dev. Brain Res 2004, 152, 129–136. [Google Scholar]
- Schroder, B.; Schlumbohm, C.; Kaune, R.; Breves, G. Role of calbindin-D9k in buffering cytosolic free Ca2+ ions in pig duodenal enterocytes. J. Physiol 1996, 492, 715–722. [Google Scholar]
- Hoenderop, J.G.; Hartog, A.; Stuiver, M.; Doucet, A.; Willems, P.H.; Bindels, R.J. Localization of the epithelial Ca2+ channel in rabbit kidney and intestine. J. Am. Soc. Nephrol 2000, 11, 1171–1178. [Google Scholar]
- Varghese, S.; Lee, S.; Huang, Y.C.; Christakos, S. Analysis of rat vitamin D-dependent calbindin-D28k gene expression. J. Biol. Chem 1988, 263, 9776–9784. [Google Scholar]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol 1963, 17, 375–412. [Google Scholar]
- Gumbiner, B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 1996, 84, 345–357. [Google Scholar]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol 2001, 2, 285–293. [Google Scholar]
- Anderson, J.M.; van Itallie, C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol 1995, 269, 467–475. [Google Scholar]
- Guillemot, L.; Paschoud, S.; Pulimeno, P.; Foglia, A.; Citi, S. The cytoplasmic plaque of tight junctions: A scaffolding and signalling center. Biochim. Biophys. Acta 2008, 1778, 601–613. [Google Scholar]
- Hewitt, K.J.; Agarwal, R.; Morin, P.J. The claudin gene family: Expression in normal and neoplastic tissues. BMC Cancer 2006, 6, 186. [Google Scholar] [CrossRef]
- Krause, G.; Winkler, L.; Piehl, C.; Blasig, I.; Piontek, J.; Muller, S.L. Structure and function of extracellular claudin domains. Ann. N. Y. Acad. Sci 2009, 1165, 34–43. [Google Scholar]
- Fujita, H.; Chiba, H.; Yokozaki, H.; Sakai, N.; Sugimoto, K.; Wada, T.; Kojima, T.; Yamashita, T.; Sawada, N. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J. Histochem. Cytochem 2006, 54, 933–944. [Google Scholar]
- Inai, T.; Sengoku, A.; Guan, X.; Hirose, E.; Iida, H.; Shibata, Y. Heterogeneity in expression and subcellular localization of tight junction proteins, claudin-10 and -15, examined by RT-PCR and immunofluorescence microscopy. Arch. Histol. Cytol 2005, 68, 349–360. [Google Scholar]
- Inai, T.; Kobayashi, J.; Shibata, Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur. J. Cell Biol 1999, 78, 849–855. [Google Scholar]
- Amasheh, S.; Schmidt, T.; Mahn, M.; Florian, P.; Mankertz, J.; Tavalali, S.; Gitter, A.H.; Schulzke, J.D.; Fromm, M. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res 2005, 321, 89–96. [Google Scholar]
- Hirase, T.; Staddon, J.M.; Saitou, M.; Ando-Akatsuka, Y.; Itoh, M.; Furuse, M.; Fujimoto, K.; Tsukita, S.; Rubin, L.L. Occludin as a possible determinant of tight junction permeability in endothelial cells. J. Cell Sci 1997, 110, 1603–1613. [Google Scholar]
- Hirase, T.; Kawashima, S.; Wong, E.Y.; Ueyama, T.; Rikitake, Y.; Tsukita, S.; Yokoyama, M.; Staddon, J.M. Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J. Biol. Chem 2001, 276, 10423–10431. [Google Scholar]
- Sheng, M.; Sala, C. PDZ domains and the organization of supramolecular complexes. Annu. Rev. Neurosci 2001, 24, 1–29. [Google Scholar]
- Stevenson, B.R.; Anderson, J.M.; Braun, I.D.; Mooseker, M.S. Phosphorylation of the tight-junction protein ZO-1 in two strains of Madin-Darby canine kidney cells which differ in transepithelial resistance. Biochem. J 1989, 263, 597–599. [Google Scholar]
- Kraidith, K.; Jantarajit, W.; Teerapornpuntakit, J.; Nakkrasae, L.I.; Krishnamra, N.; Charoenphandhu, N. Direct stimulation of the transcellular and paracellular calcium transport in the rat cecum by prolactin. Pflugers Arch 2009, 458, 993–1005. [Google Scholar]
- Tordoff, M.G.; Bachmanov, A.A.; Reed, D.R. Forty mouse strain survey of voluntary calcium intake, blood calcium, and bone mineral content. Physiol. Behav 2007, 91, 632–643. [Google Scholar]
- Sim, J.Y.; Jung, E.M.; Yoo, Y.M.; Choi, K.C.; Jeung, E.B. Transcriptional and translational expression of calbindin-D9k in the duodenum, kidney and uterus of a female canine model. J. Vet. Sci 2010, 11, 15–19. [Google Scholar]
- Koo, T.H.; Yang, H.; An, B.S.; Choi, K.C.; Hyun, S.H.; Jeung, E.B. Calcium transport genes are differently regulated in maternal and fetal placenta in the knockout mice of calbindin-D(9k) and -D(28k). Mol. Reprod. Dev 2012, 79, 346–355. [Google Scholar]
- Van Itallie, C.M.; Rogan, S.; Yu, A.; Vidal, L.S.; Holmes, J.; Anderson, J.M. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am. J. Physiol. Renal Physiol 2006, 291, 1288–1299. [Google Scholar]
- Fujita, H.; Sugimoto, K.; Inatomi, S.; Maeda, T.; Osanai, M.; Uchiyama, Y.; Yamamoto, Y.; Wada, T.; Kojima, T.; Yokozaki, H.; et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell 2008, 19, 1912–1921. [Google Scholar]
- Balmain, N. Calbindin-D9k. A vitamin-D-dependent, calcium-binding protein in mineralized tissues. Clin. Orthop. Relat. Res 1991, 265, 265–276. [Google Scholar]
- Ko, S.H.; Choi, K.C.; Oh, G.T.; Jeung, E.B. Effect of dietary calcium and 1,25-(OH)2D3 on the expression of calcium transport genes in calbindin-D9k and -D28k double knockout mice. Biochem. Biophys. Res. Commun 2009, 379, 227–232. [Google Scholar]
- Ko, S.H.; Lee, G.S.; Vo, T.T.; Jung, E.M.; Choi, K.C.; Cheung, K.W.; Kim, J.W.; Park, J.G.; Oh, G.T.; Jeung, E.B. Dietary calcium and 1,25-dihydroxyvitamin D3 regulate transcription of calcium transporter genes in calbindin-D9k knockout mice. J. Reprod. Dev 2009, 55, 137–142. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hwang, I.; Yang, H.; Kang, H.-S.; Ahn, C.; Hong, E.-J.; An, B.-S.; Jeung, E.-B. Alteration of Tight Junction Gene Expression by Calcium- and Vitamin D-Deficient Diet in the Duodenum of Calbindin-Null Mice. Int. J. Mol. Sci. 2013, 14, 22997-23010. https://doi.org/10.3390/ijms141122997
Hwang I, Yang H, Kang H-S, Ahn C, Hong E-J, An B-S, Jeung E-B. Alteration of Tight Junction Gene Expression by Calcium- and Vitamin D-Deficient Diet in the Duodenum of Calbindin-Null Mice. International Journal of Molecular Sciences. 2013; 14(11):22997-23010. https://doi.org/10.3390/ijms141122997
Chicago/Turabian StyleHwang, Inho, Hyun Yang, Hong-Seok Kang, Changhwan Ahn, Eui-Ju Hong, Beum-Soo An, and Eui-Bae Jeung. 2013. "Alteration of Tight Junction Gene Expression by Calcium- and Vitamin D-Deficient Diet in the Duodenum of Calbindin-Null Mice" International Journal of Molecular Sciences 14, no. 11: 22997-23010. https://doi.org/10.3390/ijms141122997





