Nutraceuticals: Potential for Chondroprotection and Molecular Targeting of Osteoarthritis
Abstract
:1. Introduction
2. Efficacy and Mechanism of Action of Currently Used Nutraceuticals
3. Pre-Clinical and Clinical Effects of Phytoflavonoids, Polyphenols, and Bioflavonoids Nutraceuticals on OA
3.1. Green Tea
3.2. Pomegranate
3.3. Ginger
3.4. Tumeric
3.5. Rosehip Powder
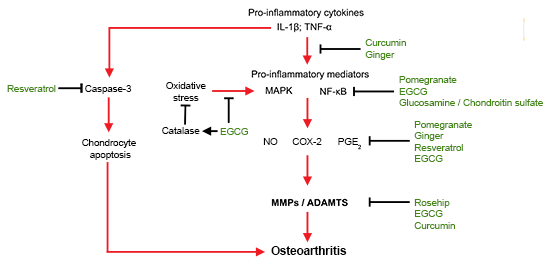
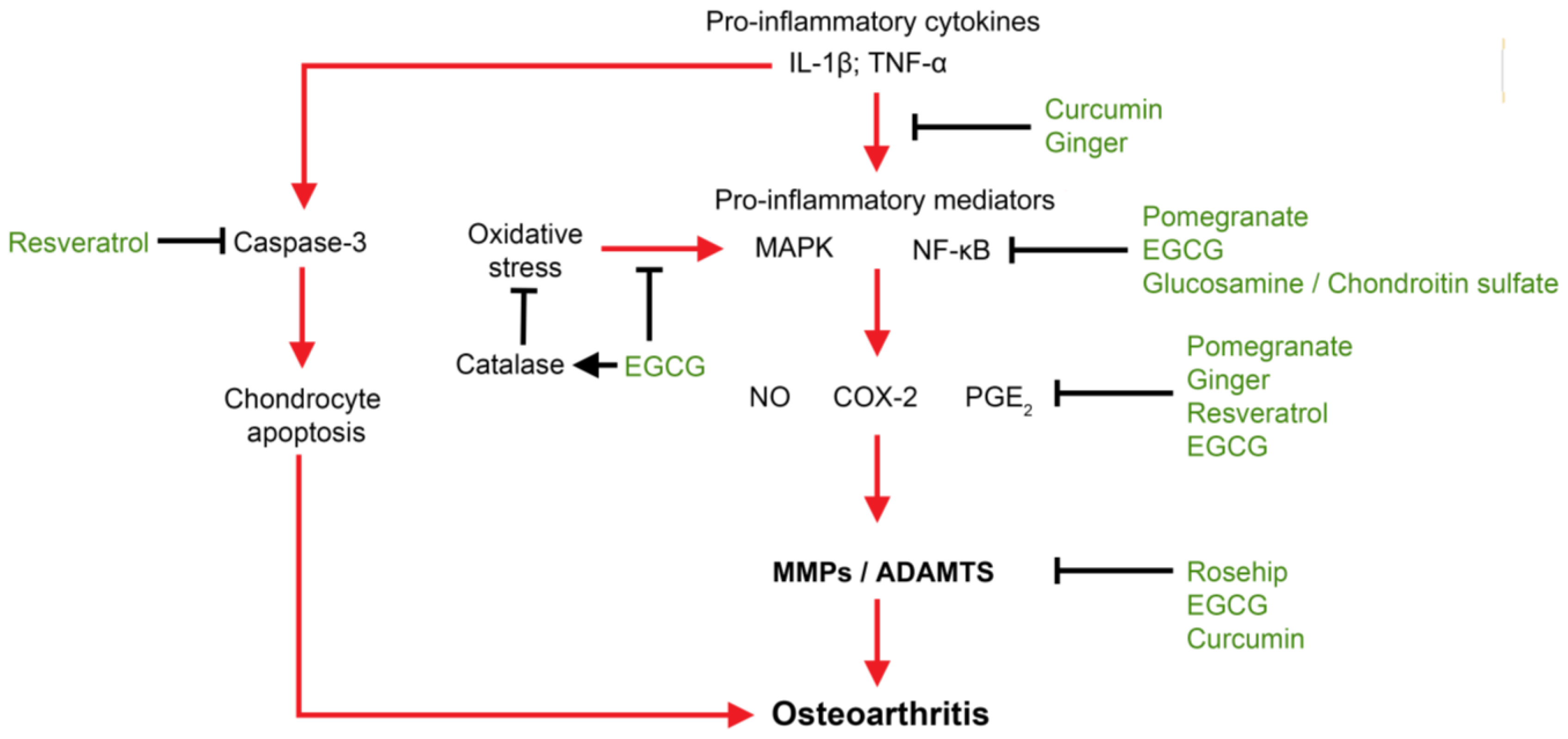
4. Nutraceuticals for Molecular Targeting of OA
4.1. Molecules in Pathology of OA Initiation and Progression
4.2. Molecular Targeting of OA by Nutraceuticals
4.3. Anti-Inflammatory
4.4. Anti-Oxidative Stress
4.5. Anti-Catabolic/Proteolytic Enzymes
5. Conclusions
| Herbal/Plant-based extracts and medicines | ||
|---|---|---|
| Nutraceuticals | Clinical efficacy | Mechanisms of action |
| Boswellia serrata | Relieved joint pain, reduced joint swelling and stiffness, increased joint flexion and walking distance [26–28] | Inhibited TNF-α-induced MMP-3 expression and protected against IL-1β-induced chondrocyte death [29] |
| Bromelain (pineapple extract) | Did not significantly relieve pain or quality-of-life symptoms [30] | Decreases PGE2 expression [31] |
| Caesalpinia Sappan extract (CSE) | Not reported | Inhibited inflammatory mediators IL-1β, iNOS, COX-2 and TNF-α expression in IL-1β stimulated primary human chondrocytes [32]. CSE also suppressed MMP-1, MMP-3, MMP-7, MMP-9 and MMP-13 gene expression [33] |
| Capsaicin | Reduced pain and stiffness and increased joint function [34–36] | Agonist for transient receptor potential vanilloid 1 (pain receptor); Prolonged exposure of capsaicin leads to desensitization of this pain pathway [37] |
| Cat’s claw | Reduced OA-associated pain [38,39] | Inhibit lipopolysaccharide (LPS)-induced PGE2 production and activation of TNF-α [38] |
| Chicory root | Improved pain and relieved joint stiffness [40] | Inhibits production of COX-2, iNOS, TNF-α, and NF-κB [41,42] |
| Diallyl sulphide (garlic extract) | Not reported | Inhibited IL-1β-induced expression of MMP-1, −3 and −13. Ameliorated OA in rabbit anterior cruciate ligament transaction mode and reduced MMP-1, −3, −13 [43]; Inhibited COX-2 expression induced by IL-1β [44] |
| Duhuo Jisheng Tang | Reduced pain and stiffness as well as improved physical function in OA patients [45] | Not reported |
| Harpogophytum procumbens (Devil’s claw) | Alleviates pain in OA patients [46–48] | Inhibited release of TNF-α, IL-1β, IL-6, and PGE2 [49] |
| Phyllanthus emblica | Not reported | Inhibited hyaluronidase and type II collagenase activities in vitro and reduced GAG release in cartilage explants from OA patients [50]. |
| Willow bark | Reduced OA-related pain [51,52] | Not reported |
| Supplements | ||
| Nutraceuticals | Clinical efficacy | Mechanisms of action |
| Aloe Vera | Protects against gastrointestinal effects of NSAIDs [53] | Not reported |
| Avocado/soybean unsaponifiables | Reduced pain in OA patients and reduced NSAID consumption [54,55] | Reduced levels of iNOS and MMP-13 [56]. Suppressed TNF-α, IL-1β, COX-2, and iNOS in LPS-activated chondrocytes [57] |
| Calcium Fructoborate | Not reported | Suppresses IL-1β, IL-6, iNOS in vivo [58] |
| Collagen hydrolysates | Alleviates OA-related pain [59,60] | Stimulate regeneration of type II collagen and increases biosynthesis of proteoglycans [59] |
| Edible Bird’s nest extract | Not reported | Reduced gene expression of MMP-1, MMP-3, IL-1, IL-6, IL-8, COX-2, PGE2, and iNOS and increased type II collagen, aggrecan and SOX-9 [61] |
| Genistein | Not reported | Reduces IL-1β and COX-2 protein synthesis in LPS-induced human chondrocytes [62]. |
| Green-Lipped Mussel extract | Improved knee joint pain, stiffness and mobility [63] | Inhibits synthesis of pro-inflammatory molecule Leukotriene B4 and production of PGE2 [64] |
| Lactobacillus casei | Not reported | Decreased TNF-α, IL-6, NF-κB, COX-2, MMP-1, −3, −13 and increased IL-4 and IL-10 [65] |
| Methylsulfonylmethane (MSM) | Improved symptoms of pain and physical function [66] | Scavenge hydroxyl free radicals [67]; sulfur content rectifies dietary deficiencies of sulfur to improve cartilage formation [68] |
| Polyunsaturated fatty acids (PUFA) | High levels of N-3 PUFA associated with less cartilage loss [69] | N-3 PUFA abolished TNF-α, IL-1β, COX-2, MMP-3, −13, ADAMTS5 expression in vitro [70] and protected against cartilage degradation in OA prone animals [71] |
| S-adenosylmethionine | Reduced OA-related pain intensity from baseline [72–74] | Increases proteoglycan synthesis [75] and chondrocyte proliferation [76] |
| Vitamins | ||
| Nutraceuticals | Clinical efficacy | Mechanisms of action |
| Niacinamide (B-complex vitamins) | Improved joint mobility [77] | Not reported |
| Vitamin C | Stimulates collagen and proteoglycan synthesis [78] | |
| Vitamin D | No effect on pain severity or MRI-assessed quantitative cartilage loss [79]; Relieved OA-associated joint pain [80] | Not reported |
| Vitamin E | Relieved OA-related pain and improved physical function [81,82] | Not reported |
| Nutraceutical | Clinical effects | Preclinical effects |
|---|---|---|
| Green tea | Not reported | |
| Pomegranate | Not reported |
|
| Ginger | Not reported | |
| Tumeric |
| Not reported |
| Rosehip powder |
| Not reported |
Conflicts of Interest
References
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008, 58, 26–35. [Google Scholar]
- Suri, P.; Morgenroth, D.C.; Hunter, D.J. Epidemiology of osteoarthritis and associated comorbidities. PM R 2012, 4, S10–S19. [Google Scholar]
- Kotlarz, H.; Gunnarsson, C.L.; Fang, H.; Rizzo, J.A. Insurer and out-of-pocket costs of osteoarthritis in the US: Evidence from national survey data. Arthritis Rheum 2009, 60, 3546–3553. [Google Scholar]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum 2012, 64, 1697–1707. [Google Scholar]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol 2012, 8, 665–673. [Google Scholar]
- Le Graverand-Gastineau, M.P. Disease modifying osteoarthritis drugs: Facing development challenges and choosing molecular targets. Curr. Drug Targets 2010, 11, 528–535. [Google Scholar]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Getting arthritis gene therapy into the clinic. Nat. Rev. Rheumatol 2011, 7, 244–249. [Google Scholar]
- Heinegard, D.; Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol 2011, 7, 50–56. [Google Scholar]
- Sun, H.B. Mechanical loading, cartilage degradation, and arthritis. Ann. N. Y. Acad. Sci 2010, 1211, 37–50. [Google Scholar]
- Hashimoto, M.; Nakasa, T.; Hikata, T.; Asahara, H. Molecular network of cartilage homeostasis and osteoarthritis. Med. Res. Rev 2008, 28, 464–481. [Google Scholar]
- Cheng, D.S.; Visco, C.J. Pharmaceutical therapy for osteoarthritis. PM&R 2012, 4, S82–S88. [Google Scholar]
- Patrignani, P.; Tacconelli, S.; Bruno, A.; Sostres, C.; Lanas, A. Managing the adverse effects of nonsteroidal anti-inflammatory drugs. Exp. Rev. Clin. Pharm 2011, 4, 605–621. [Google Scholar]
- Henrotin, Y.; Lambert, C.; Couchourel, D.; Ripoll, C.; Chiotelli, E. Nutraceuticals: Do they represent a new era in the management of osteoarthritis?—A narrative review from the lessons taken with five products. Osteoarthr. Cartilage 2011, 19, 1–21. [Google Scholar]
- Akhtar, N.; Haqqi, T.M. Current nutraceuticals in the management of osteoarthritis: A review. Ther. Adv. Musculoskelet Dis 2012, 4, 181–207. [Google Scholar]
- Shen, C.L.; Smith, B.J.; Lo, D.F.; Chyu, M.C.; Dunn, D.M.; Chen, C.H.; Kwun, I.S. Dietary polyphenols and mechanisms of osteoarthritis. J. Nutr. Biochem 2012, 23, 1367–1377. [Google Scholar]
- Guimaraes, A.G.; Xavier, M.A.; de Santana, M.T.; Camargo, E.A.; Santos, C.A.; Brito, F.A.; Barreto, E.O.; Cavalcanti, S.C.; Antoniolli, A.R.; Oliveira, R.C.; et al. Carvacrol attenuates mechanical hypernociception and inflammatory response. Naunyn. Schmiedebergs. Arch. Pharmacol 2012, 385, 253–263. [Google Scholar]
- Cavalcante Melo, F.H.; Rios, E.R.; Rocha, N.F.; Cito Mdo, C.; Fernandes, M.L.; De Sousa, D.P.; De Vasconcelos, S.M.; De Sousa, F.C. Antinociceptive activity of carvacrol (5-isopropyl-2-methylphenol) in mice. J. Pharm. Pharmacol 2012, 64, 1722–1729. [Google Scholar]
- Guimaraes, A.G.; Oliveira, G.F.; Melo, M.S.; Cavalcanti, S.C.; Antoniolli, A.R.; Bonjardim, L.R.; Silva, F.A.; Santos, J.P.; Rocha, R.F.; et al. Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin. Pharmacol. Toxicol 2010, 107, 949–957. [Google Scholar]
- Henrotin, Y.; Clutterbuck, A.L.; Allaway, D.; Lodwig, E.M.; Harris, P.; Mathy-Hartert, M.; Shakibaei, M.; Mobasheri, A. Biological actions of curcumin on articular chondrocytes. Osteoarthr. Cartilage 2010, 18, 141–149. [Google Scholar]
- Sreejayan Rao, M.N. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol 1997, 49, 105–107. [Google Scholar]
- Black, C.; Clar, C.; Henderson, R.; MacEachern, C.; McNamee, P.; Quayyum, Z.; Royle, P.; Thomas, S. The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: A systematic review and economic evaluation. Health Tech. Assess 2009, 13, 1–148. [Google Scholar]
- Roughley, P.J. The structure and function of cartilage proteoglycans. Eur. Cell Mater 2006, 12, 92–101. [Google Scholar]
- Clegg, D.O.; Reda, D.J.; Harris, C.L.; Klein, M.A.; O’Dell, J.R.; Hooper, M.M.; Bradley, J.D.; Bingham, C.O., 3rd; Weisman, M.H.; Jackson, C.G.; et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N. Engl. J. Med 2006, 354, 795–808. [Google Scholar]
- Sawitzke, A.D.; Shi, H.; Finco, M.F.; Dunlop, D.D.; Bingham, C.O., 3rd; Harris, C.L.; Singer, N.G.; Bradley, J.D.; Silver, D.; Jackson, C.G.; et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: A report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum 2008, 58, 3183–3191. [Google Scholar]
- Sawitzke, A.D.; Shi, H.; Finco, M.F.; Dunlop, D.D.; Harris, C.L.; Singer, N.G.; Bradley, J.D.; Silver, D.; Jackson, C.G.; Lane, N.E.; et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann. Rheum. Dis 2010, 69, 1459–1464. [Google Scholar]
- Gupta, P.K.; Samarakoon, S.M.; Chandola, H.M.; Ravishankar, B. Clinical evaluation of Boswellia serrata (Shallaki) resin in the management of Sandhivata (osteoarthritis). Ayu 2011, 32, 478–482. [Google Scholar]
- Krishnaraju, A.V.; Sundararaju, D.; Vamsikrishna, U.; Suryachandra, R.; Machiraju, G.; Sengupta, K.; Trimurtulu, G. Safety and toxicological evaluation of Aflapin: A novel Boswellia-derived anti-inflammatory product. Toxicol. Mech. Method 2010, 20, 556–563. [Google Scholar]
- Kimmatkar, N.; Thawani, V.; Hingorani, L.; Khiyani, R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee—A randomized double blind placebo controlled trial. Phytomedicine 2003, 10, 3–7. [Google Scholar]
- Sengupta, K.; Kolla, J.N.; Krishnaraju, A.V.; Yalamanchili, N.; Rao, C.V.; Golakoti, T.; Raychaudhuri, S.; Raychaudhuri, S.P. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: A novel Boswellia serrata extract. Mol. Cell Biochem 2011, 354, 189–197. [Google Scholar]
- Brien, S.; Lewith, G.; Walker, A.F.; Middleton, R.; Prescott, P.; Bundy, R. Bromelain as an adjunctive treatment for moderate-to-severe osteoarthritis of the knee: A randomized placebo-controlled pilot study. QJM 2006, 99, 841–850. [Google Scholar]
- Brien, S.; Lewith, G.; Walker, A.; Hicks, S.M.; Middleton, D. Bromelain as a treatment for osteoarthritis: A review of clinical studies. Evid. based Compl. Alternative Med 2004, 1, 251–257. [Google Scholar]
- Wu, S.Q.; Otero, M.; Unger, F.M.; Goldring, M.B.; Phrutivorapongkul, A.; Chiari, C.; Kolb, A.; Viernstein, H.; Toegel, S. Anti-inflammatory activity of an ethanolic Caesalpinia sappan extract in human chondrocytes and macrophages. J. Ethnopharmacol 2011, 138, 364–372. [Google Scholar]
- Toegel, S.; Wu, S.Q.; Otero, M.; Goldring, M.B.; Leelapornpisid, P.; Chiari, C.; Kolb, A.; Unger, F.M.; Windhager, R.; Viernstein, H. Caesalpinia sappan extract inhibits IL1beta-mediated overexpression of matrix metalloproteinases in human chondrocytes. Genes Nutr 2012, 7, 307–318. [Google Scholar]
- Kosuwon, W.; Sirichatiwapee, W.; Wisanuyotin, T.; Jeeravipoolvarn, P.; Laupattarakasem, W. Efficacy of symptomatic control of knee osteoarthritis with 0.0125% of capsaicin versus placebo. J. Med. Assoc. Thailand 2010, 93, 1188–1195. [Google Scholar]
- Remadevi, R.; Szallisi, A. Adlea (ALGRX-4975), an injectable capsaicin (TRPV1 receptor agonist) formulation for longlasting pain relief. IDrugs 2008, 11, 120–132. [Google Scholar]
- McKay, L.; Gemmell, H.; Jacobson, B.; Hayes, B. Effect of a topical herbal cream on the pain and stiffness of osteoarthritis: A randomized double-blind, placebo-controlled clinical trial. J. Clin. Rheumatol 2003, 9, 164–169. [Google Scholar]
- Engler, A.; Aeschlimann, A.; Simmen, B.R.; Michel, B.A.; Gay, R.E.; Gay, S.; Sprott, H. Expression of transient receptor potential vanilloid 1 (TRPV1) in synovial fibroblasts from patients with osteoarthritis and rheumatoid arthritis. Biochem. Biophys. Res. Commun 2007, 359, 884–888. [Google Scholar]
- Piscoya, J.; Rodriguez, Z.; Bustamante, S.A.; Okuhama, N.N.; Miller, M.J.; Sandoval, M. Efficacy and safety of freeze-dried cat’s claw in osteoarthritis of the knee: Mechanisms of action of the species Uncaria guianensis. Inflamm. Res 2001, 50, 442–448. [Google Scholar]
- Rosenbaum, C.C.; O’Mathuna, D.P.; Chavez, M.; Shields, K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern. Ther. Health Med 2010, 16, 32–40. [Google Scholar]
- Olsen, N.J.; Branch, V.K.; Jonnala, G.; Seskar, M.; Cooper, M. Phase 1, placebo-controlled, dose escalation trial of chicory root extract in patients with osteoarthritis of the hip or knee. BMC Musculoskelet. Disord 2010, 11, 156. [Google Scholar]
- Cavin, C.; Delannoy, M.; Malnoe, A.; Debefve, E.; Touche, A.; Courtois, D.; Schilter, B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem. Biophys. Res. Commun 2005, 327, 742–749. [Google Scholar]
- Schmidt, B.M.; Ilic, N.; Poulev, A.; Raskin, I. Toxicological evaluation of a chicory root extract. Food Chem. Toxicol 2007, 45, 1131–1139. [Google Scholar]
- Chen, W.P.; Tang, J.L.; Bao, J.P.; Hu, P.F.; Yu, C.; Shi, Z.L.; Wu, L.D. Effects of diallyl sulphide in chondrocyte and cartilage in experimental osteoarthritis in rabbit. Phytother. Res 2011, 25, 351–356. [Google Scholar]
- Lee, H.S.; Lee, C.H.; Tsai, H.C.; Salter, D.M. Inhibition of cyclooxygenase 2 expression by diallyl sulfide on joint inflammation induced by urate crystal and IL-1beta. Osteoarthr. Cartilage 2009, 17, 91–99. [Google Scholar]
- Lai, J.N.; Chen, H.J.; Chen, C.C.; Lin, J.H.; Hwang, J.S.; Wang, J.D. Duhuo jisheng tang for treating osteoarthritis of the knee: A prospective clinical observation. Chinese Med 2007, 2, 4. [Google Scholar]
- Chantre, P.; Cappelaere, A.; Leblan, D.; Guedon, D.; Vandermander, J.; Fournie, B. Efficacy and tolerance of Harpagophytum procumbens versus diacerhein in treatment of osteoarthritis. Phytomedicine 2000, 7, 177–183. [Google Scholar]
- Chrubasik, S.; Thanner, J.; Kunzel, O.; Conradt, C.; Black, A.; Pollak, S. Comparison of outcome measures during treatment with the proprietary Harpagophytum extract doloteffin in patients with pain in the lower back, knee or hip. Phytomedicine 2002, 9, 181–194. [Google Scholar]
- Chrubasik, J.E.; Roufogalis, B.D.; Chrubasik, S. Evidence of effectiveness of herbal antiinflammatory drugs in the treatment of painful osteoarthritis and chronic low back pain. Phytother. Res 2007, 21, 675–683. [Google Scholar]
- Fiebich, B.L.; Munoz, E.; Rose, T.; Weiss, G.; McGregor, G.P. Molecular targets of the antiinflammatory Harpagophytum procumbens (devil’s claw): Inhibition of TNFalpha and COX-2 gene expression by preventing activation of AP-1. Phytother. Res 2012, 26, 806–811. [Google Scholar]
- Sumantran, V.N.; Kulkarni, A.; Chandwaskar, R.; Harsulkar, A.; Patwardhan, B.; Chopra, A.; Wagh, U.V. Chondroprotective potential of fruit extracts of Phyllanthus emblica in Osteoarthritis. Evid. Base. Compl. Alternative Med 2008, 5, 329–335. [Google Scholar]
- Uehleke, B.; Muller, J.; Stange, R.; Kelber, O.; Melzer, J. Willow bark extract STW 33-I in the long-term treatment of outpatients with rheumatic pain mainly osteoarthritis or back pain. Phytomedicine 2013, 20, 980–984. [Google Scholar]
- Schmid, B.; Ludtke, R.; Selbmann, H.K.; Kotter, I.; Tschirdewahn, B.; Schaffner, W.; Heide, L. Efficacy and tolerability of a standardized willow bark extract in patients with osteoarthritis: Randomized placebo-controlled, double blind clinical trial. Phytother. Res 2001, 15, 344–350. [Google Scholar]
- Cowan, D. Oral Aloe vera as a treatment for osteoarthritis: A summary. Brit. J. Comm. Nur 2010, 15, 280–282. [Google Scholar]
- Christensen, R.; Bartels, E.M.; Astrup, A.; Bliddal, H. Symptomatic efficacy of avocado-soybean unsaponifiables (ASU) in osteoarthritis (OA) patients: A meta-analysis of randomized controlled trials. Osteoarthr. Cartilage 2008, 16, 399–408. [Google Scholar]
- Appelboom, T.; Schuermans, J.; Verbruggen, G.; Henrotin, Y.; Reginster, J.Y. Symptoms modifying effect of avocado/soybean unsaponifiables (ASU) in knee osteoarthritis. A double blind, prospective, placebo-controlled study. Scand. J. Rheumatol 2001, 30, 242–247. [Google Scholar]
- Boileau, C.; Martel-Pelletier, J.; Caron, J.; Msika, P.; Guillou, G.B.; Baudouin, C.; Pelletier, J.P. Protective effects of total fraction of avocado/soybean unsaponifiables on the structural changes in experimental dog osteoarthritis: Inhibition of nitric oxide synthase and matrix metalloproteinase-13. Arthritis Res. Ther 2009, 11, R41. [Google Scholar]
- Au, R.Y.; Al-Talib, T.K.; Au, A.Y.; Phan, P.V.; Frondoza, C.G. Avocado soybean unsaponifiables (ASU) suppress TNF-alpha, IL-1beta, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthr. Cartilage 2007, 15, 1249–1255. [Google Scholar]
- Scorei, R.I.; Rotaru, P. Calcium fructoborate—Potential anti-inflammatory agent. Biol. Trace Element Res 2011, 143, 1223–1238. [Google Scholar]
- Bello, A.E.; Oesser, S. Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: A review of the literature. Curr. Med. Res. Opin 2006, 22, 2221–2232. [Google Scholar]
- Van Vijven, J.P.; Luijsterburg, P.A.; Verhagen, A.P.; van Osch, G.J.; Kloppenburg, M.; Bierma-Zeinstra, S.M. Symptomatic and chondroprotective treatment with collagen derivatives in osteoarthritis: A systematic review. Osteoarthr. Cartilage 2012, 20, 809–821. [Google Scholar]
- Chua, K.H.; Lee, T.H.; Nagandran, K.; Md Yahaya, N.H.; Lee, C.T.; Tjih, E.T.; Abdul Aziz, R. Edible Bird’s nest extract as a chondro-protective agent for human chondrocytes isolated from osteoarthritic knee: In vitro study. BMC Compl. Alternative Med 2013, 13, 19. [Google Scholar]
- Hooshmand, S.; Soung do, Y.; Lucas, E.A.; Madihally, S.V.; Levenson, C.W.; Arjmandi, B.H. Genistein reduces the production of proinflammatory molecules in human chondrocytes. J. Nutr. Biochem 2007, 18, 609–614. [Google Scholar]
- Coulson, S.; Vecchio, P.; Gramotnev, H.; Vitetta, L. Green-lipped mussel (Perna canaliculus) extract efficacy in knee osteoarthritis and improvement in gastrointestinal dysfunction: A pilot study. Inflammopharmacology 2012, 20, 71–76. [Google Scholar]
- Halpern, G.M. Anti-inflammatory effects of a stabilized lipid extract of Perna canaliculus (Lyprinol). Allerg. Immunol 2000, 32, 272–278. [Google Scholar]
- So, J.S.; Song, M.K.; Kwon, H.K.; Lee, C.G.; Chae, C.S.; Sahoo, A.; Jash, A.; Lee, S.H.; Park, Z.Y.; Im, S.H. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci 2011, 88, 358–366. [Google Scholar]
- Kim, L.S.; Axelrod, L.J.; Howard, P.; Buratovich, N.; Waters, R.F. Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: A pilot clinical trial. Osteoarthr. Cartilage 2006, 14, 286–294. [Google Scholar]
- Fox, R.B.; Fox, W.K. Dimethyl sulfoxide prevents hydroxyl radical-mediated depolymerization of hyaluronic acid. Ann. N. Y. Acad. Sci 1983, 411, 14–18. [Google Scholar]
- Parcell, S. Sulfur in human nutrition and applications in medicine. Altern. Med. Rev 2002, 7, 22–44. [Google Scholar]
- Baker, K.R.; Matthan, N.R.; Lichtenstein, A.H.; Niu, J.; Guermazi, A.; Roemer, F.; Grainger, A.; Nevitt, M.C.; Clancy, M.; Lewis, C.E.; et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: The MOST study. Osteoarthr. Cartilage 2012, 20, 382–387. [Google Scholar]
- Curtis, C.L.; Rees, S.G.; Little, C.B.; Flannery, C.R.; Hughes, C.E.; Wilson, C.; Dent, C.M.; Otterness, I.G.; Harwood, J.L.; Caterson, B. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum 2002, 46, 1544–1553. [Google Scholar]
- Knott, L.; Avery, N.C.; Hollander, A.P.; Tarlton, J.F. Regulation of osteoarthritis by omega-3 (n-3) polyunsaturated fatty acids in a naturally occurring model of disease. Osteoarthr. Cartilage 2011, 19, 1150–1157. [Google Scholar]
- Kim, J.; Lee, E.Y.; Koh, E.M.; Cha, H.S.; Yoo, B.; Lee, C.K.; Lee, Y.J.; Ryu, H.; Lee, K.H.; Song, Y.W. Comparative clinical trial of S-adenosylmethionine versus nabumetone for the treatment of knee osteoarthritis: An 8-week, multicenter, randomized, double-blind, double-dummy, Phase IV study in Korean patients. Clin. Ther 2009, 31, 2860–2872. [Google Scholar]
- Najm, W.I.; Reinsch, S.; Hoehler, F.; Tobis, J.S.; Harvey, P.W. S-adenosyl methionine (SAMe) versus celecoxib for the treatment of osteoarthritis symptoms: A double-blind cross-over trial. [ISRCTN36233495]. BMC Musculoskelet Disord 2004, 5, 6. [Google Scholar]
- Bradley, J.D.; Flusser, D.; Katz, B.P.; Schumacher, H.R., Jr.; Brandt, K.D.; Chambers, M.A.; Zonay, L.J. A randomized, double blind, placebo controlled trial of intravenous loading with S-adenosylmethionine (SAM) followed by oral SAM therapy in patients with knee osteoarthritis. J. Rheumatol 1994, 21, 905–911. [Google Scholar]
- Harmand, M.F.; Vilamitjana, J.; Maloche, E.; Duphil, R.; Ducassou, D. Effects of S-adenosylmethionine on human articular chondrocyte differentiation. An in vitro study. Am. J. Med 1987, 83, 48–54. [Google Scholar]
- Barcelo, H.A.; Wiemeyer, J.C.; Sagasta, C.L.; Macias, M.; Barreira, J.C. Effect of S-adenosylmethionine on experimental osteoarthritis in rabbits. Am. J. Med 1987, 83, 55–59. [Google Scholar]
- Jonas, W.B.; Rapoza, C.P.; Blair, W.F. The effect of niacinamide on osteoarthritis: A pilot study. Inflamm. Res 1996, 45, 330–334. [Google Scholar]
- Clark, A.G.; Rohrbaugh, A.L.; Otterness, I.; Kraus, V.B. The effects of ascorbic acid on cartilage metabolism in guinea pig articular cartilage explants. Matrix Biol 2002, 21, 175–184. [Google Scholar]
- McAlindon, T.; LaValley, M.; Schneider, E.; Nuite, M.; Lee, J.Y.; Price, L.L.; Lo, G.; Dawson-Hughes, B. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: A randomized controlled trial. JAMA 2013, 309, 155–162. [Google Scholar]
- Sanghi, D.; Mishra, A.; Sharma, A.C.; Singh, A.; Natu, S.M.; Agarwal, S.; Srivastava, R.N. Does vitamin D improve osteoarthritis of the knee: A randomized controlled pilot trial. Clin. Orthop. Relat. Res 2013, 471, 3556–3562. [Google Scholar]
- Blankenhorn, G. Clinical effectiveness of Spondyvit (vitamin E) in activated arthroses. A multicenter placebo-controlled double-blind study. Zeitschrift fur Orthopadie und ihre Grenzgebiete 1986, 124, 340–343. [Google Scholar]
- Brand, C.; Snaddon, J.; Bailey, M.; Cicuttini, F. Vitamin E is ineffective for symptomatic relief of knee osteoarthritis: A six month double blind, randomised, placebo controlled study. Ann. Rheum. Dis 2001, 60, 946–949. [Google Scholar]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol 2005, 165, 9–28. [Google Scholar]
- Miyake, M.; Ide, K.; Sasaki, K.; Matsukura, Y.; Shijima, K.; Fujiwara, D. Oral administration of highly oligomeric procyanidins of Jatoba reduces the severity of collagen-induced arthritis. Biosci. Biotechnol. Biochem 2008, 72, 1781–1788. [Google Scholar]
- Hadipour-Jahromy, M.; Mozaffari-Kermani, R. Chondroprotective effects of pomegranate juice on monoiodoacetate-induced osteoarthritis of the knee joint of mice. Phytother. Res 2010, 24, 182–185. [Google Scholar]
- Bliddal, H.; Rosetzsky, A.; Schlichting, P.; Weidner, M.S.; Andersen, L.A.; Ibfelt, H.H.; Christensen, K.; Jensen, O.N.; Barslev, J. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthr. Cartilage 2000, 8, 9–12. [Google Scholar]
- Altman, R.D.; Marcussen, K.C. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum 2001, 44, 2531–2538. [Google Scholar]
- Kulkarni, R.R.; Patki, P.S.; Jog, V.P.; Gandage, S.G.; Patwardhan, B. Treatment of osteoarthritis with a herbomineral formulation: A double-blind, placebo-controlled, cross-over study. J. Ethnopharmacol 1991, 33, 91–95. [Google Scholar]
- Christensen, R.; Bartels, E.M.; Altman, R.D.; Astrup, A.; Bliddal, H. Does the hip powder of Rosa canina (rosehip) reduce pain in osteoarthritis patients? —A meta-analysis of randomized controlled trials. Osteoarthr. Cartilage 2008, 16, 965–972. [Google Scholar]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res. Ther 2009, 11, R165. [Google Scholar]
- Doss, M.X.; Potta, S.P.; Hescheler, J.; Sachinidis, A. Trapping of growth factors by catechins: A possible therapeutical target for prevention of proliferative diseases. J. Nutr. Biochem 2005, 16, 259–266. [Google Scholar]
- Marotte, H.; Ruth, J.H.; Campbell, P.L.; Koch, A.E.; Ahmed, S. Green tea extract inhibits chemokine production, but up-regulates chemokine receptor expression, in rheumatoid arthritis synovial fibroblasts and rat adjuvant-induced arthritis. Rheumatology 2010, 49, 467–479. [Google Scholar]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem 2005, 16, 360–367. [Google Scholar]
- Ahmed, S.; Wang, N.; Hafeez, B.B.; Cheruvu, V.K.; Haqqi, T.M. Punica granatum L. extract inhibits IL-1beta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappaB in human chondrocytes in vitro. J. Nutr. 2005, 135, 2096–2102. [Google Scholar]
- White, B. Ginger: An overview. Am. Fam. Physician 2007, 75, 1689–1691. [Google Scholar]
- Mathy-Hartert, M.; Jacquemond-Collet, I.; Priem, F.; Sanchez, C.; Lambert, C.; Henrotin, Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm. Res 2009, 58, 899–908. [Google Scholar]
- Ameye, L.G.; Chee, W.S. Osteoarthritis and nutrition. From nutraceuticals to functional foods: A systematic review of the scientific evidence. Arthritis Res. Ther 2006, 8, R127. [Google Scholar]
- Aggarwal, B.B.; Shishodia, S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: Reasoning for seasoning. Ann. N. Y. Acad. Sci 2004, 1030, 434–441. [Google Scholar]
- Mobasheri, A. Intersection of inflammation and herbal medicine in the treatment of osteoarthritis. Curr. Rheumatol. Rep 2012, 14, 604–616. [Google Scholar]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther 2009, 11, 224. [Google Scholar]
- Aigner, T.; Soder, S.; Gebhard, P.M.; McAlinden, A.; Haag, J. Mechanisms of disease: Role of chondrocytes in the pathogenesis of osteoarthritis—Structure, chaos and senescence. Nat. Clin. Pract. Rheumatol 2007, 3, 391–399. [Google Scholar]
- Pelletier, J.P.; Martel-Pelletier, J.; Abramson, S.B. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheum 2001, 44, 1237–1247. [Google Scholar]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis 2005, 64, 1263–1267. [Google Scholar]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol 2011, 7, 33–42. [Google Scholar]
- Jerosch, J. Effects of glucosamine and chondroitin sulfate on cartilage metabolism in OA: Outlook on other nutrient partners especially omega-3 fatty acids. Inter. J. Rheumatol 2011, 2011, 969012. [Google Scholar]
- Milentijevic, D.; Rubel, I.F.; Liew, A.S.; Helfet, D.L.; Torzilli, P.A. An in vivo rabbit model for cartilage trauma: A preliminary study of the influence of impact stress magnitude on chondrocyte death and matrix damage. J. Orthop. Trauma 2005, 19, 466–473. [Google Scholar]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar]
- Roman-Blas, J.A.; Jimenez, S.A. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartilage 2006, 14, 839–848. [Google Scholar]
- Saklatvala, J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr. Drug Targets 2007, 8, 305–313. [Google Scholar]
- Blom, A.B.; Brockbank, S.M.; van Lent, P.L.; van Beuningen, H.M.; Geurts, J.; Takahashi, N.; van der Kraan, P.M.; van de Loo, F.A.; Schreurs, B.W.; Clements, K.; et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: Prominent role of Wnt-induced signaling protein 1. Arthritis Rheum 2009, 60, 501–512. [Google Scholar]
- Chadjichristos, C.; Ghayor, C.; Kypriotou, M.; Martin, G.; Renard, E.; Ala-Kokko, L.; Suske, G.; de Crombrugghe, B.; Pujol, J.P.; Galera, P. Sp1 and Sp3 transcription factors mediate interleukin-1 beta down-regulation of human type II collagen gene expression in articular chondrocytes. J. Biol. Chem 2003, 278, 39762–39772. [Google Scholar]
- Goldring, M.B.; Fukuo, K.; Birkhead, J.R.; Dudek, E.; Sandell, L.J. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J. Cell Biochem 1994, 54, 85–99. [Google Scholar]
- Seguin, C.A.; Bernier, S.M. TNFalpha suppresses link protein and type II collagen expression in chondrocytes: Role of MEK1/2 and NF-kappaB signaling pathways. J. Cell Physiol 2003, 197, 356–369. [Google Scholar]
- Saklatvala, J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 1986, 322, 547–549. [Google Scholar]
- Mengshol, J.A.; Vincenti, M.P.; Coon, C.I.; Barchowsky, A.; Brinckerhoff, C.E. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: Differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum 2000, 43, 801–811. [Google Scholar]
- Lefebvre, V.; Peeters-Joris, C.; Vaes, G. Modulation by interleukin 1 and tumor necrosis factor alpha of production of collagenase, tissue inhibitor of metalloproteinases and collagen types in differentiated and dedifferentiated articular chondrocytes. Biochim. Biophys. Acta 1990, 1052, 366–378. [Google Scholar]
- Tortorella, M.D.; Malfait, A.M.; Deccico, C.; Arner, E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthr. Cartilage 2001, 9, 539–552. [Google Scholar]
- Abramson, S.B. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res. Ther 2008, 10, S2. [Google Scholar]
- Caron, J.P.; Fernandes, J.C.; Martel-Pelletier, J.; Tardif, G.; Mineau, F.; Geng, C.; Pelletier, J.P. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum 1996, 39, 1535–1544. [Google Scholar]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2009, 61, 344–352. [Google Scholar]
- Grunke, M.; Schulze-Koops, H. Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade. Ann. Rheum. Dis 2006, 65, 555–556. [Google Scholar]
- Fioravanti, A.; Fabbroni, M.; Cerase, A.; Galeazzi, M. Treatment of erosive osteoarthritis of the hands by intra-articular infliximab injections: A pilot study. Rheumatol. Int 2009, 29, 961–965. [Google Scholar]
- Magnano, M.D.; Chakravarty, E.F.; Broudy, C.; Chung, L.; Kelman, A.; Hillygus, J.; Genovese, M.C. A pilot study of tumor necrosis factor inhibition in erosive/inflammatory osteoarthritis of the hands. J. Rheumatol 2007, 34, 1323–1327. [Google Scholar]
- Renkiewicz, R.; Qiu, L.; Lesch, C.; Sun, X.; Devalaraja, R.; Cody, T.; Kaldjian, E.; Welgus, H.; Baragi, V. Broad-spectrum matrix metalloproteinase inhibitor marimastat-induced musculoskeletal side effects in rats. Arthritis Rheum 2003, 48, 1742–1749. [Google Scholar]
- Hellio Le Graverand-Gastineau, M.P. OA clinical trials: Current targets and trials for OA. Choosing molecular targets: What have we learned and where we are headed? Osteoarthr. Cartilage 2009, 17, 1393–1401. [Google Scholar]
- Loeser, R.F. The effects of aging on the development of osteoarthritis. HSS J 2012, 8, 18–19. [Google Scholar]
- Loeser, R.F. Aging processes and the development of osteoarthritis. Curr. Opin. Rheumatol 2013, 25, 108–113. [Google Scholar]
- Zhuo, Q.; Yang, W.; Chen, J.; Wang, Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol 2012, 8, 729–737. [Google Scholar]
- Aigner, T.; Richter, W. OA in 2011: Age-related OA—A concept emerging from infancy? Nat. Rev. Rheumatol 2012, 8, 70–72. [Google Scholar]
- Martin, J.A.; Buckwalter, J.A. Post-traumatic osteoarthritis: The role of stress induced chondrocyte damage. Biorheology 2006, 43, 517–521. [Google Scholar]
- Kotani, K.; Sakane, N.; Kamimoto, M.; Taniguchi, N. Levels of reactive oxygen metabolites in patients with knee osteoarthritis. Australas J. Ageing 2011, 30, 231–233. [Google Scholar]
- Loeser, R.F.; Carlson, C.S.; Del Carlo, M.; Cole, A. Detection of nitrotyrosine in aging and osteoarthritic cartilage: Correlation of oxidative damage with the presence of interleukin-1beta and with chondrocyte resistance to insulin-like growth factor 1. Arthritis Rheum 2002, 46, 2349–2357. [Google Scholar]
- Scott, J.L.; Gabrielides, C.; Davidson, R.K.; Swingler, T.E.; Clark, I.M.; Wallis, G.A.; Boot-Handford, R.P.; Kirkwood, T.B.; Taylor, R.W.; Young, D.A. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann. Rheum. Dis 2010, 69, 1502–1510. [Google Scholar]
- Regan, E.A.; Bowler, R.P.; Crapo, J.D. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthr. Cartilage 2008, 16, 515–521. [Google Scholar]
- Moon, S.J.; Woo, Y.J.; Jeong, J.H.; Park, M.K.; Oh, H.J.; Park, J.S.; Kim, E.K.; Cho, M.L.; Park, S.H.; Kim, H.Y.; et al. Rebamipide attenuates pain severity and cartilage degeneration in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. Osteoarthr. Cartilage 2012, 20, 1426–1438. [Google Scholar]
- Ramakrishnan, P.; Hecht, B.A.; Pedersen, D.R.; Lavery, M.R.; Maynard, J.; Buckwalter, J.A.; Martin, J.A. Oxidant conditioning protects cartilage from mechanically induced damage. J. Orthop. Res 2010, 28, 914–920. [Google Scholar]
- Woo, Y.J.; Joo, Y.B.; Jung, Y.O.; Ju, J.H.; Cho, M.L.; Oh, H.J.; Jhun, J.Y.; Park, M.K.; Park, J.S.; Kang, C.M.; et al. Grape seed proanthocyanidin extract ameliorates monosodium iodoacetate-induced osteoarthritis. Exp. Mol. Med 2011, 43, 561–570. [Google Scholar]
- Rasheed, Z.; Akhtar, N.; Haqqi, T.M. Pomegranate extract inhibits the interleukin-1beta-induced activation of MKK-3, p38alpha-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes. Arthritis Res. Ther 2010, 12, R195. [Google Scholar]
- Garbacki, N.; Angenot, L.; Bassleer, C.; Damas, J.; Tits, M. Effects of prodelphinidins isolated from Ribes nigrum on chondrocyte metabolism and COX activity. Naunyn. Schmiedebergs. Arch. Pharmacol 2002, 365, 434–441. [Google Scholar]
- Shen, C.L.; Hong, K.J.; Kim, S.W. Effects of ginger (Zingiber officinale Rosc.) on decreasing the production of inflammatory mediators in sow osteoarthrotic cartilage explants. J. Med. Food 2003, 6, 323–328. [Google Scholar]
- Thomson, M.; Al-Qattan, K.K.; Al-Sawan, S.M.; Alnaqeeb, M.A.; Khan, I.; Ali, M. The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostag. Leukotr. Ess 2002, 67, 475–478. [Google Scholar]
- Frondoza, C.G.; Sohrabi, A.; Polotsky, A.; Phan, P.V.; Hungerford, D.S.; Lindmark, L. An in vitro screening assay for inhibitors of proinflammatory mediators in herbal extracts using human synoviocyte cultures. In Vitro Cell Dev. Biol. Anim 2004, 40, 95–101. [Google Scholar]
- Shakibaei, M.; Csaki, C.; Nebrich, S.; Mobasheri, A. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol 2008, 76, 1426–1439. [Google Scholar]
- Csaki, C.; Keshishzadeh, N.; Fischer, K.; Shakibaei, M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 2008, 75, 677–687. [Google Scholar]
- Shakibaei, M.; John, T.; Seifarth, C.; Mobasheri, A. Resveratrol inhibits IL-1 beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann. N. Y. Acad. Sci. 2007, 1095, 554–563. [Google Scholar]
- Schulze-Tanzil, G.; Mobasheri, A.; Sendzik, J.; John, T.; Shakibaei, M. Effects of curcumin (diferuloylmethane) on nuclear factor kappaB signaling in interleukin-1beta-stimulated chondrocytes. Ann. N. Y. Acad. Sci 2004, 1030, 578–586. [Google Scholar]
- Sreejayan, N.; Rao, M.N. Free radical scavenging activity of curcuminoids. Arzneimittelforschung 1996, 46, 169–171. [Google Scholar]
- Chan, P.S.; Caron, J.P.; Orth, M.W. Short-term gene expression changes in cartilage explants stimulated with interleukin beta plus glucosamine and chondroitin sulfate. J. Rheumatol 2006, 33, 1329–1340. [Google Scholar]
- Largo, R.; Alvarez-Soria, M.A.; Diez-Ortego, I.; Calvo, E.; Sanchez-Pernaute, O.; Egido, J.; Herrero-Beaumont, G. Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthr. Cartilage 2003, 11, 290–298. [Google Scholar]
- Henrotin, Y.; Deby-Dupont, G.; Deby, C.; De Bruyn, M.; Lamy, M.; Franchimont, P. Production of active oxygen species by isolated human chondrocytes. Br. J. Rheumatol 1993, 32, 562–567. [Google Scholar]
- Lo, Y.Y.; Wong, J.M.; Cruz, T.F. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J. Biol. Chem 1996, 271, 15703–15707. [Google Scholar]
- Song, D.U.; Jung, Y.D.; Chay, K.O.; Chung, M.A.; Lee, K.H.; Yang, S.Y.; Shin, B.A.; Ahn, B.W. Effect of drinking green tea on age-associated accumulation of Maillard-type fluorescence and carbonyl groups in rat aortic and skin collagen. Arch. Biochem. Biophys 2002, 397, 424–429. [Google Scholar]
- Bordoni, A.; Hrelia, S.; Angeloni, C.; Giordano, E.; Guarnieri, C.; Caldarera, C.M.; Biagi, P.L. Green tea protection of hypoxia/reoxygenation injury in cultured cardiac cells. J. Nutr. Biochem 2002, 13, 103–111. [Google Scholar]
- Katiyar, S.K.; Afaq, F.; Azizuddin, K.; Mukhtar, H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol. Appl. Pharmacol 2001, 176, 110–117. [Google Scholar]
- Meng, Q.; Velalar, C.N.; Ruan, R. Effects of epigallocatechin-3-gallate on mitochondrial integrity and antioxidative enzyme activity in the aging process of human fibroblast. Free Radic. Biol. Med 2008, 44, 1032–1041. [Google Scholar]
- Ippoushi, K.; Azuma, K.; Ito, H.; Horie, H.; Higashio, H. [6]-Gingerol inhibits nitric oxide synthesis in activated J774.1 mouse macrophages and prevents peroxynitrite-induced oxidation and nitration reactions. Life Sci 2003, 73, 3427–3437. [Google Scholar]
- Pan, M.H.; Hsieh, M.C.; Hsu, P.C.; Ho, S.Y.; Lai, C.S.; Wu, H.; Sang, S.; Ho, C.T. 6-Shogaol suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. Mol. Nutr. Food Res 2008, 52, 1467–1477. [Google Scholar]
- Seeram, N.P.; Nair, M.G. Inhibition of lipid peroxidation and structure-activity-related studies of the dietary constituents anthocyanins, anthocyanidins, and catechins. J. Agric. Food Chem 2002, 50, 5308–5312. [Google Scholar]
- Sudheesh, S.; Vijayalakshmi, N.R. Flavonoids from Punica granatum—Potential antiperoxidative agents. Fitoterapia 2005, 76, 181–186. [Google Scholar]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and −13 in human chondrocytes. J. Pharmacol. Exp. Ther 2004, 308, 767–773. [Google Scholar]
- Vankemmelbeke, M.N.; Jones, G.C.; Fowles, C.; Ilic, M.Z.; Handley, C.J.; Day, A.J.; Knight, C.G.; Mort, J.S.; Buttle, D.J. Selective inhibition of ADAMTS-1, −4 and −5 by catechin gallate esters. Eur. J. Biochem 2003, 270, 2394–2403. [Google Scholar]
- Yun, H.J.; Yoo, W.H.; Han, M.K.; Lee, Y.R.; Kim, J.S.; Lee, S.I. Epigallocatechin-3-gallate suppresses TNF-alpha-induced production of MMP-1 and −3 in rheumatoid arthritis synovial fibroblasts. Rheumatol. Int 2008, 29, 23–29. [Google Scholar]
- Lee, J.H.; Chung, J.H.; Cho, K.H. The effects of epigallocatechin-3-gallate on extracellular matrix metabolism. J. Dermatol. Sci 2005, 40, 195–204. [Google Scholar]
- Cheng, X.W.; Kuzuya, M.; Kanda, S.; Maeda, K.; Sasaki, T.; Wang, Q.L.; Tamaya-Mori, N.; Shibata, T.; Iguchi, A. Epigallocatechin-3-gallate binding to MMP-2 inhibits gelatinolytic activity without influencing the attachment to extracellular matrix proteins but enhances MMP-2 binding to TIMP-2. Arch. Biochem. Biophys 2003, 415, 126–132. [Google Scholar]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol 2007, 73, 1434–1445. [Google Scholar]
- Clutterbuck, A.L.; Mobasheri, A.; Shakibaei, M.; Allaway, D.; Harris, P. Interleukin-1beta-induced extracellular matrix degradation and glycosaminoglycan release is inhibited by curcumin in an explant model of cartilage inflammation. Ann. N. Y. Acad. Sci 2009, 1171, 428–435. [Google Scholar]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: Role of the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem 2011, 286, 28556–28566. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Leong, D.J.; Choudhury, M.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Nutraceuticals: Potential for Chondroprotection and Molecular Targeting of Osteoarthritis. Int. J. Mol. Sci. 2013, 14, 23063-23085. https://doi.org/10.3390/ijms141123063
Leong DJ, Choudhury M, Hirsh DM, Hardin JA, Cobelli NJ, Sun HB. Nutraceuticals: Potential for Chondroprotection and Molecular Targeting of Osteoarthritis. International Journal of Molecular Sciences. 2013; 14(11):23063-23085. https://doi.org/10.3390/ijms141123063
Chicago/Turabian StyleLeong, Daniel J., Marwa Choudhury, David M. Hirsh, John A. Hardin, Neil J. Cobelli, and Hui B. Sun. 2013. "Nutraceuticals: Potential for Chondroprotection and Molecular Targeting of Osteoarthritis" International Journal of Molecular Sciences 14, no. 11: 23063-23085. https://doi.org/10.3390/ijms141123063