The Homeobox Only Protein Homeobox (HOPX) and Colorectal Cancer
Abstract
:1. Introduction
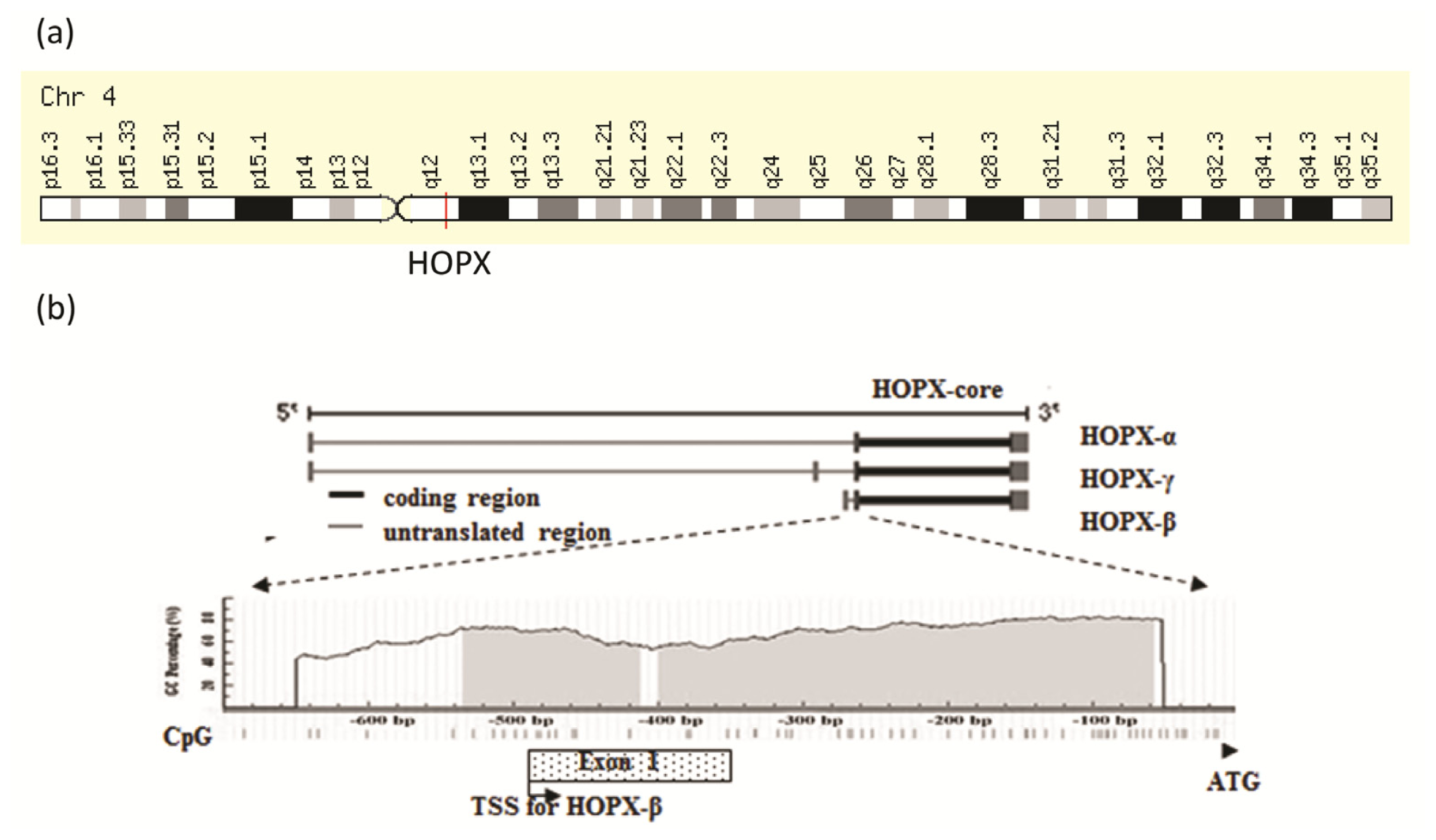
2. Genomic Structure of HOPX Gene
3. Colorectal Cancer (CRC) and HOPX Gene Expression
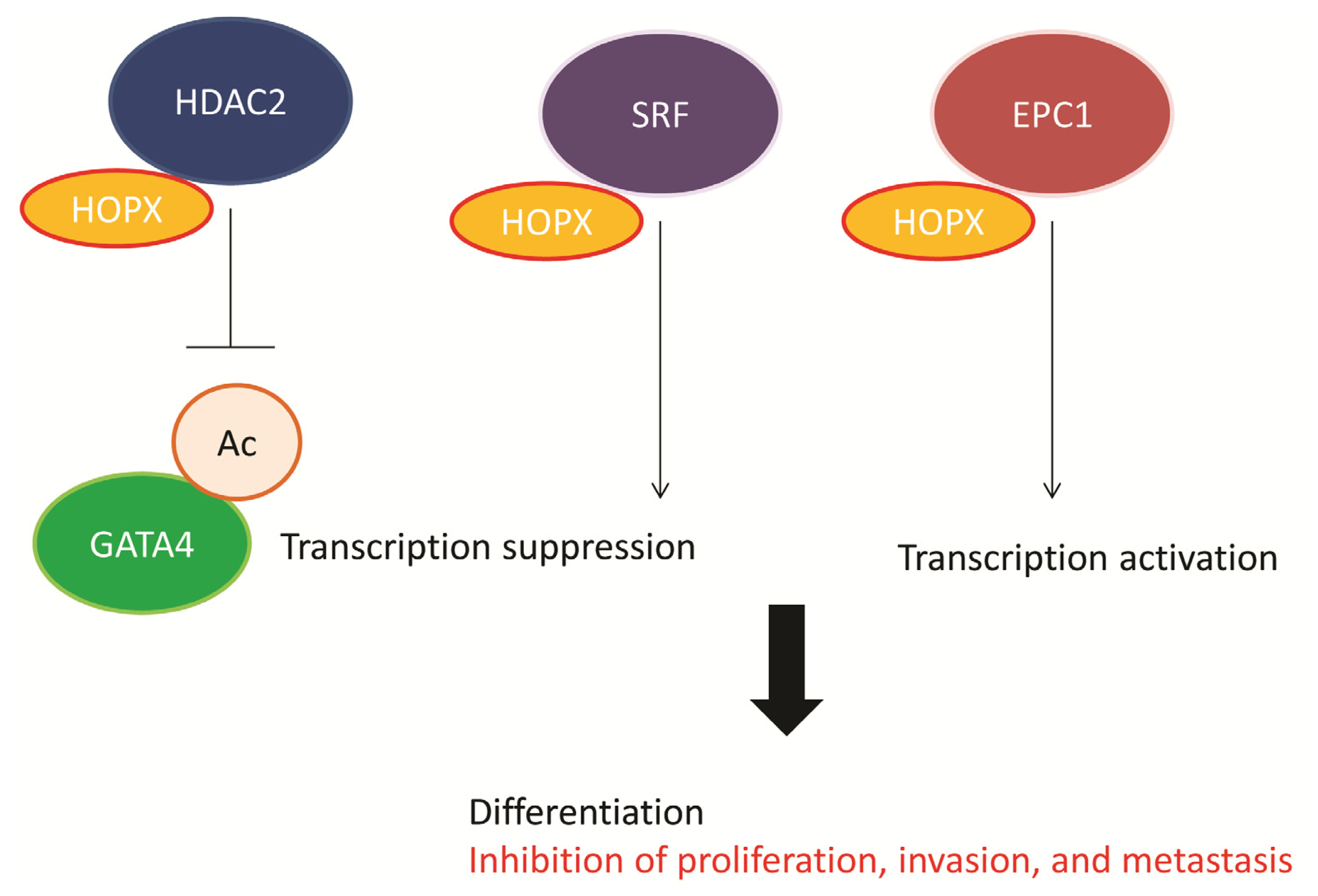
4. Functional Role of HOPX in CRC
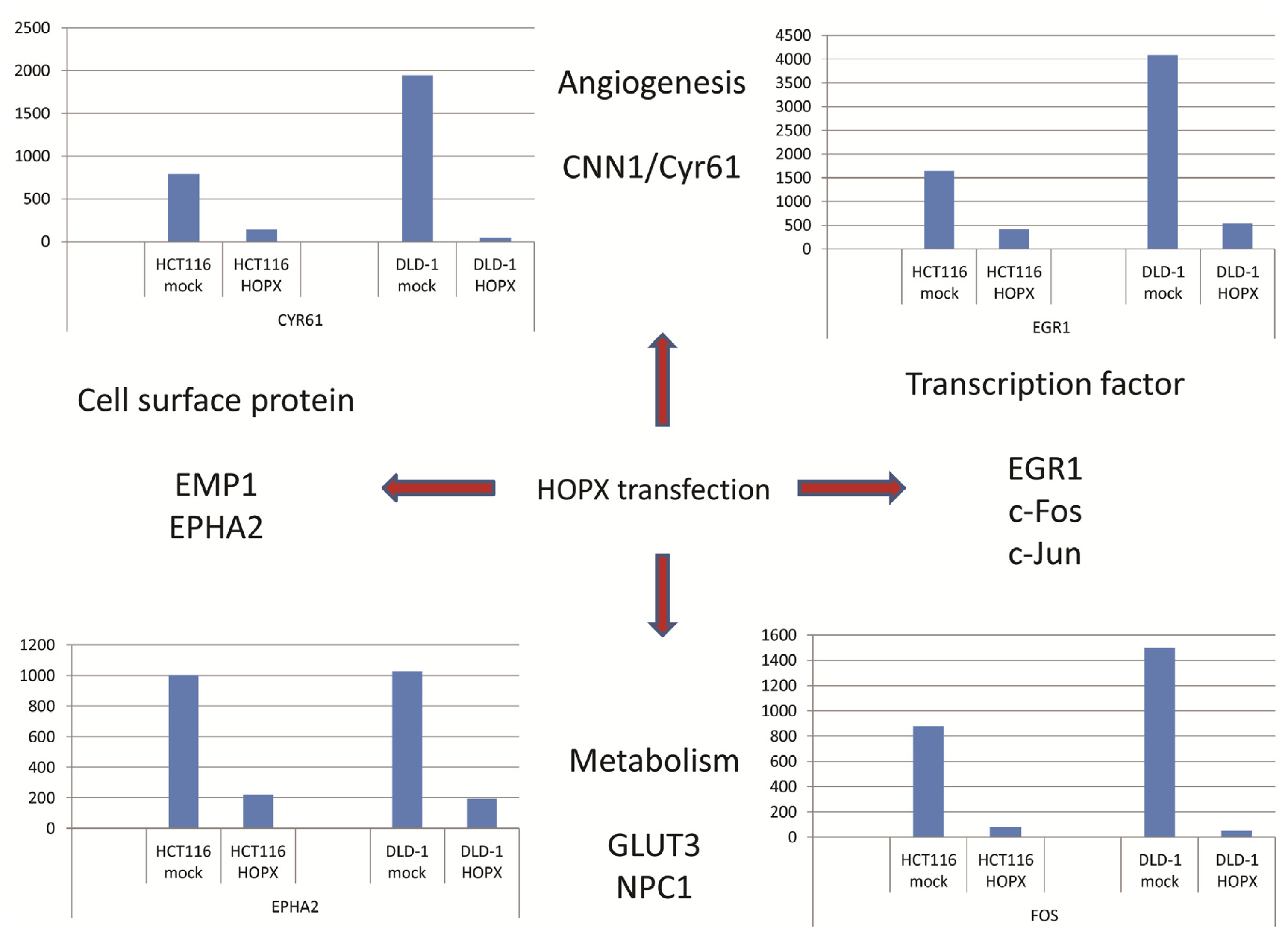
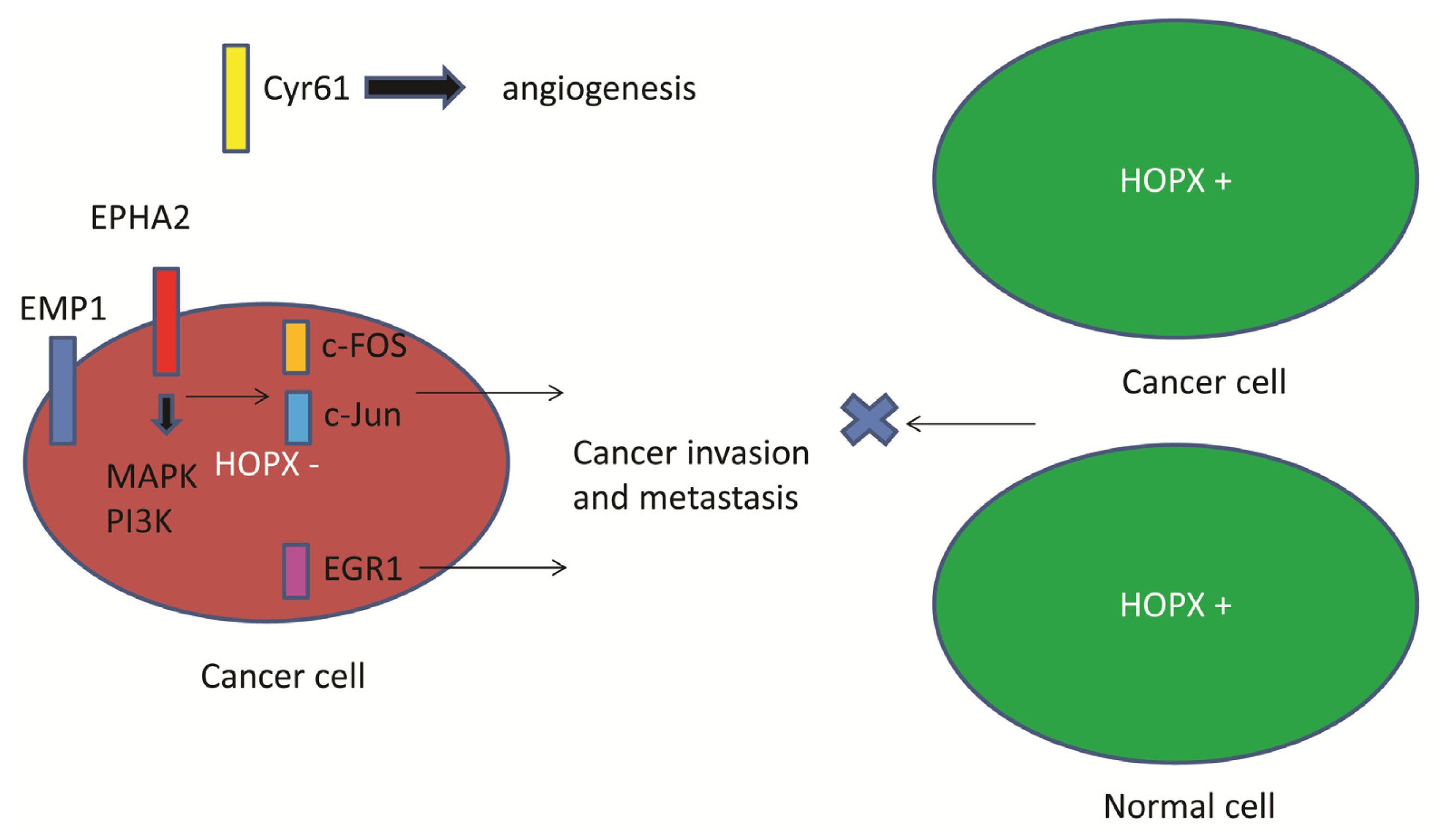
5. Downstream Gene of HOPX in CRC
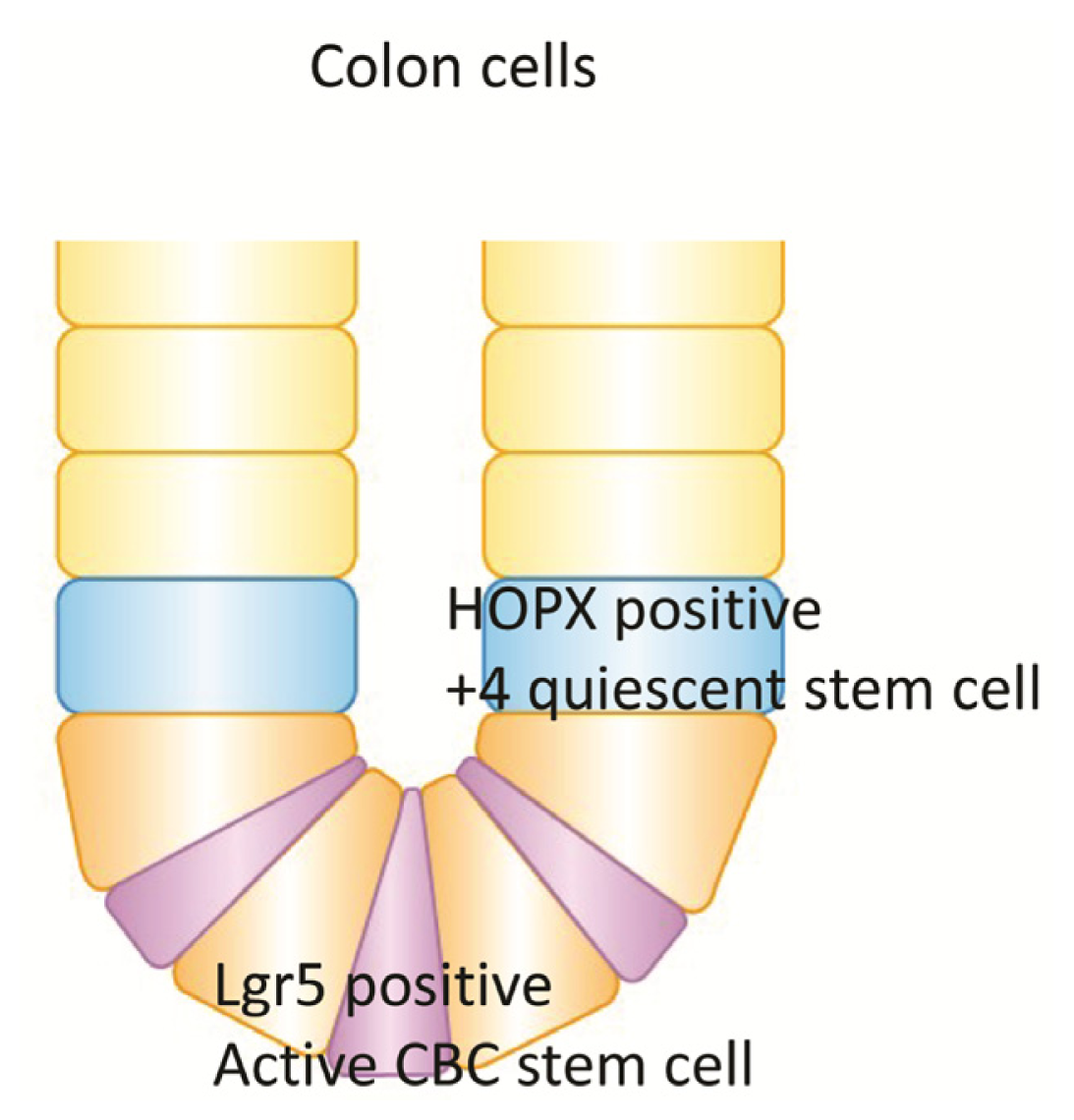
6. Quiescent Stem Cell (+4) and HOPX in Colon Goblet Cells
7. Conclusions
Conflicts of Interest
References
- Chen, F.; Kook, H.; Milewski, R.; Gitler, A.D.; Lu, M.M.; Li, J.; Nazarian, R.; Schnepp, R.; Jen, K.; Biben, C.; et al. Hop is an unusual homeobox gene that modulates cardiac development. Cell 2002, 110, 713–723. [Google Scholar]
- Shin, C.H.; Liu, Z.P.; Passier, R.; Zhang, C.L.; Wang, D.Z.; Harris, T.M.; Yamagishi, H.; Richardson, J.A.; Childs, G.; Olson, E.N. Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell 2002, 110, 725–735. [Google Scholar]
- Kook, H.; Lepore, J.J.; Gitler, A.D.; Lu, M.M.; Wing-Man Yung, W.; Mackay, J.; Zhou, R.; Ferrari, V.; Gruber, P.; Epstein, J.A. Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop. J. Clin. Investig 2003, 112, 863–871. [Google Scholar]
- Trivedi, C.M.; Zhu, W.; Wang, Q.; Jia, C.; Kee, H.J.; Li, L.; Hannenhalli, S.; Epstein, J.A. Hopx and Hdac2 interact to modulate Gata4 acetylation and embryonic cardiac myocyte proliferation. Dev. Cell 2010, 19, 450–459. [Google Scholar]
- Kee, H.J.; Kim, J.R.; Nam, K.I.; Park, H.Y.; Shin, S.; Kim, J.C.; Shimono, Y.; Takahashi, M.; Jeong, M.H.; Kim, N.; et al. Enhancer of polycomb1, a novel homeodomain only protein-binding partner, induces skeletal muscle differentiation. J. Biol. Chem 2007, 282, 7700–7709. [Google Scholar]
- Asanoma, K.; Kato, H.; Inoue, T.; Matsuda, T.; Wake, N. Analysis of a candidate gene associated with growth suppression of choriocarcinoma and differentiation of trophoblasts. J. Reprod. Med 2004, 49, 617–626. [Google Scholar]
- Asanoma, K.; Kato, H.; Yamaguchi, S.; Shin, C.H.; Liu, Z.P.; Kato, K.; Inoue, T.; Miyanari, Y.; Yoshikawa, K.; Sonoda, K.; et al. HOP/NECC1, a novel regulator of mouse trophoblast differentiation. J. Biol. Chem 2007, 282, 24065–24074. [Google Scholar]
- Yang, J.M.; Sim, S.M.; Kim, H.Y.; Park, G.T. Expression of the homeobox gene, HOPX, is modulated by cell differentiation in human keratinocytes and is involved in the expression of differentiation markers. Eur. J. Cell Biol 2010, 89, 537–546. [Google Scholar]
- Obarzanek-Fojt, M.; Favre, B.; Kypriotou, M.; Ryser, S.; Huber, M.; Hohl, D. Homeodomain-only protein HOP is a novel modulator of late differentiation in keratinocytes. Eur. J. Cell Biol 2011, 90, 279–290. [Google Scholar]
- Hawiger, D.; Wan, Y.Y.; Eynon, E.E.; Flavell, R.A. The transcription cofactor Hopx is required for regulatory T cell function in dendritic cell-mediated peripheral T cell unresponsiveness. Nat. Immunol 2010, 11, 962–968. [Google Scholar]
- Albrecht, I.; Niesner, U.; Janke, M.; Menning, A.; Loddenkemper, C.; Kühl, A.A.; Lepenies, I.; Lexberg, M.H.; Westendorf, K.; Hradilkova, K.; et al. Persistence of effector memory Th1 cells is regulated by Hopx. Eur. J. Immunol 2010, 40, 2993–3006. [Google Scholar]
- Cheung, W.K.; Zhao, M.; Liu, Z.; Stevens, L.E.; Cao, P.D.; Fang, J.E.; Westbrook, T.F.; Nguyen, D.X. Control of alveolar differentiation by the lineage transcription factors GATA6 and HOPX inhibits lung adenocarcinoma metastasis. Cancer Cell 2013, 23, 725–738. [Google Scholar]
- Takeda, N.; Jain, R.; LeBoeuf, M.R.; Wang, Q.; Lu, M.M.; Epstein, J.A. Interconversion between intestinal stem cell populations in distinct niches. Science 2011, 334, 1420–1424. [Google Scholar]
- Takeda, N.; Jain, R.; Leboeuf, M.R.; Padmanabhan, A.; Wang, Q.; Li, L.; Lu, M.M.; Millar, S.E.; Epstein, J.A. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+ niche cells. Development 2013, 140, 1655–1664. [Google Scholar]
- Sakakura, C.; Hagiwara, A.; Taniguchi, H.; Yamaguchi, T.; Yamagishi, H.; Takahashi, T.; Koyama, K.; Nakamura, Y.; Abe, T.; Inazawa, J. Chromosomal aberrations in human hepatocellular carcinomas associated with hepatitis C virus infection detected by comparative genomic hybridization. Br. J. Cancer 1999, 80, 2034–2039. [Google Scholar]
- Wong, N.; Lai, P.; Lee, S.W.; Fan, S.; Pang, E.; Liew, C.T.; Sheng, Z.; Lau, J.W.; Johnson, P.J. Assessment of genetic changes in hepatocellular carcinoma by comparative genomic hybridization analysis: Relationship to disease stage, tumor size, and cirrhosis. Am. J. Pathol 1999, 154, 37–43. [Google Scholar]
- Burger, A.M.; Zhang, X.; Li, H.; Ostrowski, J.L.; Beatty, B.; Venanzoni, M.; Papas, T.; Seth, A. Down-regulation of T1A12/mac25, a novel insulin-like growth factor binding protein related gene, is associated with disease progression in breast carcinomas. Oncogene 1998, 16, 2459–2467. [Google Scholar]
- Leon, M.; Kew, M.C. Loss of heterozygosity in chromosome 4q12–q13 in hepatocellular carcinoma in southern African blacks. Anticancer Res 1996, 16, 349–351. [Google Scholar]
- Chen, Y.; Pacyna-Gengelbach, M.; Deutschmann, N.; Niesporek, S.; Petersen, I. Homeobox gene HOP has a potential tumor suppressive activity in human lung cancer. Int. J. Cancer 2007, 121, 1021–1027. [Google Scholar]
- Ooki, A.; Yamashita, K.; Kikuchi, S.; Sakuramoto, S.; Katada, N.; Kokubo, K.; Kobayashi, H.; Kim, M.S.; Sidransky, D.; Watanabe, M. Potential utility of HOP homeobox gene promoter methylation as a marker of tumor aggressiveness in gastric cancer. Oncogene 2010, 29, 3263–3275. [Google Scholar]
- Waraya, M.; Yamashita, K.; Katoh, H.; Ooki, A.; Kawamata, H.; Nishimiya, H.; Nakamura, K.; Ema, A.; Watanabe, M. Cancer specific promoter CpG Islands hypermethylation of HOP homeobox (HOPX) gene and its potential tumor suppressive role in pancreatic carcinogenesis. BMC Cancer 2012, 12. [Google Scholar] [CrossRef]
- Yamashita, K.; Upadhyay, S.; Osada, M.; Hoque, M.O.; Xiao, Y.; Mori, M.; Sato, F.; Meltzer, S.J.; Sidransky, D. Pharmacologic unmasking of epigenetically silenced tumor suppressor genes in esophageal squamous cell carcinoma. Cancer Cell 2002, 2, 485–495. [Google Scholar]
- Yamashita, K.; Kim, M.S.; Park, H.L.; Tokumaru, Y.; Osada, M.; Inoue, H.; Mori, M.; Sidransky, D. HOP/OB1/NECC1 promoter DNA is frequently hypermethylated and involved in tumorigenic ability in esophageal squamous cell carcinoma. Mol. Cancer Res 2008, 6, 31–41. [Google Scholar]
- Yamashita, K.; Sakuramoto, S.; Watanabe, M. Genomic and epigenetic profiles of gastric cancer: Potential diagnostic and therapeutic applications. Surg. Today 2011, 41, 24–38. [Google Scholar]
- Lemaire, F.; Millon, R.; Muller, D.; Rabouel, Y.; Bracco, L.; Abecassis, J.; Wasylyk, B. Loss of HOP tumour suppressor expression in head and neck squamous cell carcinoma. Br. J. Cancer 2004, 91, 258–261. [Google Scholar]
- Katoh, H.; Yamashita, K.; Waraya, M.; Margalit, O.; Ooki, A.; Tamaki, H.; Sakagami, H.; Kokubo, K.; Sidransky, D.; Watanabe, M. Epigenetic silencing of HOPX promotes cancer progression in colorectal cancer. Neoplasia 2012, 14, 559–571. [Google Scholar]
- Chen, Y.; Petersen, S.; Pacyna-Gengelbach, M.; Pietas, A.; Petersen, I. Identification of a novel homeobox-containing gene, LAGY, which is downregulated in lung cancer. Oncology 2003, 64, 450–458. [Google Scholar]
- Asanoma, K.; Matsuda, T.; Kondo, H.; Kato, K.; Kishino, T.; Niikawa, N.; Wake, N.; Kato, H. NECC1, a candidate choriocarcinoma suppressor gene that encodes a homeodomain consensus motif. Genomics 2003, 81, 15–25. [Google Scholar]
- Yamaguchi, S.; Asanoma, K.; Takao, T.; Kato, K.; Wake, N. Homeobox gene HOPX is epigenetically silenced in human uterine endometrial cancer and suppresses estrogen-stimulated proliferation of cancer cells by inhibiting serum response factor. Int. J. Cancer 2009, 124, 2577–2588. [Google Scholar]
- Harada, Y.; Kijima, K.; Shinmura, K.; Sakata, M.; Sakuraba, K.; Yokomizo, K.; Kitamura, Y.; Shirahata, A.; Goto, T.; Mizukami, H.; et al. Methylation of the homeobox gene, HOPX, is frequently detected in poorly differentiated colorectal cancer. Anticancer Res 2011, 31, 2889–2892. [Google Scholar]
- Kim, M.S.; Yamashita, K.; Baek, J.H.; Park, H.L.; Carvalho, A.L.; Osada, M.; Hoque, M.O.; Upadhyay, S.; Mori, M.; Moon, C.; et al. N-methyl-d-aspartate receptor type 2B is epigenetically inactivated and exhibits tumor-suppressive activity in human esophageal cancer. Cancer Res 2006, 66, 3409–3418. [Google Scholar]
- Kim, M.S.; Lee, J.; Sidransky, D. DNA methylation markers in colorectal cancer. Cancer Metastasis Rev 2010, 29, 181–206. [Google Scholar]
- Kovarova, D.; Plachy, J.; Kosla, J.; Trejbalova, K.; Cermak, V.; Hejnar, J. Downregulation of the HOPX gene decreases metastatic activity in a chicken sarcoma cell line model and identifies genes associated with metastasis. Mol. Cancer Res 2013, 11, 1235–1247. [Google Scholar]
- Crowe, D.L.; Brown, T.N. Transcriptional inhibition of matrix metalloproteinase 9 (MMP-9) activity by a c-fos/estrogen receptor fusion protein is mediated by the proximal AP-1 site of the MMP-9 promoter and correlates with reduced tumor cell invasion. Neoplasia 1999, 1, 368–372. [Google Scholar]
- Kajanne, R.; Miettinen, P.; Mehlem, A.; Leivonen, S.K.; Birrer, M.; Foschi, M.; Kähäri, V.M.; Leppä, S. EGF-R regulates MMP function in fibroblasts through MAPK and AP-1 pathways. J. Cell Physiol 2007, 212, 489–497. [Google Scholar]
- Dong, W.; Li, Y.; Gao, M.; Hu, M.; Li, X.; Mai, S.; Guo, N.; Yuan, S.; Song, L. IKKα contributes to UVB-induced VEGF expression by regulating AP-1 transactivation. Nucleic Acids Res 2012, 40, 2940–2955. [Google Scholar]
- Bae, S.K.; Bae, M.H.; Ahn, M.Y.; Son, M.J.; Lee, Y.M.; Bae, M.K.; Lee, O.H.; Park, B.C.; Kim, K.W. Egr-1 mediates transcriptional activation of IGF-II gene in response to hypoxia. Cancer Res 1999, 59, 5989–5994. [Google Scholar]
- Worden, B.; Yang, X.P.; Lee, T.L.; Bagain, L.; Yeh, N.T.; Cohen, J.G.; Van Waes, C.; Chen, Z. Hepatocyte growth factor/scatter factor differentially regulates expression of proangiogenic factors through Egr-1 in head and neck squamous cell carcinoma. Cancer Res 2005, 65, 7071–7080. [Google Scholar]
- Baron, V.; De Gregorio, G.; Krones-Herzig, A.; Virolle, T.; Calogero, A.; Urcis, R.; Mercola, D. Inhibition of Egr-1 expression reverses transformation of prostate cancer cells in vitro and in vivo. Oncogene 2003, 22, 4194–4204. [Google Scholar]
- Herath, N.I.; Boyd, A.W. The role of Eph receptors and ephrin ligands in colorectal cancer. Int. J. Cancer 2010, 126, 2003–2011. [Google Scholar]
- Biao-Xue, R.; Xi-Guang, C.; Shuan-ying, Y.; Wei, L.; Zong-juan, M. EphA2-dependent molecular targeting therapy for malignant tumors. Curr. Cancer Drug Targets 2011, 11, 1082–1097. [Google Scholar]
- Binda, E.; Visioli, A.; Giani, F.; Lamorte, G.; Copetti, M.; Pitter, K.L.; Huse, J.T.; Cajola, L.; Zanetti, N.; di Meco, F.; et al. The EphA2 receptor drives self-renewal and tumorigenicity in stem-like tumor-propagating cells from human glioblastomas. Cancer Cell 2012, 22, 765–780. [Google Scholar]
- Salaita, K.; Nair, P.M.; Petit, R.S.; Neve, R.M.; Das, D.; Gray, J.W.; Groves, J.T. Restriction of receptor movement alters cellular response: Physical force sensing by EphA2. Science 2010, 327, 1380–1385. [Google Scholar]
- Lee, J.W.; Han, H.D.; Shahzad, M.M.; Kim, S.W.; Mangala, L.S.; Nick, A.M.; Lu, C.; Langley, R.R.; Schmandt, R.; Kim, H.S.; et al. EphA2 immunoconjugate as molecularly targeted chemotherapy for ovarian carcinoma. J. Natl. Cancer Inst 2009, 101, 1193–1205. [Google Scholar]
- Miao, H.; Li, D.Q.; Mukherjee, A.; Guo, H.; Petty, A.; Cutter, J.; Basilion, J.P.; Sedor, J.; Wu, J.; Danielpour, D.; et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell 2009, 16, 9–20. [Google Scholar]
- Jain, A.; Tindell, C.A.; Laux, I.; Hunter, J.B.; Curran, J.; Galkin, A.; Afar, D.E.; Aronson, N.; Shak, S.; Natale, R.B.; et al. Epithelial membrane protein-1 is a biomarker of gefitinib resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 11858–11863. [Google Scholar]
- Karapetis, C.S.; Khambata-Ford, S.; Jonker, D.J.; O’Callaghan, C.J.; Tu, D.; Tebbutt, N.C.; Simes, R.J.; Chalchal, H.; Shapiro, J.D.; Robitaille, S.; et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med 2008, 359, 1757–1765. [Google Scholar]
- Carrato, A.; Gómez, A.; Escudero, P.; Chaves, M.; Rivera, F.; Marcuello, E.; González, E.; Grávalos, C.; Constenla, M.; Manzano, J.L.; et al. Panitumumab and irinotecan every 3 weeks is an active and convenient regimen for second-line treatment of patients with wild-type K-RAS metastatic colorectal cancer. Clin. Transl. Oncol 2013, 15, 705–711. [Google Scholar]
- Harris, L.G.; Pannell, L.K.; Singh, S.; Samant, R.S.; Shevde, L.A. Increased vascularity and spontaneous metastasis of breast cancer by hedgehog signaling mediated upregulation of cyr61. Oncogene 2012, 31, 3370–3380. [Google Scholar]
- Haque, I.; De, A.; Majumder, M.; Mehta, S.; McGregor, D.; Banerjee, S.K.; van Veldhuizen, P.; Banerjee, S. The matricellular protein CCN1/Cyr61 is a critical regulator of Sonic Hedgehog in pancreatic carcinogenesis. J. Biol. Chem 2012, 287, 38569–38579. [Google Scholar]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar]
- Takahashi, H.; Ishii, H.; Nishida, N.; Takemasa, I.; Mizushima, T.; Ikeda, M.; Yokobori, T.; Mimori, K.; Yamamoto, H.; Sekimoto, M.; et al. Significance of Lgr5(+ve) cancer stem cells in the colon and rectum. Ann. Surg. Oncol 2011, 18, 1166–1174. [Google Scholar]

| Cancer Kinds | Histology | HOPX expression | DNA methylation | Prognostic relevance | References | Published year |
|---|---|---|---|---|---|---|
| Lung | SCC | Reduced in cancer | Not assessed | Not assessed | [27] | 2003 |
| Adeno | Reduced in cancer | Not assessed | Yes | [12] | 2013 | |
| Placenta | Tropho | Reduced in cancer | Not assessed | Not assessed | [28] | 2003 |
| Head and Neck | SCC | Reduced in cancer | Not assessed | Not assessed | [25] | 2004 |
| Esophagus | SCC | Reduced in cancer | Cancer-prone | Yes | [23] | 2008 |
| Uterine | Endo | Reduced in cancer | Cancer-prone | Not assessed | [29] | 2009 |
| Stomach | Adeno | Reduced in cancer | Cancer-prone | Yes | [20] | 2010 |
| Colon/Rectum | Adeno | Not assessed | Cancer-prone | Not assessed | [30] | 2011 |
| Adeno | Reduced in cancer | Cancer-prone | Yes | [26] | 2012 | |
| Pancreas | Adeno | Reduced in cancer | Cancer-prone | No | [21] | 2012 |
| Cancer Kinds | In vivo tumorigenesis | In vitro tumorigenesis | Proliferaion | Apoptosis | Invasion | Angiogenesis | Mets in animial | Ref. | Published year |
|---|---|---|---|---|---|---|---|---|---|
| Placenta | Yes | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | [28] | 2003 |
| Esophagus | Not assessed | Yes | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | [23] | 2008 |
| Head and Neck | Not assessed | Yes | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | [23] | 2008 |
| Lung | Yes | Yes | Yes | Not assessed | Not assessed | Not assessed | Not assessed | [19] | 2007 |
| Not assessed | Not assessed | No | No | Yes | Not assessed | Yes | [12] | 2013 | |
| Uterine | Yes | Not assessed | Yes | Not assessed | Not assessed | Not assessed | Not assessed | [28] | 2009 |
| Breast | Yes | Not assessed | Yes | Not assessed | Not assessed | Not assessed | Not assessed | [29] | 2009 |
| Stomach | Not assessed | Yes | Yes | Yes | Yes | Not assessed | Not assessed | [20] | 2010 |
| Colon/Rectum | Yes | Yes | Yes | Yes | Yes | Yes | Not assessed | [26] | 2012 |
| Pancreas | Not assessed | Yes | Yes | Yes | Yes | Not assessed | Not assessed | [21] | 2012 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamashita, K.; Katoh, H.; Watanabe, M. The Homeobox Only Protein Homeobox (HOPX) and Colorectal Cancer. Int. J. Mol. Sci. 2013, 14, 23231-23243. https://doi.org/10.3390/ijms141223231
Yamashita K, Katoh H, Watanabe M. The Homeobox Only Protein Homeobox (HOPX) and Colorectal Cancer. International Journal of Molecular Sciences. 2013; 14(12):23231-23243. https://doi.org/10.3390/ijms141223231
Chicago/Turabian StyleYamashita, Keishi, Hiroshi Katoh, and Masahiko Watanabe. 2013. "The Homeobox Only Protein Homeobox (HOPX) and Colorectal Cancer" International Journal of Molecular Sciences 14, no. 12: 23231-23243. https://doi.org/10.3390/ijms141223231




