Tyrosol and Its Analogues Inhibit Alpha-Melanocyte-Stimulating Hormone Induced Melanogenesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
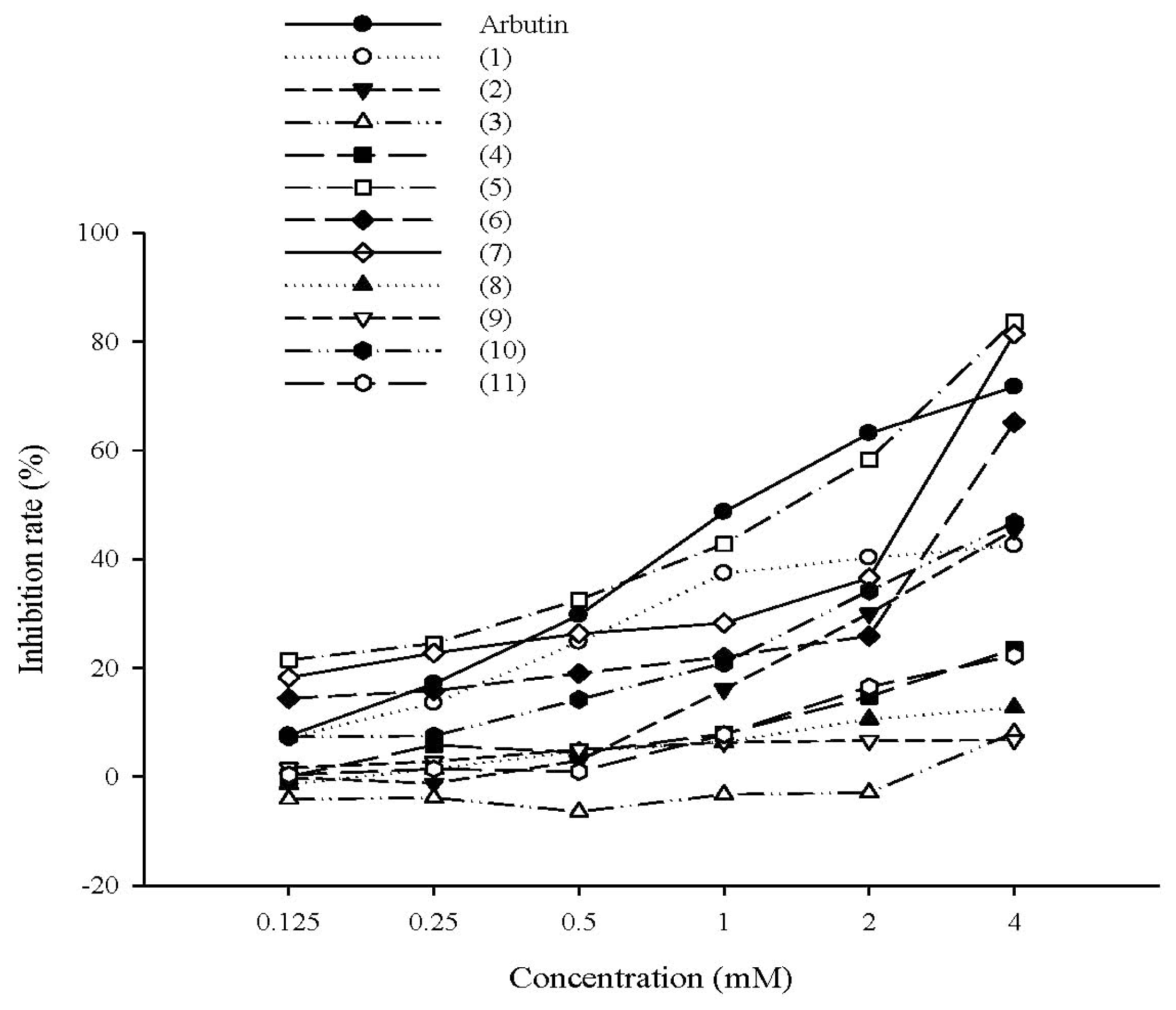
2.1.1. The Effect of Tyrosol and Its Analogues on Inhibition of Mushroom Tyrosinase Activity
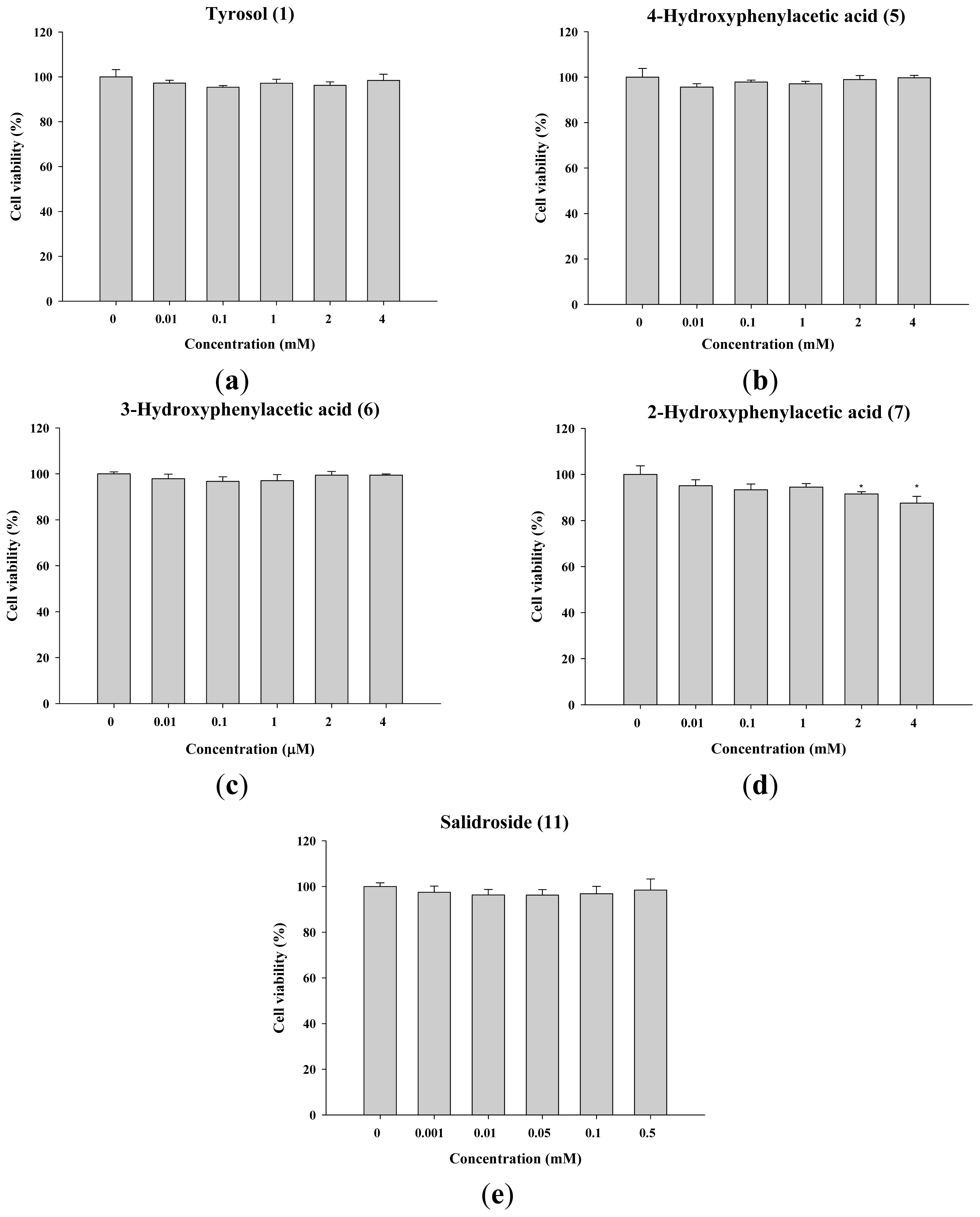
2.1.2. The Effect of Tyrosol and Its Analogues on the Viability of B16F0 Cells
2.1.3. Inhibitory Effect of Tyrosol and Its Analogues on Melanin Synthesis in B16F0 Cells
2.1.4. Inhibition of Tyrosinase Activity in B16F0 Cells Exposed to Tyrosol and Its Analogues
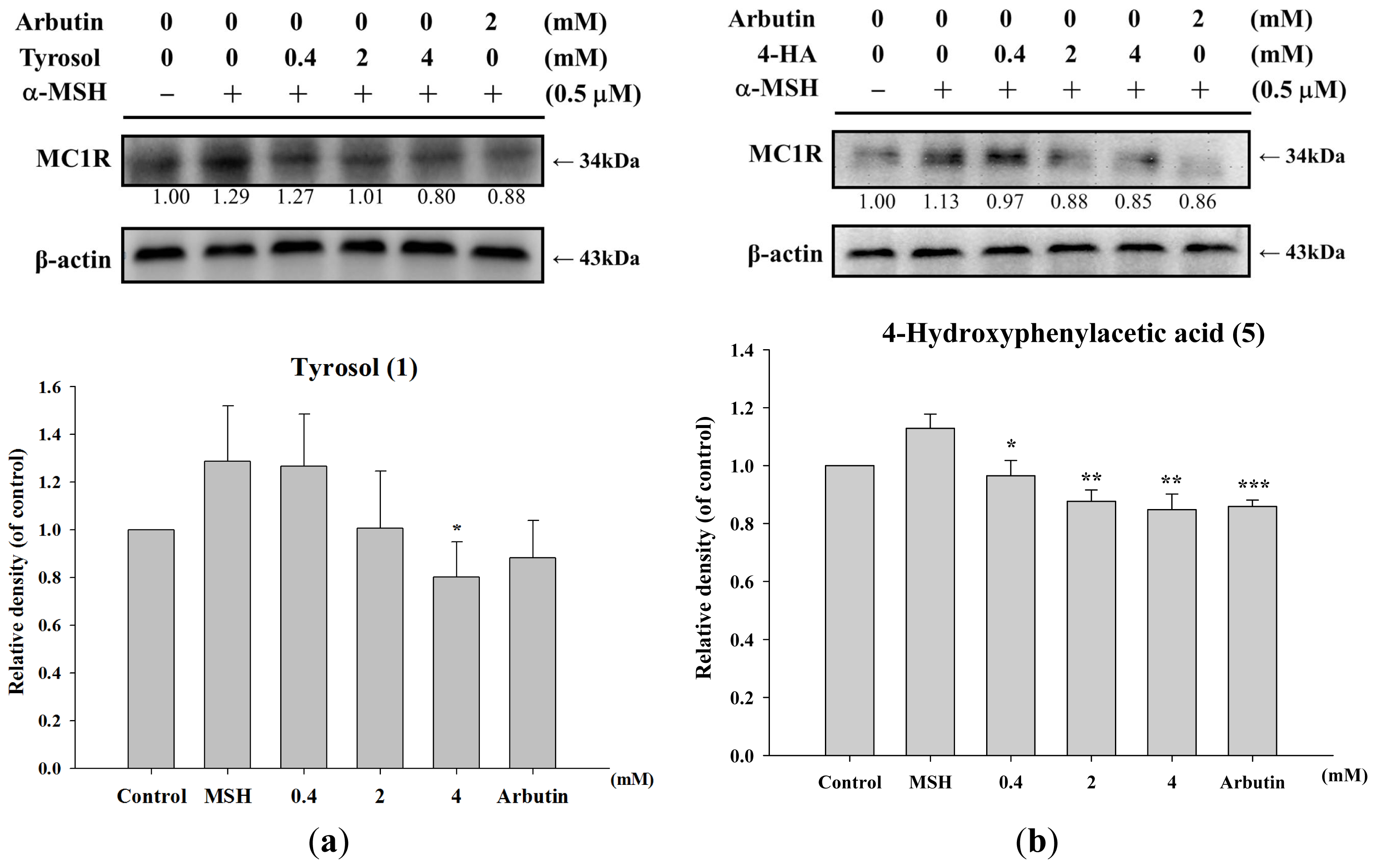
2.1.5. Effect of Tyrosol and Its Analogues on MC1R Protein Expression
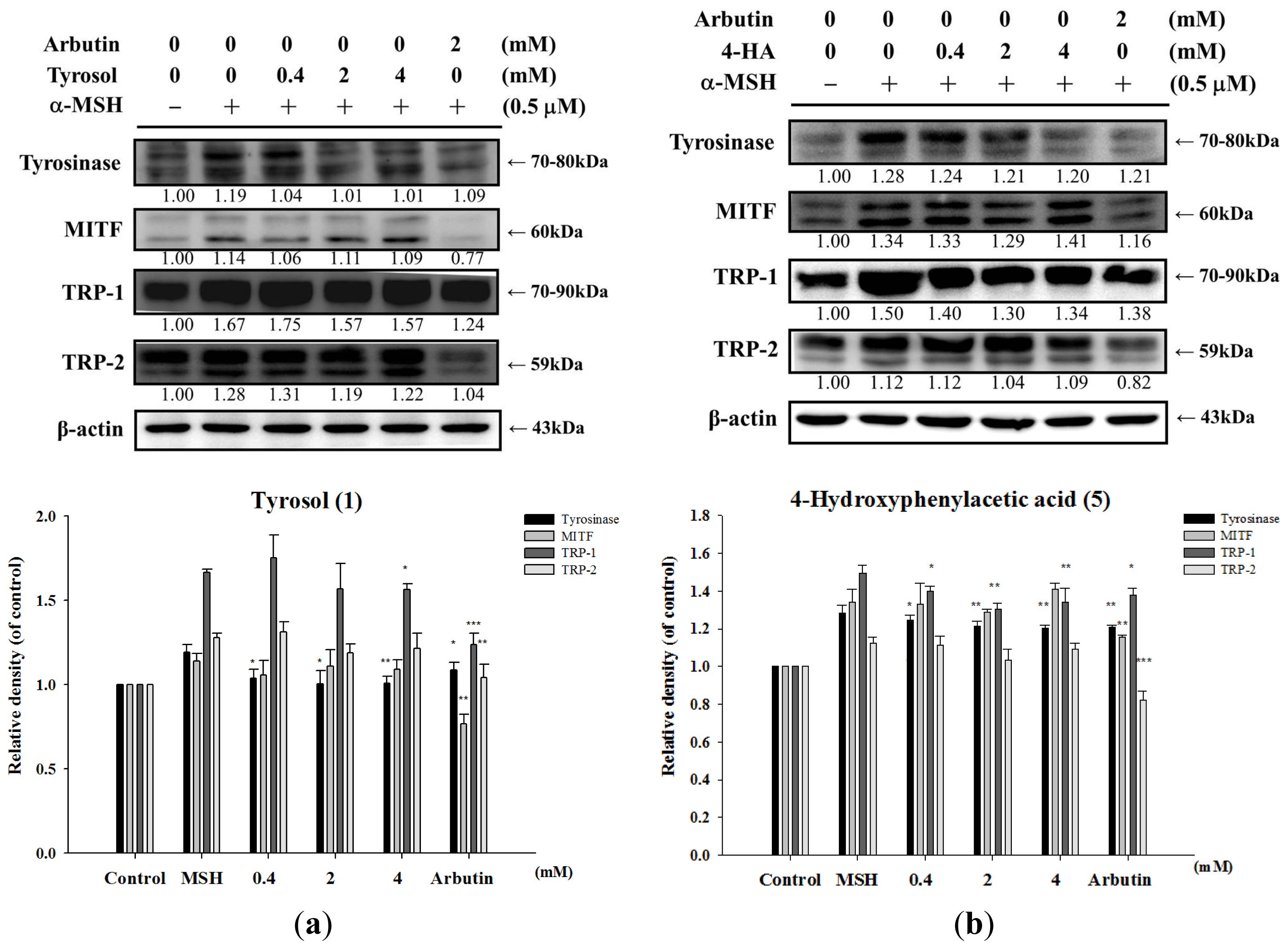
2.1.6. Effect of Tyrosol and Its Analogues on Melanogenesis Related Proteins
2.1.7. Inhibitory Effect of Tyrosol and Its Analogues on α-MSH Induced Melanin Synthesis in B16F0 Cells
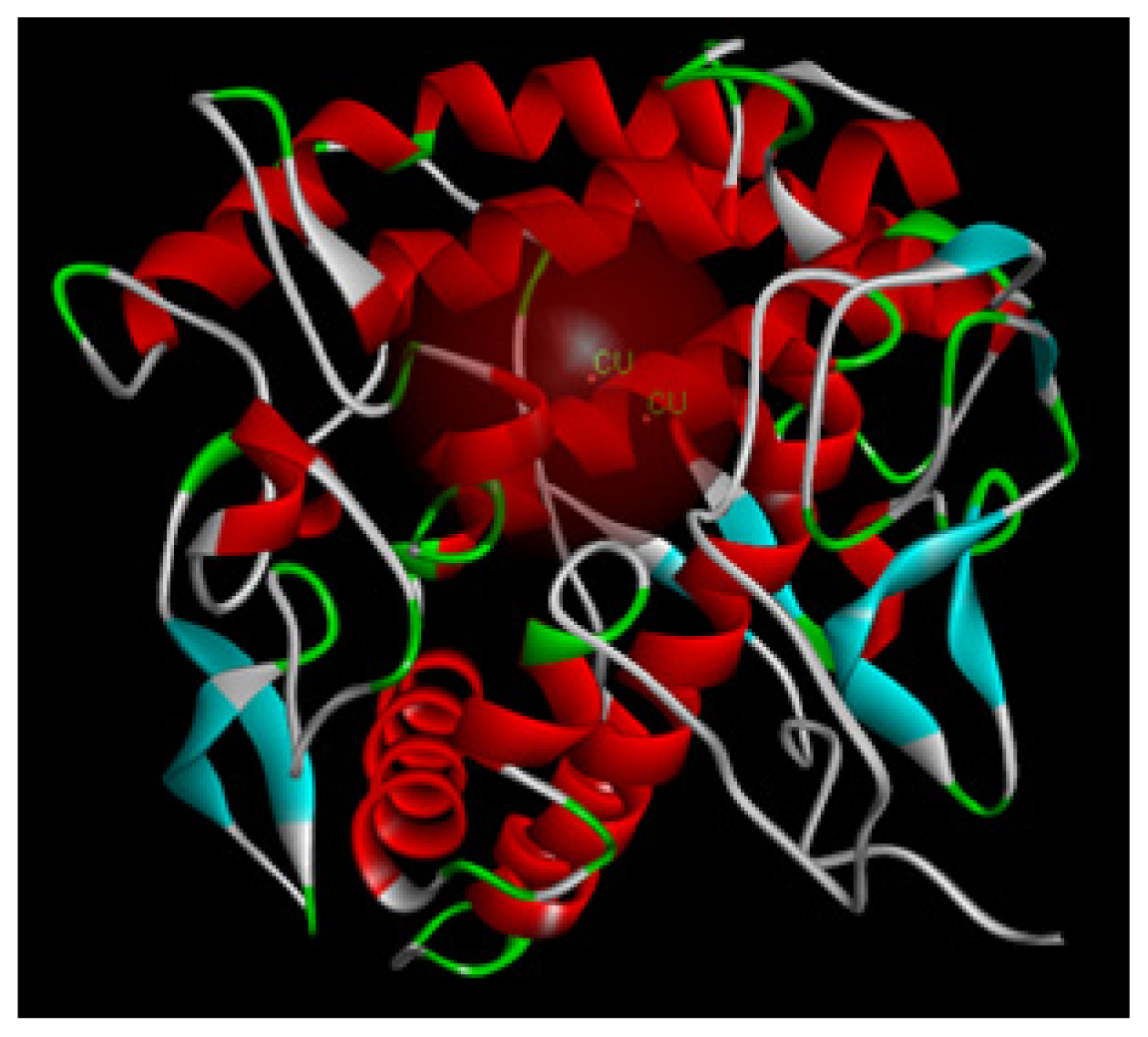
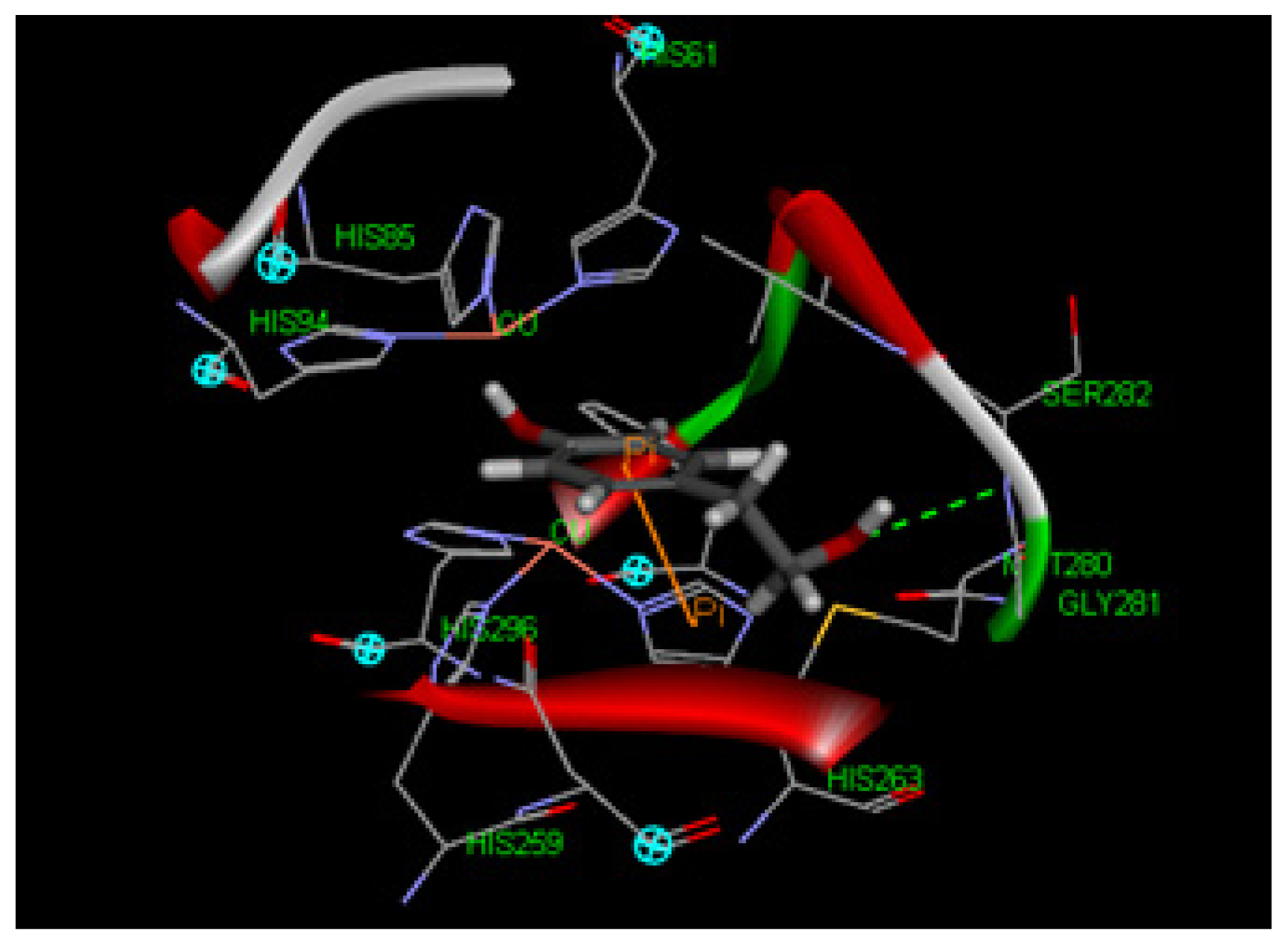
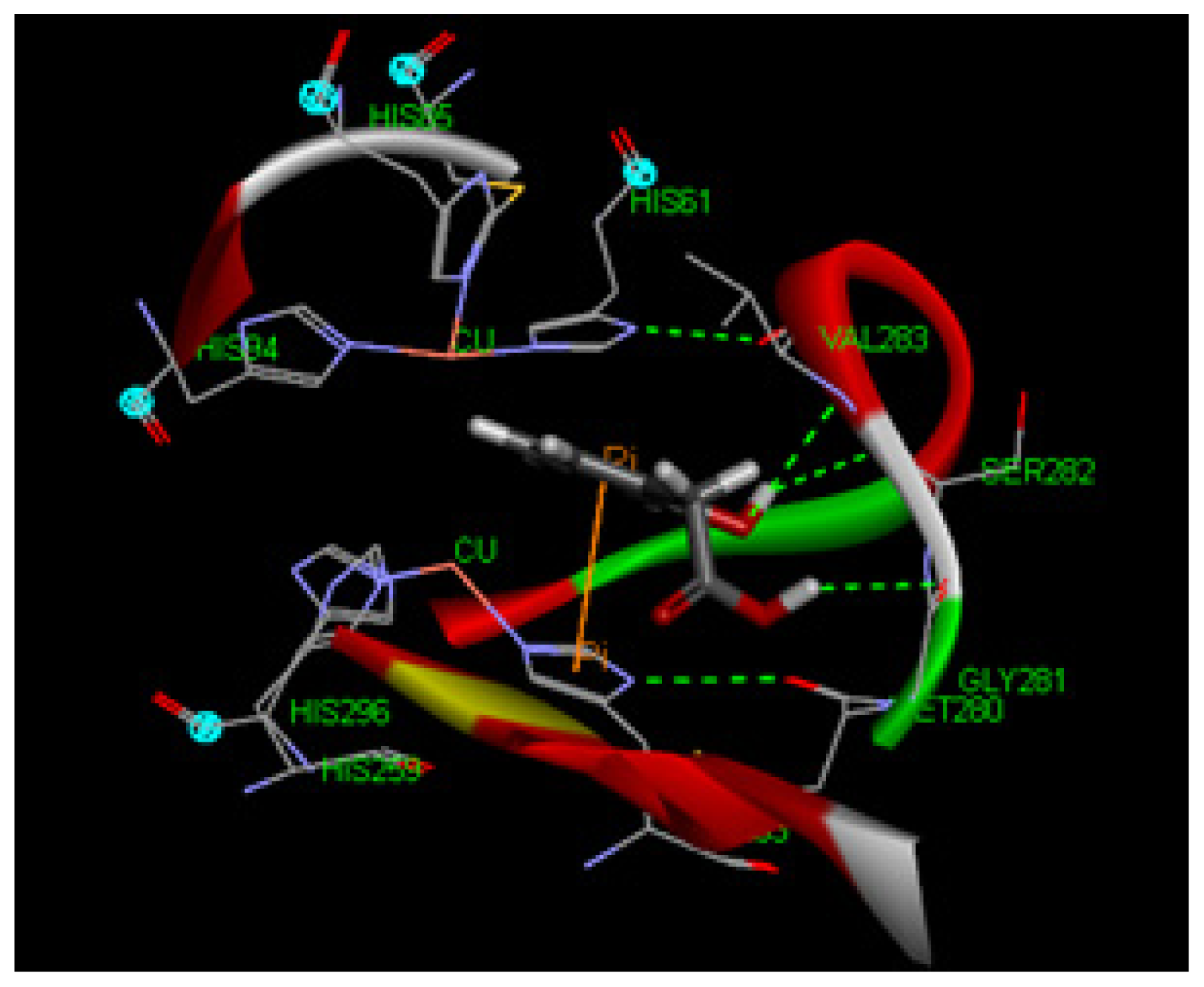
2.1.8. Molecular Docking Study
2.2. Discussion
3. Materials and Methods
3.1. Materials and Reagents
3.2. Inhibitory Effects of Tyrosol and Its Analogues on Mushroom Tyrosinase
3.3. Cell Cultures
3.4. Cell Viability Assay
3.5. Assay of Cellular Tyrosinase Activity
3.6. The Effect of Tyrosol Analogues on Cellular Melanin Content
3.7. Western Blot Analysis
3.8. Molecular Modeling Study
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Abbreviation
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| FBS | fetal bovine serum |
| MC1R | melanocortin 1 receptor |
| MITF | microphthalmia-associated transcription factor |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| TRP-1 | tyrosinase-related protein 1 |
| TRP-2 | tyrosinase-related protein 2 |
| α-MSH | α-melanocyte-stimulating hormone |
Conflicts of Interest
References
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res 2006, 19, 550–571. [Google Scholar]
- Cheung, F.W.; Guo, J.; Ling, Y.H.; Che, C.T.; Liu, W.K. Anti-melanogenic property of geoditin A in murine B16 melanoma cells. Mar. Drugs 2012, 10, 465–476. [Google Scholar]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci 2005, 62, 1707–1723. [Google Scholar]
- Kondo, T.; Hearing, V.J. Update on the regulation of melanocyte function and skin pigmentation. Expert Rev. Dermatol 2011, 6, 97–108. [Google Scholar]
- Chiang, H.M.; Chen, H.W.; Huang, Y.H.; Chan, S.Y.; Chen, C.C.; Wu, W.C.; Wen, K.C. Melanogenesis and Natural Hypopigmentation Agents. In Melanin: Biosynthesis, Functions and Health Effects; Ma, X., Sun, X., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 1–76. [Google Scholar]
- Busca, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res 2000, 13, 60–69. [Google Scholar]
- Kadekaro, A.L.; Kavanagh, R.J.; Wakamatsu, K.; Ito, S.; Pipitone, M.A.; Abdel-Malek, Z.A. Cutaneous photobiology. The melanocyte vs. the sun: Who will win the final round? Pigment Cell Res 2003, 16, 434–447. [Google Scholar]
- Ye, Y.; Chu, J.H.; Wang, H.; Xu, H.; Chou, G.X.; Leung, A.K.; Fong, W.F.; Yu, Z.L. Involvement of p38 MAPK signaling pathway in the anti-melanogenic effect of San-bai-tang, a Chinese herbal formula, in B16 cells. J. Ethnopharmacol 2010, 132, 533–535. [Google Scholar]
- Griffiths, C.E.; Finkel, L.J.; Ditre, C.M.; Hamilton, T.A.; Ellis, C.N.; Voorhees, J.J. Topical tretinoin (retinoic acid) improves melasma. A vehicle-controlled, clinical trial. Br. J. Dermatol 1993, 129, 415–421. [Google Scholar]
- Haddad, A.L.; Matos, L.F.; Brunstein, F.; Ferreira, L.M.; Silva, A.; Costa, D., Jr. A clinical, prospective, randomized, doubleblind trial comparing skin whitening complex with hydroquinone vs. placebo in the treatment of melasma. Int. J. Dermatol 2003, 42, 153–156. [Google Scholar]
- Draelos, Z.D. The combination of 2% 4-hydroxyanisole (mequinol) and 0.01% tretinoin effectively improves the appearance of solar lentigines in ethnic groups. J. Cosmet. Dermatol 2006, 5, 239–244. [Google Scholar]
- Kucinskaite, A.; Briedis, V.; Savickas, A. Experimental analysis of therapeutic properties of Rhodiola rosea L. and its possible application in medicine. Medicina 2004, 40, 614–619. [Google Scholar]
- Kanupriya, D.; Prasad Sai Ram, M.; Kumar, R.; Sawhney, R.C.; Sharma, S.K.; Ilavazhagan, G.; Kumar, D.; Banerjee, P.K. Cytoprotective and antioxidant activity of Rhodiola imbricata against tert-butyl hydroperoxide induced oxidative injury in U-937 human macrophages. Mol. Cell. Biochem 2005, 275, 1–6. [Google Scholar]
- Bolshakova, I.V.; Lozovskaia, E.L.; Sapezhinskii, I.I. Antioxidant properties of a series of extracts from medicinal plants. Biofizika 1997, 42, 480–483. [Google Scholar]
- Ma, J.B. The Biological Effect of Salidroside on Melanocytes and the Discussion about the Selection Processes of Whitening Chinese Herbal. Ph.D. Thesis, Fudan University, Shanghai, China, 2003. [Google Scholar]
- Van Sumere, C.F. Phenols and Phenolic Acids in Plant Biochemistry; Academic Press: New York, NY, USA, 1989; Volume 1, pp. 29–73. [Google Scholar]
- Kahn, V.; Ben-shalom, N.; Zakin, V. p-hydroxyphenylacetic acid and -3,4-dihydroxyphenylacetic acid as substrates for mushroom tyrosinase. J. Food Biochem 2000, 24, 1–19. [Google Scholar]
- Lee, J.Y.; Jang, Y.W.; Kang, H.S.; Moon, H.; Sim, S.S.; Kim, C.J. Anti-inflammatory action of phenolic compounds from Gastrodia elata root. Arch. Pharm. Res 2006, 29, 849–858. [Google Scholar]
- Nevado, J.J.; Peñalvo, G.C.; Robledo, V.R.; Martínez, G.V. New CE-ESI-MS analytical method for the separation, identification and quantification of seven phenolic acids including three isomer compounds in virgin olive oil. Talanta 2009, 79, 1238–1246. [Google Scholar]
- Kadekaro, A.L.; Kavanagh, R.; Kanto, H.; Terzieva, S.; Hauser, J.; Kobayashi, N.; Schwemberger, S.; Cornelius, J.; Babcock, G.; Shertzer, H.G.; et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res 2005, 65, 4292–4299. [Google Scholar]
- Iwasawa, A.; Ayaki, M.; Niwano, Y. Cell viability score (CVS) as a good indicator of critical concentration of benzalkonium chloride for toxicity in cultured ocular surface cell lines. Reg. Toxicol. Pharmacol 2013, 66, 177–183. [Google Scholar]
- Ancans, J.; Tobin, D.J.; Hoogduijn, M.J.; Smit, N.P.; Wakamatsu, K.; Thody, A.J. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp. Cell Res 2001, 268, 26–35. [Google Scholar]
- Fuller, B.B.; Spaulding, D.T.; Smith, D.R. Regulation of the catalytic activity of preexisting tyrosinase in black and Caucasian human melanocyte cell cultures. Exp. Cell Res 2001, 262, 197–208. [Google Scholar]
- Chakraborty, A.K.; Pawelek, J.M. Up-regulation of MSH receptors by MSH in Cloudman melanoma cells. Biochem. Biophys. Res. Commun 1992, 188, 1325–1331. [Google Scholar]
- Rouzaud, F.; Annereau, J.; Valencia, J.C.; Costin, G.; Hearing, V.J. Regulation of melanocortin 1 receptor expression at the mRNA and protein levels by its natural agonist and antagonist. FASEB J 2003, 17, 2154–2156. [Google Scholar]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Investig. Dermatol 2007, 127, 751–761. [Google Scholar]
- Gaggioli, C.; Busca, R.; Abbe, P.; Ortonne, J.P.; Ballotti, R. Microphthalmia-associated transcription factor (MITF) is required but is not sufficient to induce the expression of melanogenicgenes. Pigment Cell Res 2003, 16, 374–382. [Google Scholar]
- Khatib, S.; Nerya, O.; Musa, R.; Shmuel, M.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The importance of a 2,4-substituted resorcinol moiety. Bioorg. Med. Chem 2005, 13, 433–441. [Google Scholar]
- Yoshimura, M.; Watanabe, Y.; Kasai, K.; Yamakoshi, J.; Koga, T. Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci. Biotechnol. Biochem 2005, 69, 2368–2373. [Google Scholar]
- Maeda, K.; Naganuma, M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J. Photochem. Photobiol. B 1998, 47, 136–141. [Google Scholar]
- Kong, Y.H.; Jo, Y.O.; Cho, C.W.; Son, D.; Park, S.; Rho, J.; Choi, S.Y. Inhibitory effects of cinnamic acid on melanin biosynthesis in skin. Biol. Pharm. Bull 2008, 31, 946–948. [Google Scholar]
- An, S.M.; Lee, S.I.; Choi, S.W.; Moon, S.W.; Boo, Y.C. p-Coumaric acid, a constituent of Sasa quelpaertensis Nakai, inhibits cellular melanogenesis stimulated by a-melanocyte stimulating hormone. Br. J. Dermatol 2008, 159, 292–299. [Google Scholar]
- Kim, D.S.; Park, S.H.; Kwon, S.B.; Na, J.I.; Huh, C.H.; Park, K.C. Additive effects of heat and p38 MAPK inhibitor treatment on melanin synthesis. Arch. Pharm. Res 2007, 30, 581–586. [Google Scholar]
- Ito, S.; Suzuki, N.; Takebayashi, S.; Commo, S.; Wakamatsu, K. Neutral pH and copper ions promote eumelanogenesis after the dopachrome stage. Pigment Cell Melanoma Res 2013, 26, 817–825. [Google Scholar]
- Khemis, A.; Kaiafa, A.; Queille-Roussel, C.; Duteil, L.; Ortonne, J.P. Evaluation of efficacy and safety of rucinol serum in patients with melasma: A randomized controlled trial. Br. J. Dermatol 2007, 156, 997–1004. [Google Scholar]
- Shirasugi, I.; Kamada, M.; Matsui, T.; Sakakibara, Y.; Liu, M.C.; Suiko, M. Sulforaphane inhibited melanin synthesis by regulating tyrosinase gene expression in B16 mouse melanoma cells. Biosci. Biotechnol. Biochem 2010, 74, 579–582. [Google Scholar]
- Hirata, N.; Naruto, S.; Ohguchi, K.; Akao, Y.; Nozawa, Y.; Iinuma, M.; Matsuda, H. Mechanism of the melanogenesis stimulation activity of (−)-cubebin in murine B16 melanoma cells. Bioorg. Med. Chem 2007, 15, 4897–4902. [Google Scholar]
- Jimenez-Cervantes, C.; Solano, F.; Kobayashi, T.; Urabe, K.; Hearing, V.J.; Lozano, J.A.; Garcia-Borron, J.C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J. Biol. Chem 1994, 269, 17993–18000. [Google Scholar]
- Olivares, C.; Jimenez-Cervantes, C.; Lozano, J.A.; Solano, F.; Garcia-Borron, J.C. The 5,6-dihydroxyindole-2-carboxylic acid (DHICA) oxidase activity of human tyrosinase. Biochem. J 2001, 354, 131–139. [Google Scholar]
- Chiang, H.M.; Lin, J.W.; Hsiao, P.L.; Tsai, S.Y.; Wen, K.C. Hydrolysates of citrus plants stimulate melanogenesis protecting UV-induced dermal damage. Phytother. Res 2011, 25, 569–576. [Google Scholar]
- Chiang, H.M.; Lin, T.J.; Chiu, C.Y.; Chang, C.W.; Hsu, K.C.; Fan, P.C.; Wen, K.C. Coffea arabica extract and its constituents prevent photoaging by suppressing MMPs expression and MAP kinase pathway. Food Chem. Toxicol 2011, 49, 309–318. [Google Scholar]
- Chiang, H.M.; Chen, H.C.; Lin, T.J.; Shih, I.J.; Wen, K.C. Michelia alba extract attenuates UVB-induced expression of matrix metalloproteinases via MAP kinase pathway in human dermal fibroblast. Food Chem. Toxicol 2012, 50, 4250–4269. [Google Scholar]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar]







© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wen, K.-C.; Chang, C.-S.; Chien, Y.-C.; Wang, H.-W.; Wu, W.-C.; Wu, C.-S.; Chiang, H.-M. Tyrosol and Its Analogues Inhibit Alpha-Melanocyte-Stimulating Hormone Induced Melanogenesis. Int. J. Mol. Sci. 2013, 14, 23420-23440. https://doi.org/10.3390/ijms141223420
Wen K-C, Chang C-S, Chien Y-C, Wang H-W, Wu W-C, Wu C-S, Chiang H-M. Tyrosol and Its Analogues Inhibit Alpha-Melanocyte-Stimulating Hormone Induced Melanogenesis. International Journal of Molecular Sciences. 2013; 14(12):23420-23440. https://doi.org/10.3390/ijms141223420
Chicago/Turabian StyleWen, Kuo-Ching, Chih-Shiang Chang, Yin-Chih Chien, Hsiao-Wen Wang, Wan-Chen Wu, Chin-Sheng Wu, and Hsiu-Mei Chiang. 2013. "Tyrosol and Its Analogues Inhibit Alpha-Melanocyte-Stimulating Hormone Induced Melanogenesis" International Journal of Molecular Sciences 14, no. 12: 23420-23440. https://doi.org/10.3390/ijms141223420




