Anti-Inflammatory Effects of 4-Methylcyclopentadecanone on Edema Models in Mice
Abstract
:1. Introduction
2. Results
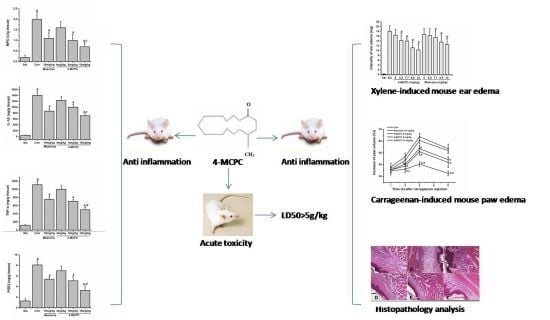
2.1. Acute Toxicity
2.2. Effects on Xylene-Induced Mouse Ear Edema
2.3. Effects on Carrageenan-Induced Mouse Paw Edema
2.4. Effects on Myeloperoxidase (MPO) Activity in Carrageenan-Induced Mouse Paws
2.5. Effects on IL-1β, TNF-α and PGE2 Levels in Carrageenan-Induced Mouse Paws
2.6. Histopathology Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Acute Oral Toxicity Study
4.4. Xylene-Induced Mouse Ear Edema
4.5. Carrageenan-Induced Mouse Paw Edema
4.6. Determination of the IL-1β, TNF-α, PGE2 and MPO Levels in Mouse Paw
4.7. Histopathologic Examination
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lawrence, T.; Willoughby, D.A.; Gilroy, D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol 2002, 2, 787–795. [Google Scholar]
- Kuncha, M.; Boyapati, S.; Vegi, G.M.N.; Sistla, R.; Banda, N.; Akkinepally, R.R.; Prakash, V.D. Anti-inflammatory potential of thienopyridines as possible alternative to NSAIDs. Eur. J. Pharmacol 2012, 678, 48–54. [Google Scholar]
- Yang, J.H.; Li, S.C.; Xie, C.F.; Ye, H.Y.; Tang, H.; Chen, L.J.; Peng, A.H. Anti-inflammatory activity of ethyl acetate fraction of the seeds of Brucea Javanica. J. Ethnopharmacol 2013, 147, 442–446. [Google Scholar]
- Posadas, I.; Terencio, M.C.; Guillen, I.; Ferrandiz, M.L.; Coloma, J.; Paya, M.; Alcaraz, M.J. Co-regulation between cyclo-oxygenase-2 and inducible nitric oxide synthase expression in the time-course of murine inflammation. Naunyn Schmiedeberg Arch. Pharmacol 2000, 361, 98–106. [Google Scholar]
- Zhang, C.X.; Dai, Z.R.; Cai, Q.X. Anti-inflammatory and anti-nociceptive activities of Sipunculus nudus L. extract. J. Ethnopharmacol 2011, 137, 1177–1182. [Google Scholar]
- Lin, D.L.; Chang, H.C.; Huang, S.H. Characterization of allegedly musk-containing medicinal products in Taiwan. J. Forensic Sci 2001, 49, 1187–1193. [Google Scholar]
- Morishita, S.; Mishima, Y.; Shoji, M. Pharmacological properties of musk. Gen. Pharmacol 1987, 18, 253–261. [Google Scholar]
- Luo, H.M.; Dai, R.H.; Wang, S.Y. Sdudy on heart-protecting musk pill in improving myocardial ischemia evaluated by nuclear myocardial imaging. Chin. J. Integr. Tradit. West. Med 1996, 16, 323–325. [Google Scholar]
- Liang, Q.Q.; Zhang, M.; Zhou, Q.; Shi, Q.; Wang, Y.J. Muscone protects vertebral end-plate degeneration by antiinflammatory property. Clin. Orthop. Relat. Res 2010, 468, 1600–1610. [Google Scholar]
- Song, J.J.; Zhou, A.M. 4-Methylcyclopentadecanone and Its Application. Patent Authorization Number: CN 200610068879.9 3 June 2009. [Google Scholar]
- Kassuya, C.A.; Cremoneze, A.; Barros, L.F.; Simas, A.S.; Lapa, F.R.; Mello-Silva, R.; Stefanenello, M.E.; Zampronio, A.R. Antipytetic and anti-inflammatory properties of the ethanolic extract, dichloromethane fraction and costunolide from Magnolia ovate (Magnoliaceae). J. Ethnopharmacol 2009, 124, 369–376. [Google Scholar]
- Dewanjee, S.; Dua, T.K.; Sahu, R. Potential anti-inflammatory effect of Leea macrophylla Roxb. leaves: A wild edible plant. Food. Chem. Toxicol 2013, 59, 514–520. [Google Scholar]
- Sowemimo, A.; Samuel, F.; Fageyinbo, M.S. Anti-inflammatory activity of Markhamia tomentosa (Benth.) K. Schum. Ex Engl. ethanolic leaf extract. J. Ethnopharmacol 2013, 149, 191–194. [Google Scholar]
- Kumawat, R.; Sharma, S.; Vasudeva, N.; Kumar, S. In vivo anti-inflamatory potential of various extracts of Sida tiagii Bhandari. Asian. Pac. J. Trop. Biomed 2012, 2, S947–S952. [Google Scholar]
- Yonathan, M.; Assefa, A.; Bucar, F. In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinose. J. Ethnopharmacol 2006, 108, 462–470. [Google Scholar]
- Sadeghi, H.; Hajhashemi, V.; Minaiyan, M.; Movahedian, A.; Talebi, A. Further studies on anti-inflammatory activity of maprotiline in carrageenan-induced paw edema in rat. Int. Immunopharmacol 2013, 15, 505–510. [Google Scholar]
- Liao, J.C.; Tsai, J.C.; Peng, W.H.; Chiu, Y.J.; Sung, P.J.; Tsuzoki, M.; Kuo, Y.H. Anti-inflammatory activity of N-(3-florophenyl)ethylcaffeamide in mice. Int. J. Mol. Sci 2013, 14, 15199–15211. [Google Scholar]
- Li, Y.C.; Xian, Y.F.; Ip, S.P.; Su, Z.R.; Su, J.Y.; He, J.J.; Xie, Q.F.; Lai, X.P.; Lin, Z.X. Anti-inflammatory activity of patchouli alcohol isolated from Pogostemonis Herba in animal models. Fitoterapia 2011, 82, 1295–1301. [Google Scholar]
- Santos, J.A.; Arruda, A.; Silva, M.A.; Cardoso, C.A.; Vieira Mdo, C.; Kassuya, C.A.; Arena, A.C. Anti-inflammatory effects and acute toxicity of hydroethanolic extract of Jacaranda decurrens roots in adult male rats. J. Ethnopharmacol 2012, 144, 802–805. [Google Scholar]
- Di Rosa, M.; Giroud, J.P.; Willoughby, D.A. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J. Pathol 1971, 104, 15–29. [Google Scholar]
- Li, W.F.; Huang, H.M.; Zhang, Y.M.; Fan, T.; Liu, X.; Xing, W.; Niu, X.F. Anti-inflammatory effect of tetrahydrocoptisine from Corydalis impatiens is a function of possible inhibition of TNF-α, IL-6 and NO production in lipopolysaccharide-stimulated peritoneal macrophages through inhibiting NF-κB activation and MAPK pathway. Eur. J. Pharmacol 2013, 715, 62–71. [Google Scholar]
- Andonegui, G.; Bonder, C.S.; Green, F.; Mullaly, S.C.; Zbytnuik, L.; Raharjo, E.; Kubes, P. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J. Clin. Investig 2003, 111, 1011–1020. [Google Scholar]
- Davis, R.J. MAPKs: New JNK expands the group. Trends Biochem. Sci 1994, 19, 470–473. [Google Scholar]
- Xie, C.; Kang, J.; Li, Z.; Schauss, A.G.; Badger, T.M.; Nagarajan, S.; Wu, T.; Wu, X. The acai flavonoid velutin is a potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J. Nutr. Biochem 2012, 23, 1184–1191. [Google Scholar]
- Bhat, N.R.; Zhang, P.; Lee, J.C.; Hogan, E.L. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J. Neurosci 1998, 18, 1633–1641. [Google Scholar]
- Park, Y.G.; Kang, S.K.; Kim, W.J.; Lee, Y.C.; Kim, C.H. Effects of TGF-β, TNF-α, IL-β and IL-6 alone or in combination, and tyrosine kinase inhibitor on cyclooxygenase expression, prostaglandin E2 production and bone resorption in mouse calvarial bone cells. Int. J. Biochem 2004, 36, 2270–2280. [Google Scholar]
- Swiergiel, A.H.; Dunn, A.J. Distinct roles for cyclooxygenases 1 and 2 in interleukin-1-induced behavioral changes. J. Pharmacol. Exp. Ther 2002, 302, 1031–1036. [Google Scholar]
- Hosseinzadeh, A.; Khoshdet, M.; Ghorbani, M. Antinociceptive, anti-inflammatory effects and acute toxicity of aqueous and ethanolic extracts of Myrtus communis L. Aerial Parts in mice. J. Acupunct. Meridian Stud 2011, 4, 242–247. [Google Scholar]
- Wallace, J.L. Nonsteroidal anti-inflammatroy drugs and gastroenteropathy: The second hundred years. Gastroenterology 1997, 112, 1000–1016. [Google Scholar]
- Wallace, J.L. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract. Res. Clin. Endocrinol. Metab 2001, 15, 691–703. [Google Scholar]
- Berenguer, B.; Sanchez, L.M.; Quilez, A.; Lopez-Barreiro, M.; de Haro, M.O.; Galvez, J.; Martin, M.J. Protective and antioxidant effects of Rhizophora mangle L. against NSAID-induced gastric ulcers. J. Ethnopharmacol 2006, 103, 194–200. [Google Scholar]
- Sairam, K.; Rao, C.V.; Babu, M.D.; Goel, R.K. Prophylactic and curative effects of Bacopa monniera in gastric ulcer models. Phytomedicine 2001, 8, 423–430. [Google Scholar]
- Tan, P.V.; Penlap, V.B.; Nyasse, B.; Joseph, D.B.; Nguemo, J.D.B. Anti-ulcer actions of the bark methanol extract of Voacanga africana in different experimental ulcer models in rats. J. Ethnopharmacol 2000, 73, 423–428. [Google Scholar]
- Backhouse, N.; Delporte, C.; Bakhouse, N.; Erazo, S.; Negrete, R.; Vildal, P.; Silva, X.; Lopez-Perez, J.L.; San Feliciano, A.; Munoz, O. Analgesic-antiinflammatory properties of Proustia pyrifolia. J. Ethnopharmacol 2005, 99, 119–124. [Google Scholar]





© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ma, Y.; Li, Y.; Li, X.; Wu, Y. Anti-Inflammatory Effects of 4-Methylcyclopentadecanone on Edema Models in Mice. Int. J. Mol. Sci. 2013, 14, 23980-23992. https://doi.org/10.3390/ijms141223980
Ma Y, Li Y, Li X, Wu Y. Anti-Inflammatory Effects of 4-Methylcyclopentadecanone on Edema Models in Mice. International Journal of Molecular Sciences. 2013; 14(12):23980-23992. https://doi.org/10.3390/ijms141223980
Chicago/Turabian StyleMa, Yukui, Yue Li, Xiufeng Li, and Yingliang Wu. 2013. "Anti-Inflammatory Effects of 4-Methylcyclopentadecanone on Edema Models in Mice" International Journal of Molecular Sciences 14, no. 12: 23980-23992. https://doi.org/10.3390/ijms141223980