Thermotropic and Barotropic Phase Behavior of Phosphatidylcholine Bilayers
Abstract
:1. Introduction
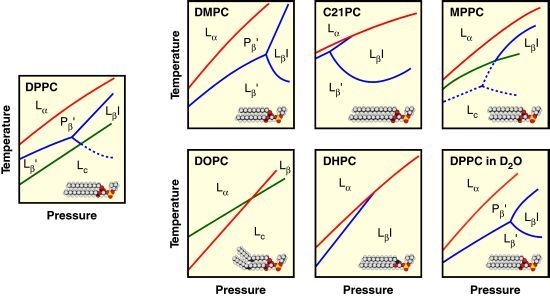
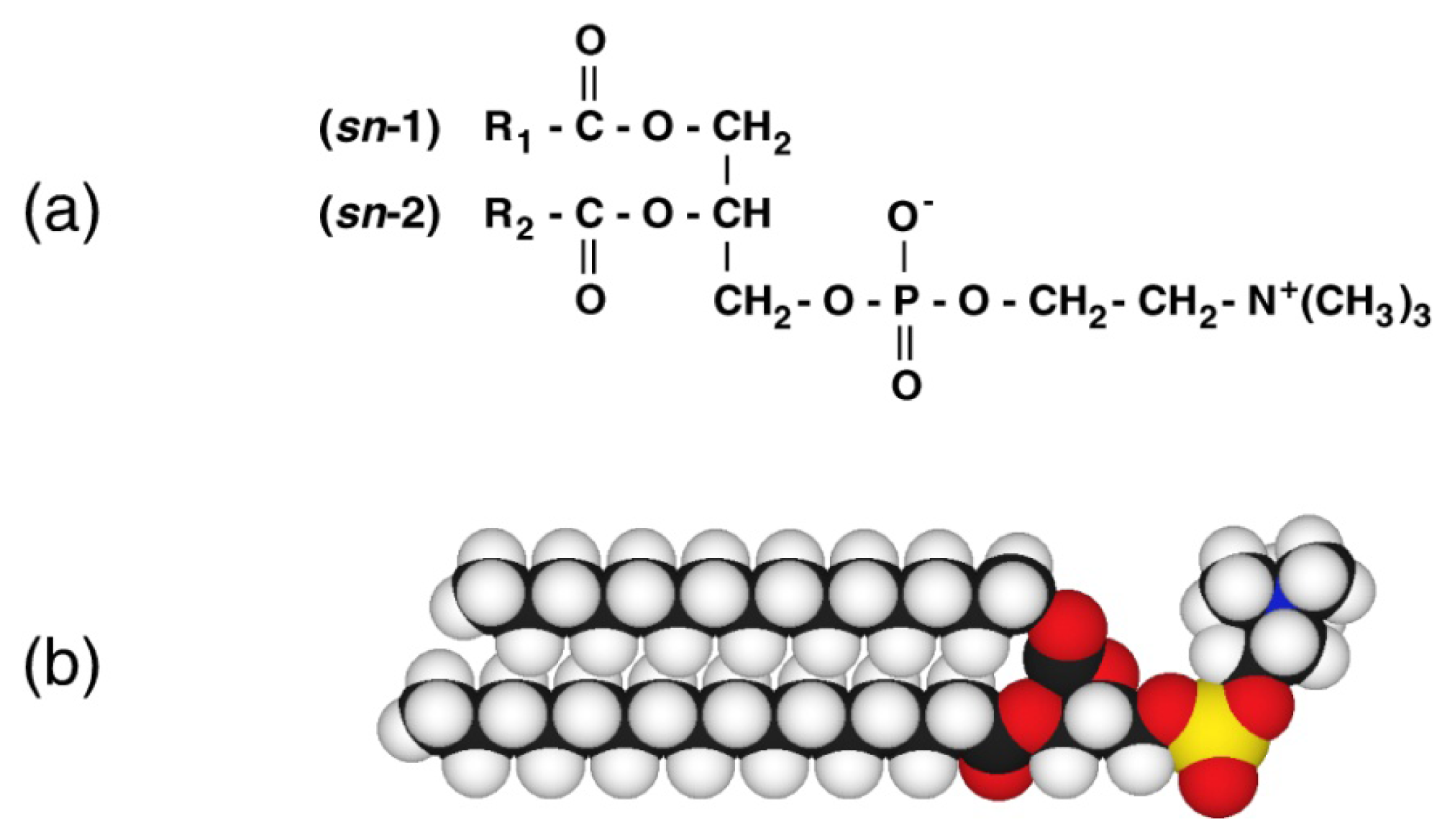
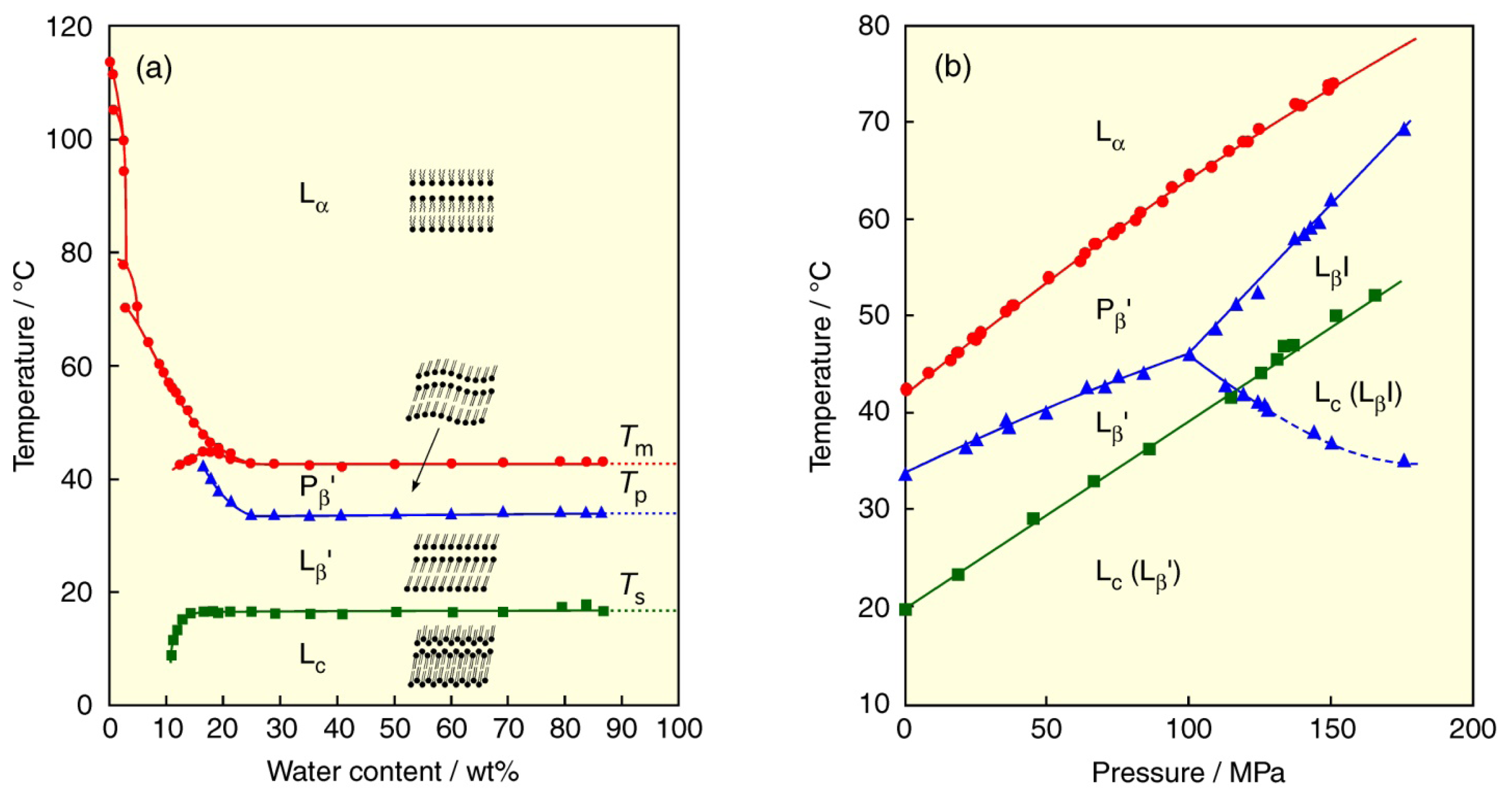
2. Lyotropic, Thermotropic and Barotropic Phase Transitions of DPPC Bilayer
3. Effect of Acyl Chain Structure on the Phase Behavior of PC Bilayer Membranes
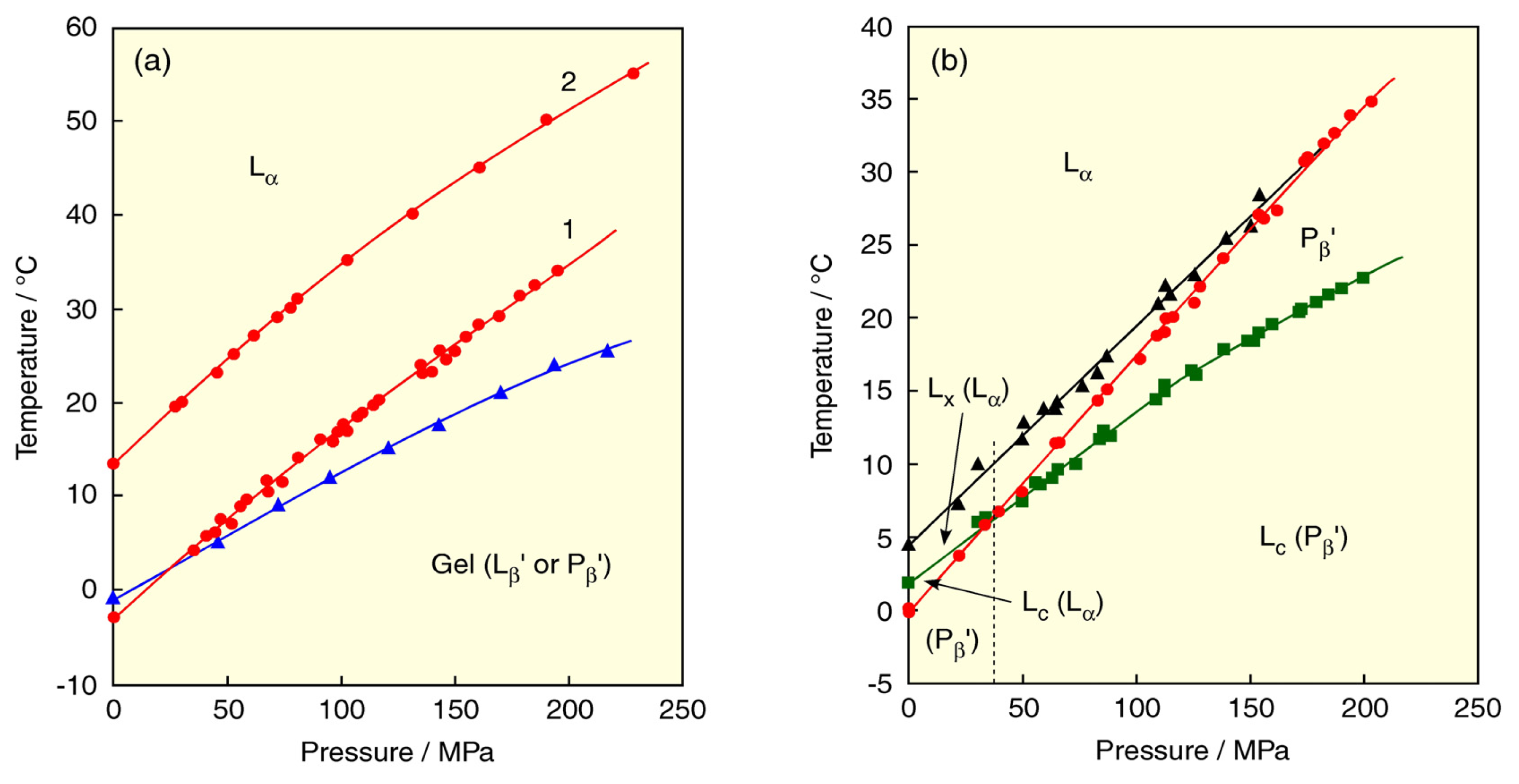
3.1. Length of Acyl Chain
3.2. Asymmetry of Saturated Acyl Chain
3.3. Unsaturated Acyl Chain
3.4. Asymmetry of Unsaturated Mixed Acyl Chains
4. Effect of Linkage of Glycerol Backbone on the Phase Behavior of PC Bilayer Membranes
5. Effect of Solvent on the Phase Behavior of DPPC Bilayer Membrane
6. Conclusions
Acknowledgments
References
- Yeagle, P.L. The Structure of Biological Membranes, 2nd ed; CRC Press: New York, NY, USA, 2005. [Google Scholar]
- Dopico, A.M. Methods in Membrane Lipids (Methods in Molecular Biology 400); Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Heimberg, T. Thermal Biophysics of Membranes; Wiley-VCH: Weiheim, Germany, 2007. [Google Scholar]
- Caffrey, M. LIPIDAT, A Database of Thermodynamic Data and Associated Information on Lipid Mesomorphic and Polymorphic Transitions; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Wilkinson, D.A.; Nagle, J.F. Thermodynamics of Lipid Bilayers. In Liposomes: from Physical Structure to Therapeutic Applications; Kninght, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1981; pp. 273–297. [Google Scholar]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta 1998, 1376, 91–145. [Google Scholar]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta 2000, 1469, 159–195. [Google Scholar]
- Trudell, J.R.; Payan, D.G.; Chin, J.H.; Cohen, E.N. Pressure-induced elevation of phase transition temperature in dipalmitoylphosphatidylcholine bilayers. An electron spin resonance measurement of the enthalpy of phase transiton. Biochim. Biophys. Acta 1974, 373, 436–443. [Google Scholar]
- Srinivasan, K.R.; Kay, R.L.; Nagle, J.F. The pressure dependence of the lipid bilayer phase transition. Biochemistry 1974, 13, 3494–3496. [Google Scholar]
- Chong, P.L.G.; Weber, G. Pressure dependence of 1,6-diphenyl-1,3,5-hexatrien fluorescence in single-component phosphatidylcholine liposomes. Biochemistry 1983, 22, 5544–5550. [Google Scholar]
- Wong, P.T.T.; Mantsch, H.H. Effects of hydrostatic pressure on the molecular structure and endothermic phase transitions of phosphatidylcholine bilayers: A Raman scattering study. Biochemistry 1985, 24, 4091–4096. [Google Scholar]
- Bragnza, L.F.; Worcester, D.L. Hydrostatic pressure induces hydrocarbon chain interdigitation in single component phospholipid bilayers. Biochemistry 1986, 25, 2591–2596. [Google Scholar]
- Prasad, S.K.; Shashidhar, R.; Gaber, B.P.; Chandrasekhar, S.C. Pressure studies on two hydrated phospholipids—1,2-dimristoyl-phosphatidylcholine and 1,2-dipalmitoyl-phosphatidylcholine. Chem. Phys. Lipids 1987, 43, 227–235. [Google Scholar]
- Winter, R.; Pilgrim, W.C. A SANS study of high pressure phase transitions in model biomembranes. Ber. Bunsenges. Phys. Chem 1989, 93, 708–717. [Google Scholar]
- Driscoll, D.A.; Samarasinghe, J.; Adamy, S.; Jonas, J.; Jonas, A. Pressure effects on dipalmitoylphosphatidylcholine bilayers measured by 2H nuclear magnetic resonance. Biochemistry 1991, 30, 3322–3327. [Google Scholar]
- Hauser, H.; Pascher, I.; Pearson, R.H.; Sundell, S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim. Biophys. Acta 1981, 650, 21–51. [Google Scholar]
- Kodama, M.; Kato, H.; Aoki, H. Comparison of differently bound molecules in the gel and subgel phases of a phospholipids bilyaer system. J. Therm. Anal. Cal 2001, 64, 219–230. [Google Scholar]
- Kodama, M.; Kuwabara, M.; Seki, S. Successive phase-transition phenomena and phase diagram of the phosphatidylcholine-water system as revealed by differential scanning calorimetry. Biochim. Biophys. Acta 1982, 689, 567–570. [Google Scholar]
- Kodama, M. Phase transition phenomena induced by the successive appearances of new types of aggregation states of water molecules in the “l-dipalmitoylphosphatidylcholine-water” system. Thermchim. Acta 1986, 109, 81–89. [Google Scholar]
- Kusube, M.; Matsuki, H.; Kaneshina, S. Thermotropic and barotropic phase transitions of N-methylated dipalmitoylphosphatidylethanolamine bilayers. Biochim. Biophys. Acta 2005, 1668, 25–32. [Google Scholar]
- Ichimori, H.; Hata, T.; Matsuki, H.; Kaneshina, S. Baroropic phase transitions and pressure-induced interdigitation on bilayer membranes of phospholipids with varying acyl chain-lengths. Biochim. Biophys. Acta 1998, 1414, 165–174. [Google Scholar]
- Ichimori, H.; Hata, T.; Yoshioka, T.; Matsuki, H.; Kaneshina, S. Thermotropic and barotropic phase transition on bilayer membranes of phospholipids with varying acyl chain-lengths. Chem. Phys. Lipids 1997, 89, 97–105. [Google Scholar]
- Nagle, J.F.; Wilkinson, D.A. Density measurements and molecular interactions. Biophys. J 1978, 23, 159–175. [Google Scholar]
- Blume, A. Apparent molar heat capacities of phospholipids in aqueous dispersion. Effects of chain length and head group structure. Biochemistry 1983, 22, 5436–5442. [Google Scholar]
- Stümpel, J.; Eibl, H.; Nichsch, A. X-ray analysis and calorimetry on phosphatidylcholine model membranes. The influence of length and position of acyl chains upon structure and phase behaviour. Biochim. Biophys. Acta 1983, 727, 246–254. [Google Scholar]
- Lewis, R.N.A.H.; Mak, N.; McElhaney, R.N. A differential scanning calorimetric study of the thermotropic phase behavior of model membranes composed of phosphatidylcholines containing linear saturated fatty acyl chains. Biochemistry 1987, 26, 6118–6126. [Google Scholar]
- Goto, M.; Wilk, A.; Kazama, A.; Chodankar, S.; Kohlbrecher, J.; Matsuki, H. Chain elongation of diacylphosphatidylcholine induces fully bilayer interdigitation under atmospheric pressure. Colloids Surf. B 2011, 84, 44–48. [Google Scholar]
- Finegold, L.; Shaw, W.A.; Singer, M.A. Unusual phase properties of dilauryl phosphatidylcholine (C12PC). Chem. Phys. Lipids 1990, 53, 177–184. [Google Scholar]
- Hatta, I.; Matsuoka, S.; Singer, M.A.; Finegold, L. A new liquid crystalline phase in phosphatidylcholine bilayers as studied by X-ray diffraction. Chem. Phys. Lipids 1994, 69, 129–136. [Google Scholar]
- Tada, K.; Goto, M.; Tamai, N.; Matsuki, H.; Kaneshina, S. Thermotropic and barotropic phase transitions of dilauroylphosphatidylcholine bilayer. Chem. Phys. Lipids 2008, 153, 138–143. [Google Scholar]
- Huang, C.; Mason, J.T. Structure and properties of mixed-chain phospholipid assemblies. Biochim. Biophys. Acta 1986, 864, 423–470. [Google Scholar]
- Bultmann, T.; Lin, H.; Wang, Z.; Huang, C. Thermotropic and mixing behavior of mixed-chain phosphatidylcholines with molecular weights identical with that of l-α-dipalmitoylphosphatidylcholine. Biochemistry 1991, 30, 7194–7202. [Google Scholar]
- Matsuki, H.; Goto, M.; Kusube, M.; Tamai, N.; Kaneshina, S. Barotropic phase transitions of 1-palmitoyl-2-stearoylphosphatidylcholine bilayer membrane. Chem. Lett 2005, 34, 270–271. [Google Scholar]
- Goto, M.; Kusube, M.; Tamai, N.; Matsuki, H.; Kaneshina, S. Effect of hydrostatic pressure on the bilayer phase behavior of symmetric and asymmetric phospholipids with the same total chain length. Biochim. Biophys. Acta 2008, 1778, 1067–1078. [Google Scholar]
- Goto, M.; Ishida, S.; Tamai, N.; Matsuki, H.; Kaneshina, S. Chain asymmetry alters thermotropic and barotropic properties of phospholipid bilayer membranes. Chem. Phys. Lipids 2009, 161, 65–76. [Google Scholar]
- Stümpel, J.; Nichsch, A.; Eibl, H. Calorimetric studies on saturated mixed-chain lecithin-water systems. Nonequivalence of acyl chains in the thermotropic phase transition. Biochemistry 1981, 20, 662–665. [Google Scholar]
- Lin, H.; Wang, Z.; Huang, C. The influence of acyl chain-length asymmetry on the phase transition parameters of phosphatidylcholine dispersions. Biochim. Biophys. Acta 1991, 1067, 17–28. [Google Scholar]
- Goto, M.; Wilk, A.; Kataoka, K.; Chodankar, S.; Tamai, N.; Fukui, M.; Kohlbrecher, J.; Ito, H.; Matsuki, H. Study on the subgel-phase formation using an asymmetric phospholipidb membrane by high-pressure fluorometry. Langmuir 2012, 28, 12191–12198. [Google Scholar]
- Goto, M.; Matsui, T.; Tamai, N.; Matsuki, H.; Kaneshina, S. Prodan fluorescence detects the bilayer packing of asymmetric phospholipids. Colloids Surf. B 2011, 84, 55–62. [Google Scholar]
- Kaneshina, S.; Ichimori, H.; Hata, T.; Matsuki, H. Barotropic phase transitions of dioleoylphosphatidylcholine and stearoyl-oleoylphosphatidylcholine bilayer membranes. Biochim. Biophys. Acta 1998, 1374, 1–8. [Google Scholar]
- Ichimori, H.; Hata, T.; Matsuki, H.; Kaneshina, S. Effect of unsaturated acyl chains on the thermotropic and barotropic phase transitions of phospholipid bilayer membranes. Chem. Phys. Lipids 1999, 100, 151–154. [Google Scholar]
- Kusube, M.; Goto, M.; Tamai, N.; Matsuki, H.; Kaneshina, S. Bilayer phase transitions of N-methylated dioleoylphosphatidylethanolamines under high pressure. Chem. Phys. Lipids 2006, 142, 94–102. [Google Scholar]
- Lewis, R.N.A.H.; McElhaney, R.N. The Mesomorphic Phase Behavior of Lipid Bilayers. In The Structure of Biological Membranes, 2nd ed; Yeagle, PL, Ed.; CRC Press: New York, NY, USA, 2005; pp. 66–69. [Google Scholar]
- Silvius, J.R.; McElhaney, R.N. Effects of phospholipid acyl chain structure on thermotropic phase properties. 2: Phosphatidylcholines with unsaturated or cyclopropane acyl chains. Chem. Phys. Lipids 1979, 25, 125–134. [Google Scholar]
- Seelig, J.; Waespe-Sarcevic, N. Molecular order in cis and trans unsaturated phospholipid bilayers. Biochemistry 1978, 17, 3310–3315. [Google Scholar]
- Siminovitch, D.J.; Wong, P.T.T.; Mantsch, H.H. Effects of cis and trans unsaturation on the structure of phospholipid bilayer: A high-pressure infrared spectroscopic study. Biochemistry 1987, 26, 3277–3287. [Google Scholar]
- Lewis, R.N.A.H.; Sykes, B.D.; McElhaney, R.N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing cis-monounsaturated acyl chain homologues of oleic acid: Differential scanning calorimetric and 31P NMR spectroscopic studies. Biochemistry 1998, 27, 880–887. [Google Scholar]
- Davis, P.J.; Fleming, B.D.; Coolbear, K.P.; Keough, K.M.W. Gel to liquid crystalline transition temperatures of water dispersions of two pairs of positional isomers of unsaturated mixed-acid phosphatidylcholines. Biochemistry 1981, 20, 3633–3636. [Google Scholar]
- Coolbear, K.P.; Berde, C.B.; Keough, K.M. Gel to liquid-crystalline phase transitions of aqueous dispersions of polyunsaturated mixed-acid phosphatidylcholines. Biochemistry 1983, 22, 1466–1473. [Google Scholar]
- Keough, K.M.W.; Griffin, B.; Kariel, N. The influence of unsaturation on the phase transition temperatures of a series of heteroacid phosphatidylcholines containing twenty-carbon chains. Biochim. Biophys. Acta 1987, 902, 1–10. [Google Scholar]
- Tada, K.; Saito, K.; Goto, M.; Tamai, N.; Matsuki, H.; Kaneshina, S. High-pressure study on bilayer phase behavior of oleoylmyristoyl- and myristoyloleoyl-phosphatidylcholines. Biophys. Chem 2008, 138, 36–41. [Google Scholar]
- Tada, K.; Miyazaki, E.; Goto, M.; Tamai, N.; Matsuki, H.; Kaneshina, S. Barotropic and thermotropic bilayer phase behavior of positional isomers of unsaturated mixed-chain phosphatidylcholines. Biochim. Biophys. Acta 2009, 1788, 1056–1063. [Google Scholar]
- Tada, K.; Goto, M.; Tamai, N.; Matsuki, H.; Kaneshina, S. Pressure effect on the bilayer phase transition of asymmetric lipids with an unsaturated acyl chain. Ann. N.Y. Acad. Sci 2010, 1189, 77–85. [Google Scholar]
- Holte, L.L.; Separovic, F.; Gawrisch, K. Nuclear magnetic resonance investigation of hydrocarbon chain packing in bilayers of polyunsaturated phospholipids. Lipids 1996, 31, 199–203. [Google Scholar]
- Koenig, B.W.; Strey, H.H.; Gawrisch, K. Membrane lateral compressibility determined by NMR and X-ray diffraction: Effect of acyl chain polyunsaturation. Biophys. J 1997, 73, 1954–1966. [Google Scholar]
- Applegate, K.R.; Glomset, T.A. Computer-based modeling of the conformation and packing properties of docosahexaenoic acid. J. Lipid Res 1986, 27, 658–680. [Google Scholar]
- Paltauf, F. Ether lipids in biomembranes. Chem. Phys. Lipids 1994, 74, 101–139. [Google Scholar]
- Han, X.; Gross, R.W. Plasmenylcholine and phosphatidylcholine membrane bilayers possess distinct conformational motifs. Biochemistry 1990, 29, 4992–4996. [Google Scholar]
- Lohner, K. Is the high propensity of ethanolamine plasmalogens to form non-lamellar lipid structures manifested in the properties of biomembranes? Chem. Phys. Lipids 1996, 81, 167–184. [Google Scholar]
- Broniec, A.; Goto, M.; Matsuki, H. A peculiar phase transition of plasmalogen bilayer membrane under high pressure. Langmuir 2009, 25, 11265–11268. [Google Scholar]
- Chong, P.L.G. Archaebacterial bipolar tetraether lipids; physico-chemical and membrane properties. Chem. Phys. Lipids 2010, 163, 253–265. [Google Scholar]
- Ruocco, M.J.; Siminovitch, D.J.; Griffin, R.G. Comparative study of the gel phases of ether-and ester-linked phosphatidylcholines. Biochemistry 1985, 24, 2406–2411. [Google Scholar]
- Laggner, P.; Lohner, K.; Degovics, G.; Müller, K.; Schuster, A. Structure and thermodynamics of the dihexadecylphosphatidylcholine-water system. Chem. Phys. Lipids 1987, 44, 31–60. [Google Scholar]
- Kim, J.T.; Mattai, J.; Shipley, G.G. Gel phase polymorphism in ether-linked dihexadecylphosphatidylcholine bilayers. Biochemstry 1987, 26, 6592–6598. [Google Scholar]
- Maruyama, S.; Matsuki, H.; Ichimori, I.; Kaneshina, S. Thermotropic and barotropic phase behavior of dihexadecylphosphatidylcholine bilayer membrane. Chem. Phys. Lipids 1996, 82, 125–132. [Google Scholar]
- Matsuki, H.; Miyazaki, E.; Sakano, F.; Tamai, N; Kaneshina, S. Thermotropic and barotropic phase transitions in bilayer membranes of ether-linked phospholipids with varying alkyl chain lengths. Biochim. Biophys. Acta 2007, 1768, 479–489. [Google Scholar]
- Usui, K.; Hiraki, T.; Kawamoto, J.; Kurihara, T.; Nogi, Y.; Abe, F. Eicosapentaenoic acid plays a role in stabilizing dynamic membrane structure in the deep-sea piezophile Shewanella violacea: A study employing high-pressure time-resolved fluorescence anisotropy measurement. Biochim. Biophys. Acta 2012, 1818, 574–583. [Google Scholar]
- Rowe, E. Lipid chain length and temperature dependence of ethanol-phosphatidylcholine interactions. Biochemistry 1983, 22, 3329–3305. [Google Scholar]
- Adachi, T.; Takahashi, H.; Ohki, K.; Hatta, I. Interdigitated structure of phospholipid-alcohol systems studied by X-ray diffraction. Biophys. J 1995, 68, 1850–1855. [Google Scholar]
- Kinoshita, K.; Yamazaki, M. Organic solvents induce interdigitated gel structures in multilamellar vesicles of dipalmitoylphosphatidylcholine. Biochim. Biophys. Acta 1996, 1284, 233–239. [Google Scholar]
- Li, S.J.; Kinoshita, K.; Furuike, S.; Yamazaki, M. Effects of solvents interacting favorably with hydrophilic segments of the membrane surface of phosphatidylcholine on their gel-phase membranes in water. Biophys. Chem 1999, 81, 191–196. [Google Scholar]
- Matsuki, H.; Okuno, H.; Sakano, F.; Kusube, M.; Kaneshina, S. Effect of deuterium oxide on the thermodynamic quantities associated with phase transitions of lipid bilayer membranes. Biochim. Biophys. Acta 2005, 1712, 92–100. [Google Scholar]
- Kaneshina, S.; Tamura, K.; Kawakami, H.; Matsuki, H. Effects of pressure and ethanol on the phase behavior of dipalmitoylphosphatidylcholine multilamellar vesicles. Chem. Lett 1992, 21, 1963–1966. [Google Scholar]
- Nemethy, G.; Scheraga, H.A. Structure of water and hydrogen bonding in proteins. I. A model for the thermodynamic properties of liquid water. J. Chem. Phys 1962, 36, 3382–3400. [Google Scholar]
- Nemethy, G.; Scheraga, H.A. Structure of water and hydrogen bonding in proteins. IV. The thermodynamic properties of liquid deuterium oxide. J. Chem. Phys 1964, 41, 680–689. [Google Scholar]
- Ruocco, M.J.; Shipley, G.G. Characterization of the sub-transition of hydrated dipalmitoylphosphatidylcholine bilayers: Kinetics, hydration, and structural study. Biochim. Biophys. Acta 1982, 691, 309–320. [Google Scholar]

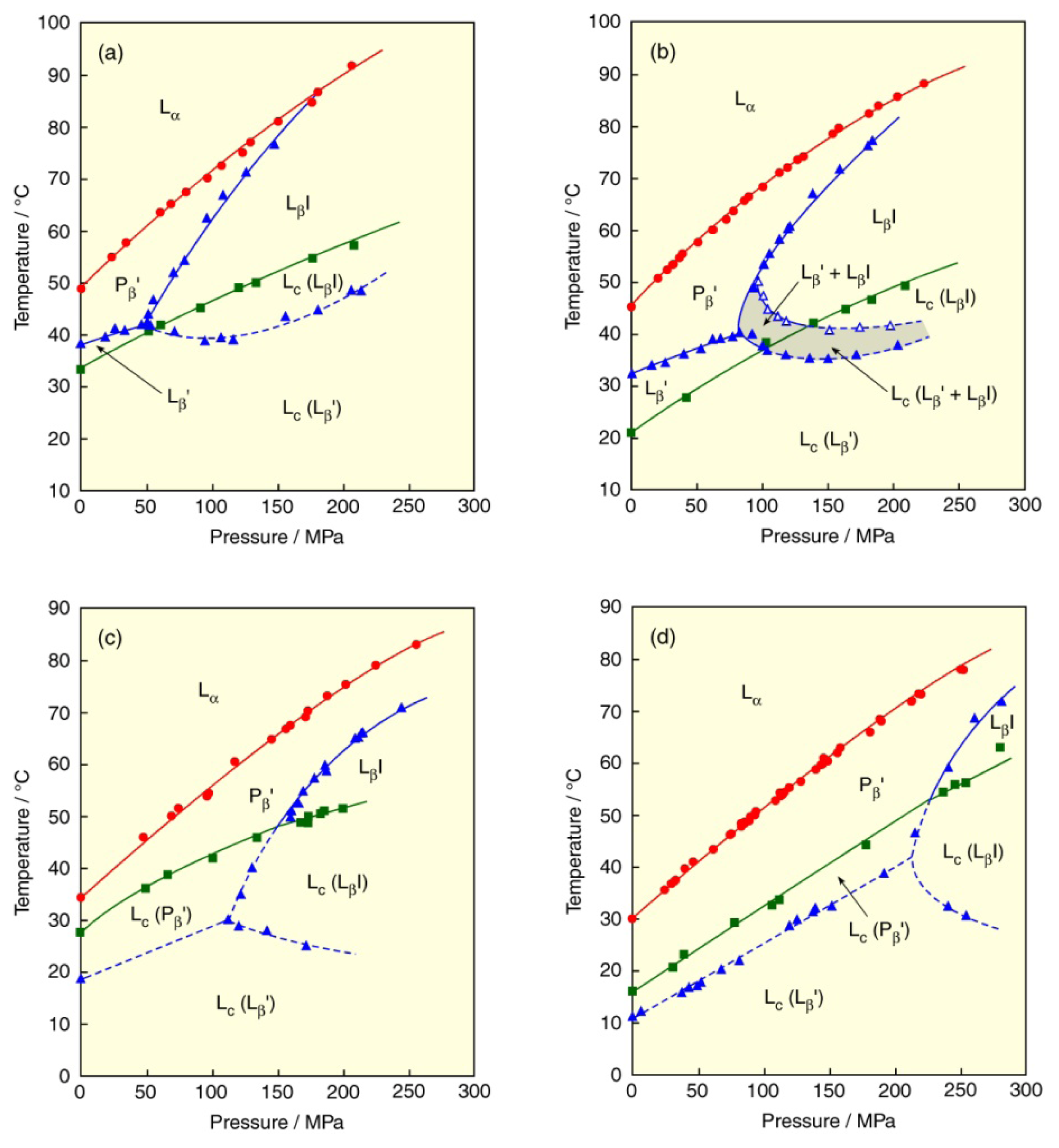

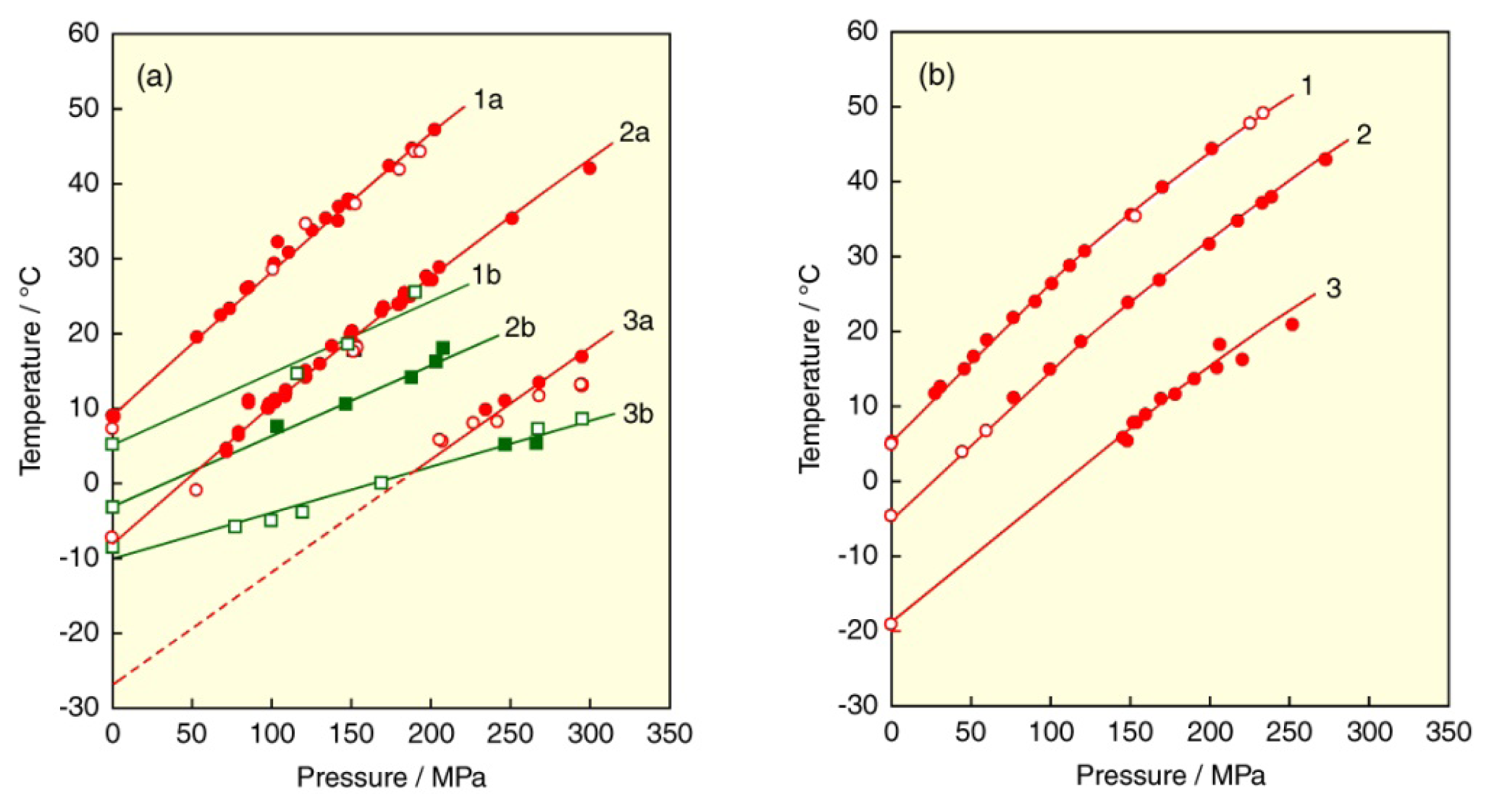
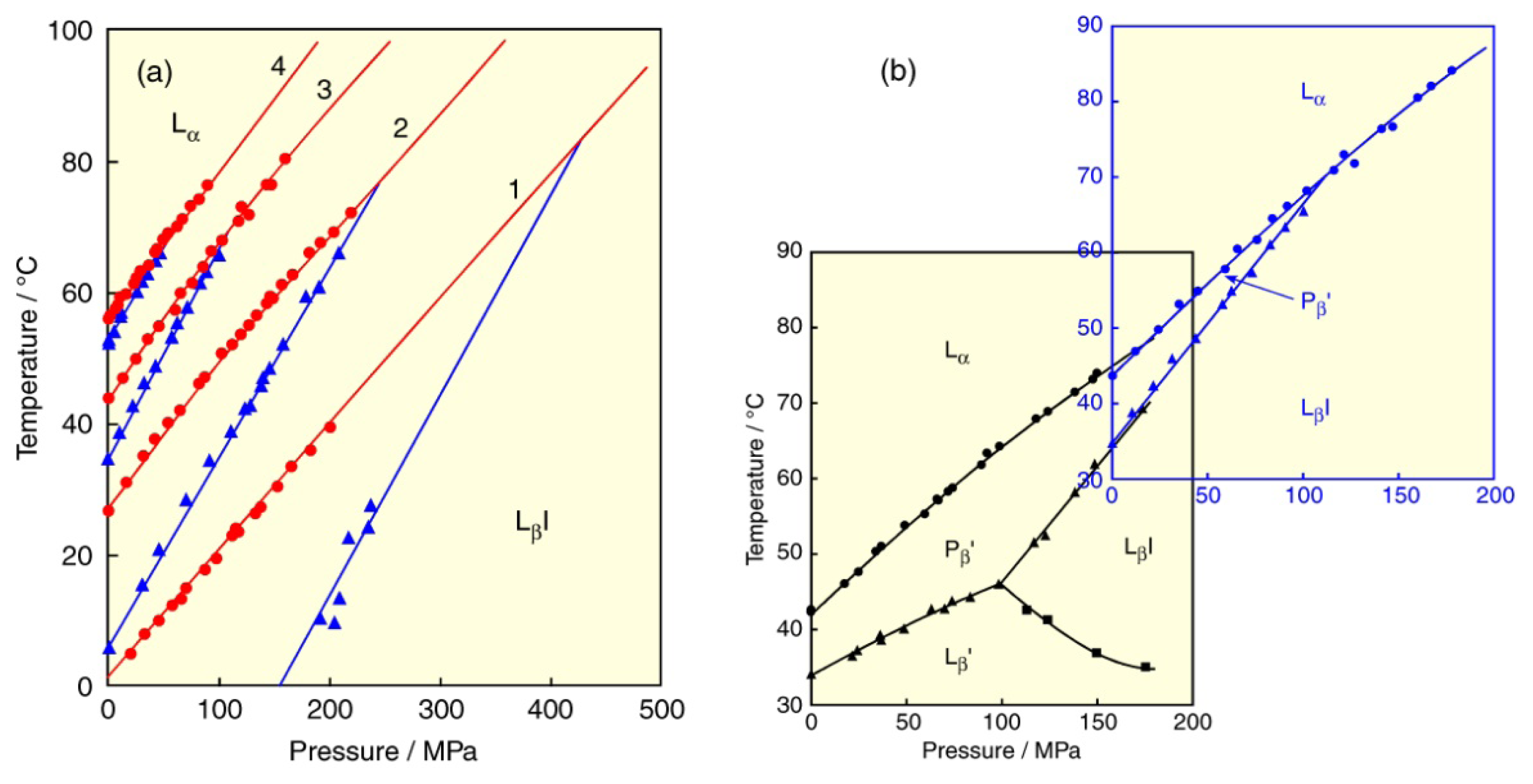
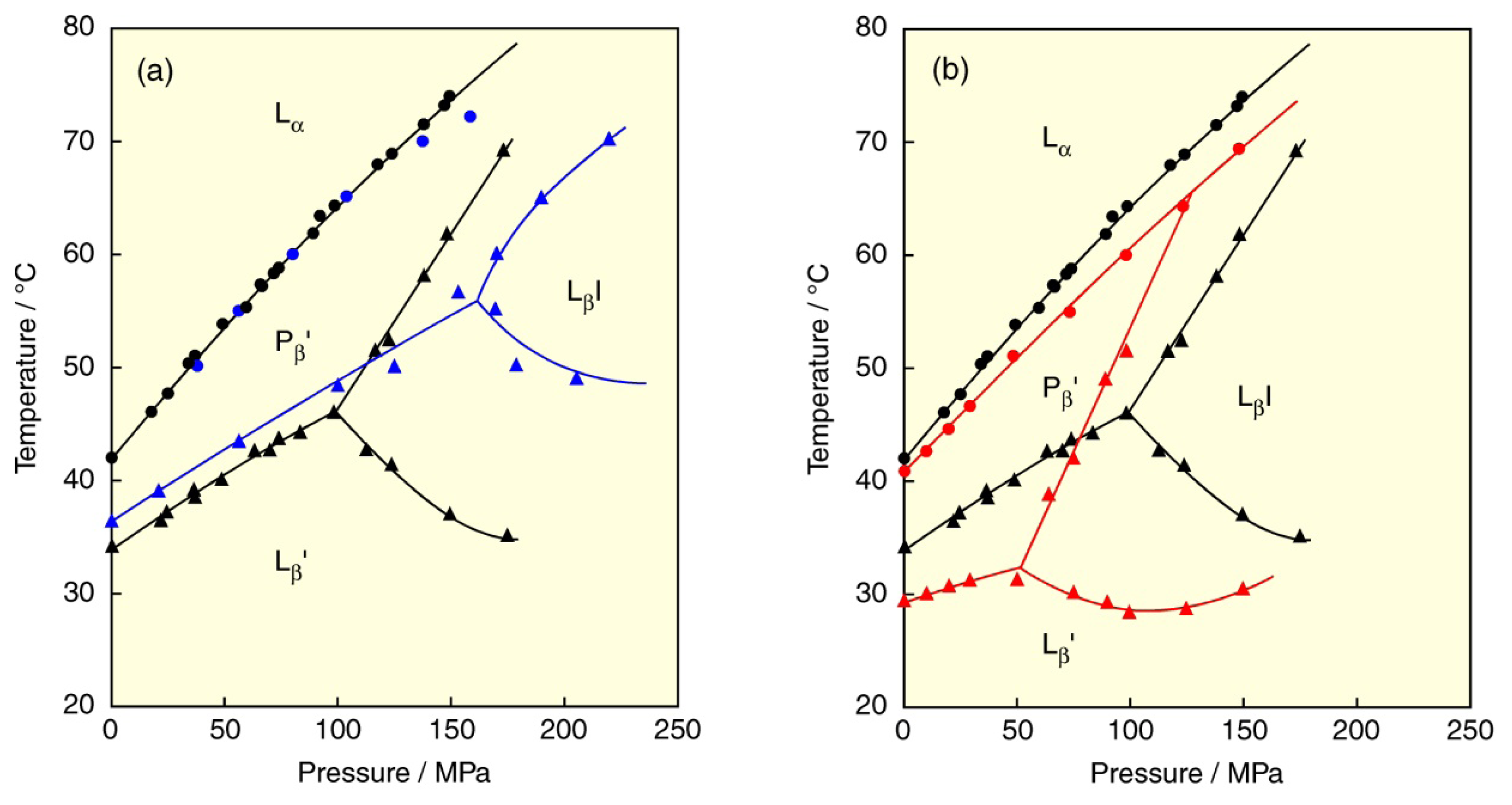
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matsuki, H.; Goto, M.; Tada, K.; Tamai, N. Thermotropic and Barotropic Phase Behavior of Phosphatidylcholine Bilayers. Int. J. Mol. Sci. 2013, 14, 2282-2302. https://doi.org/10.3390/ijms14022282
Matsuki H, Goto M, Tada K, Tamai N. Thermotropic and Barotropic Phase Behavior of Phosphatidylcholine Bilayers. International Journal of Molecular Sciences. 2013; 14(2):2282-2302. https://doi.org/10.3390/ijms14022282
Chicago/Turabian StyleMatsuki, Hitoshi, Masaki Goto, Kaori Tada, and Nobutake Tamai. 2013. "Thermotropic and Barotropic Phase Behavior of Phosphatidylcholine Bilayers" International Journal of Molecular Sciences 14, no. 2: 2282-2302. https://doi.org/10.3390/ijms14022282