Epidermal Growth Factor Stimulates Extracellular-Signal Regulated Kinase Phosphorylation of a Novel Site on Cytoplasmic Dynein Intermediate Chain 2
Abstract
:1. Introduction
2. Results
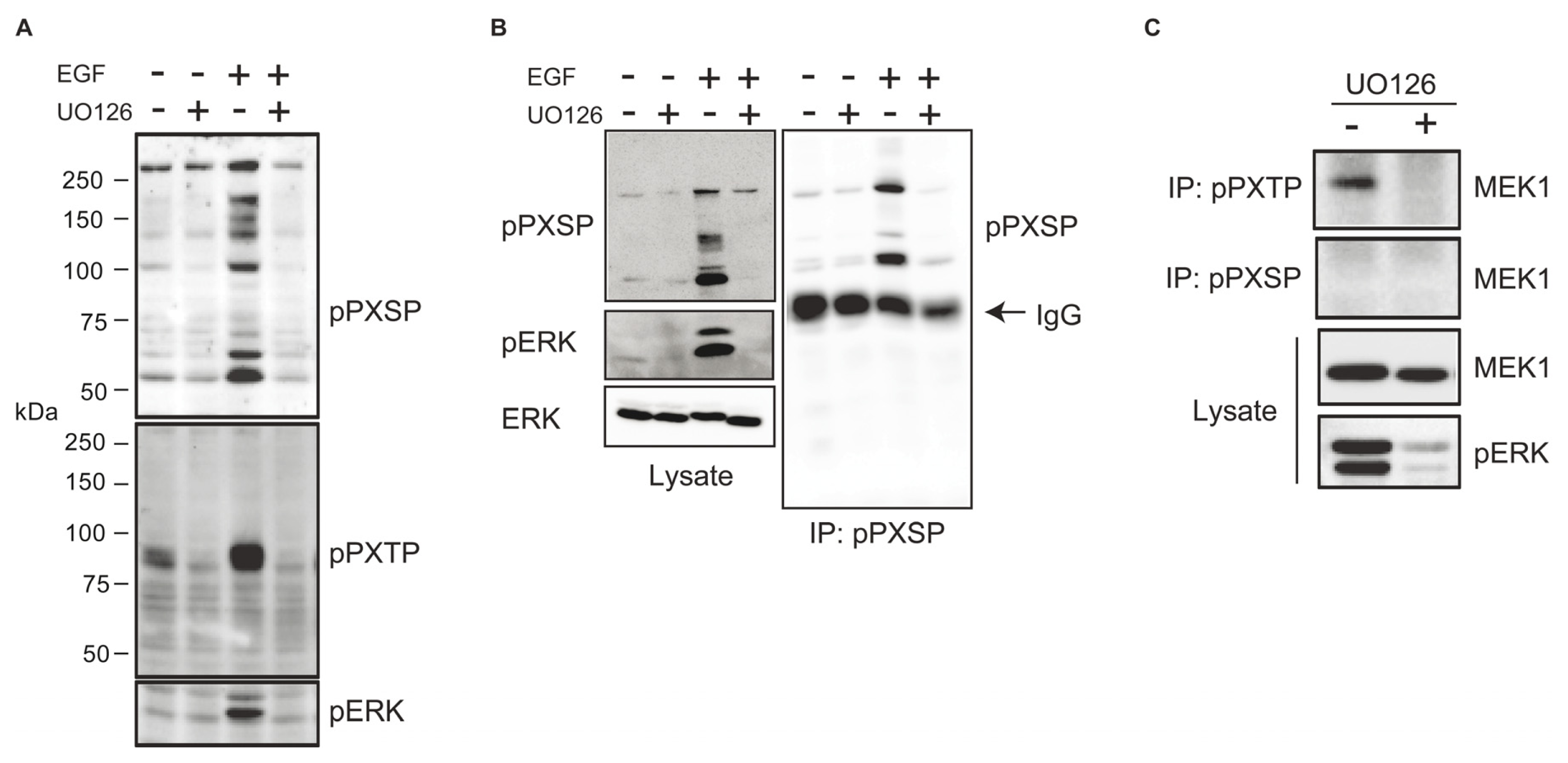
2.1. Identification of Potential ERK Substrates Using Phosphomotif-Specific Antisera
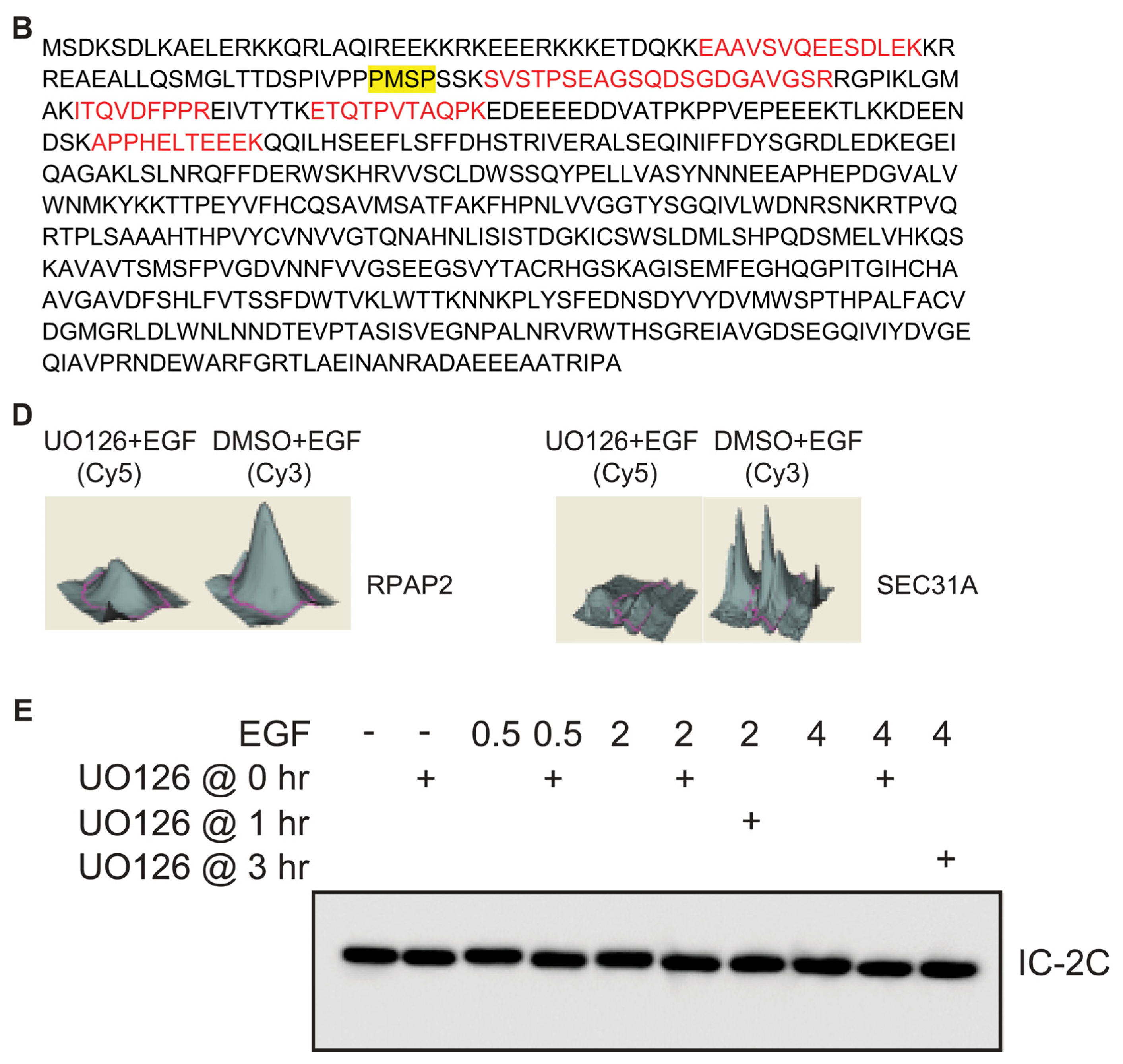
2.2. Identification of Dynein Intermediate Chain 2 as a Potential ERK Substrate
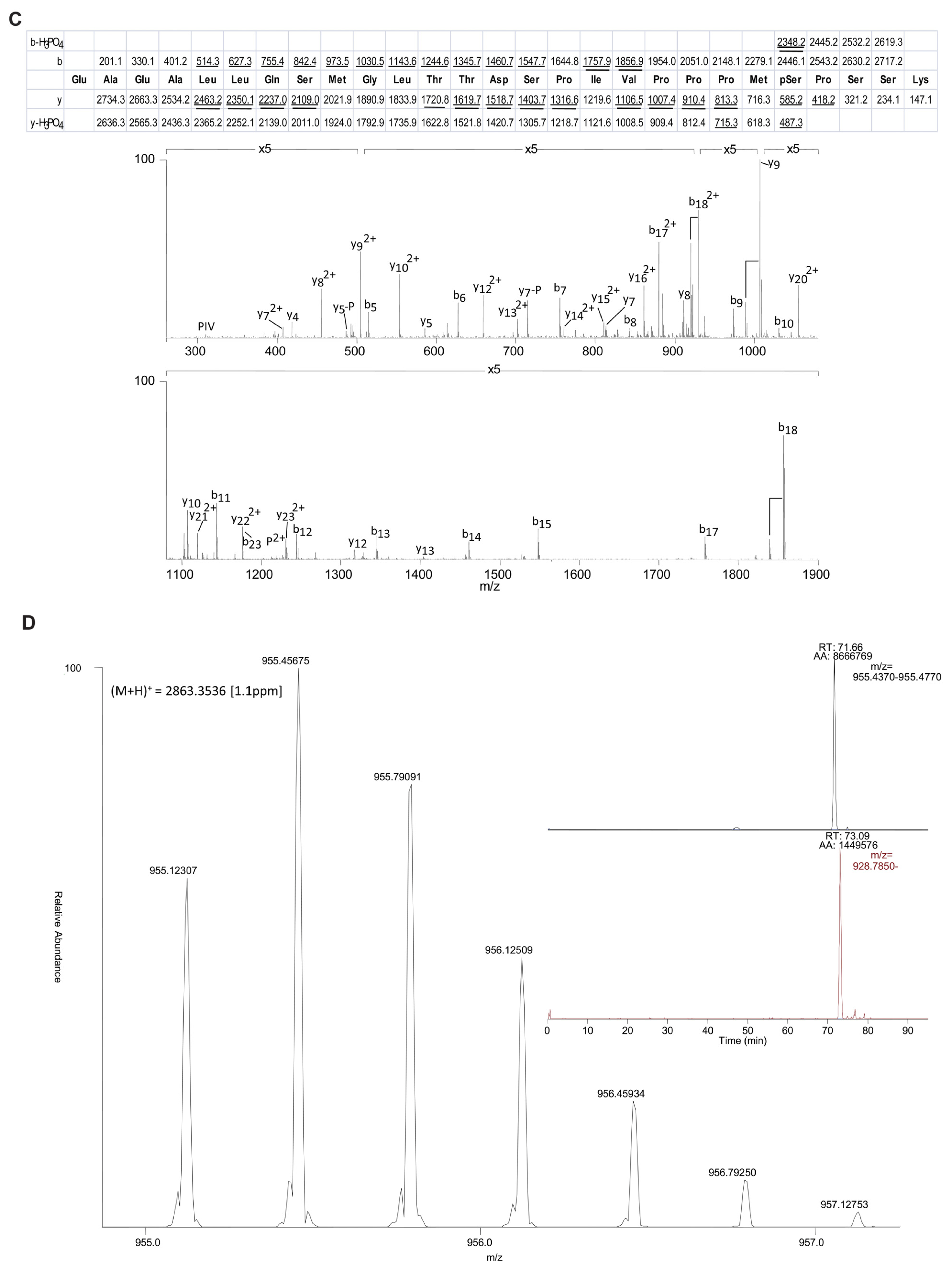
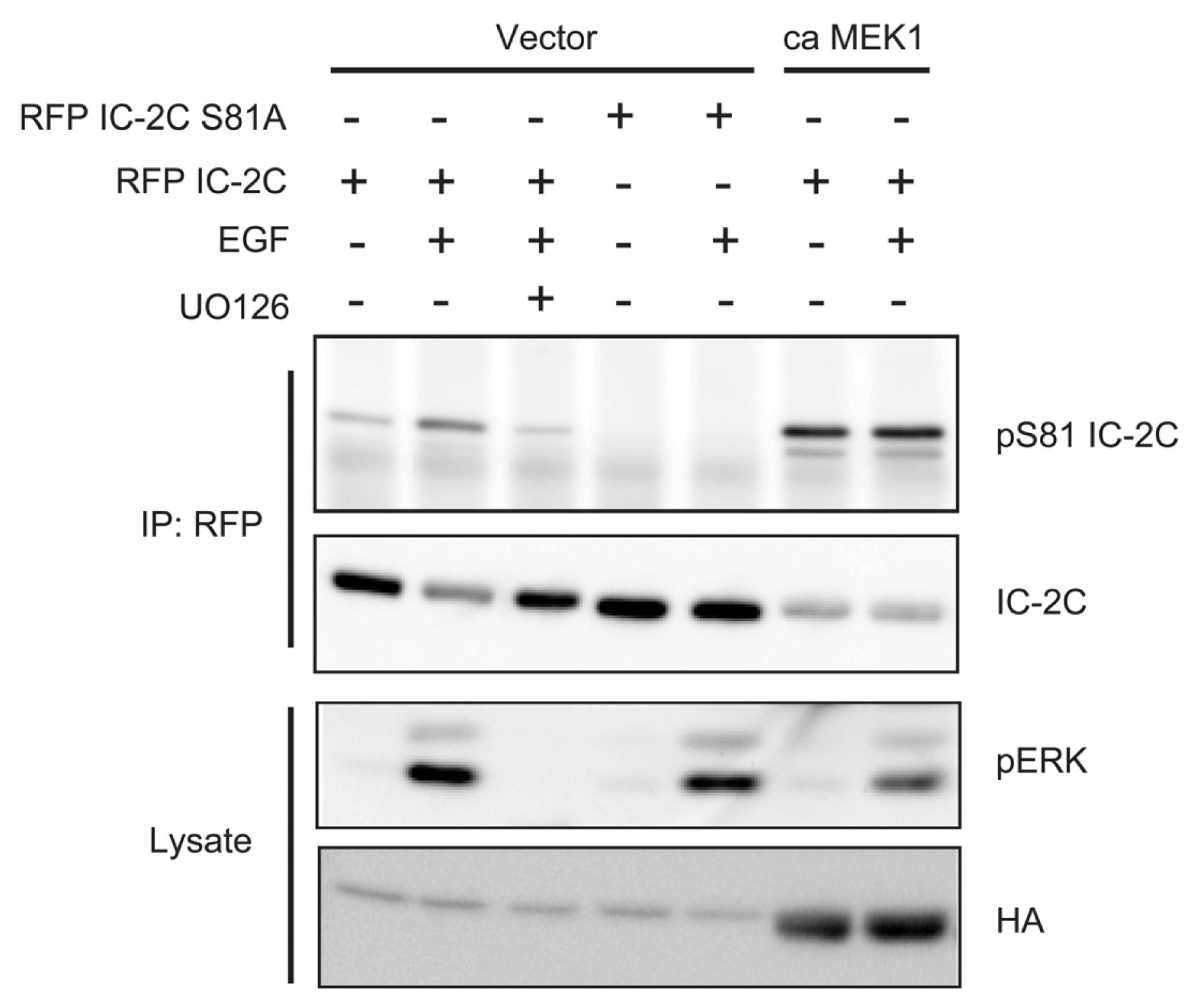
2.3. EGF Stimulates Phosphorylation of Serine 81 of Dynein Intermediate Chain 2C
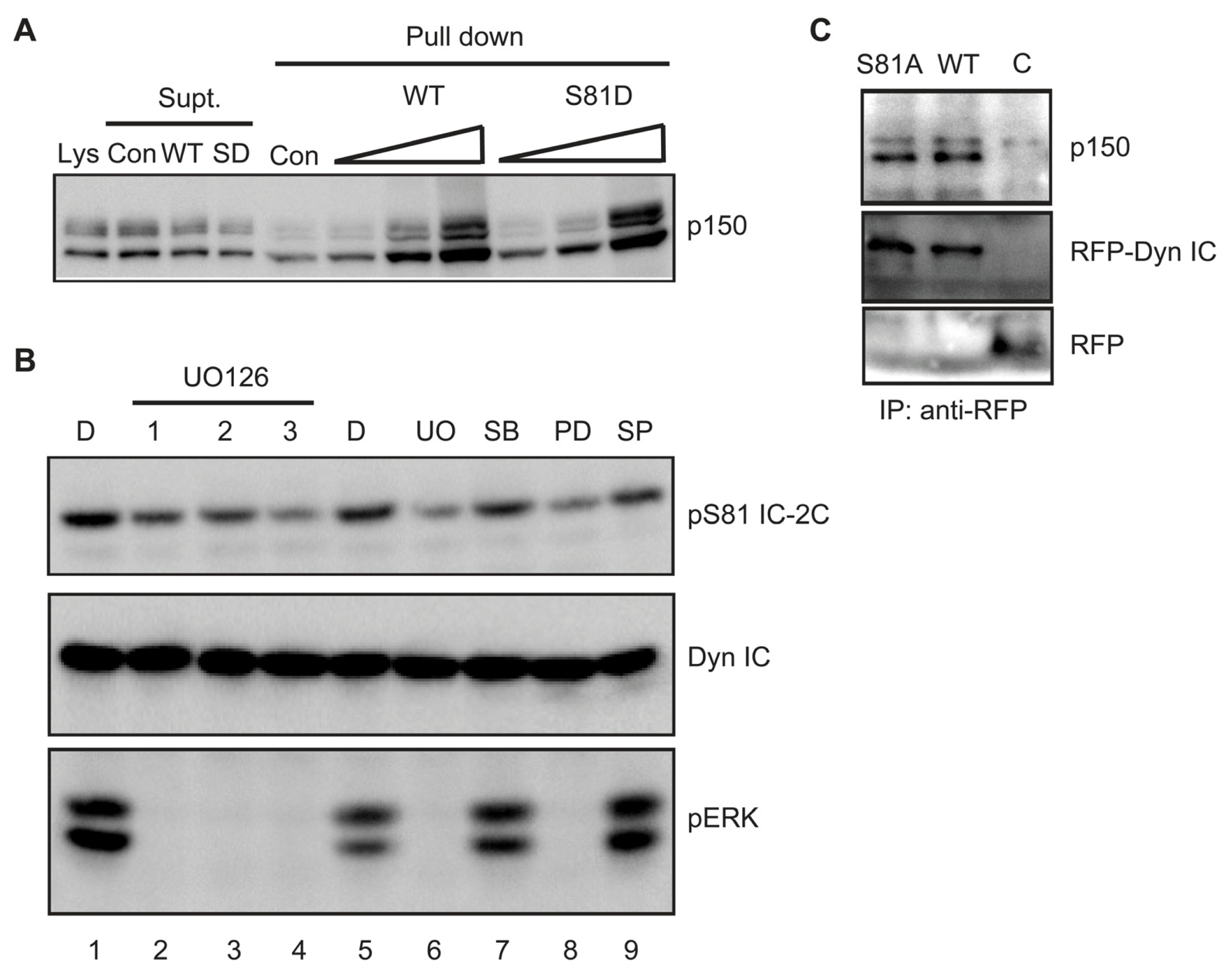
2.4. ERK Activity Is Necessary and Sufficient to Stimulate Serine 81 Phosphorylation of Dynein Intermediate Chain 2C
2.5. Phosphorylation Does Not Regulate Binding of Dynein Intermediate Chain to p150Glued
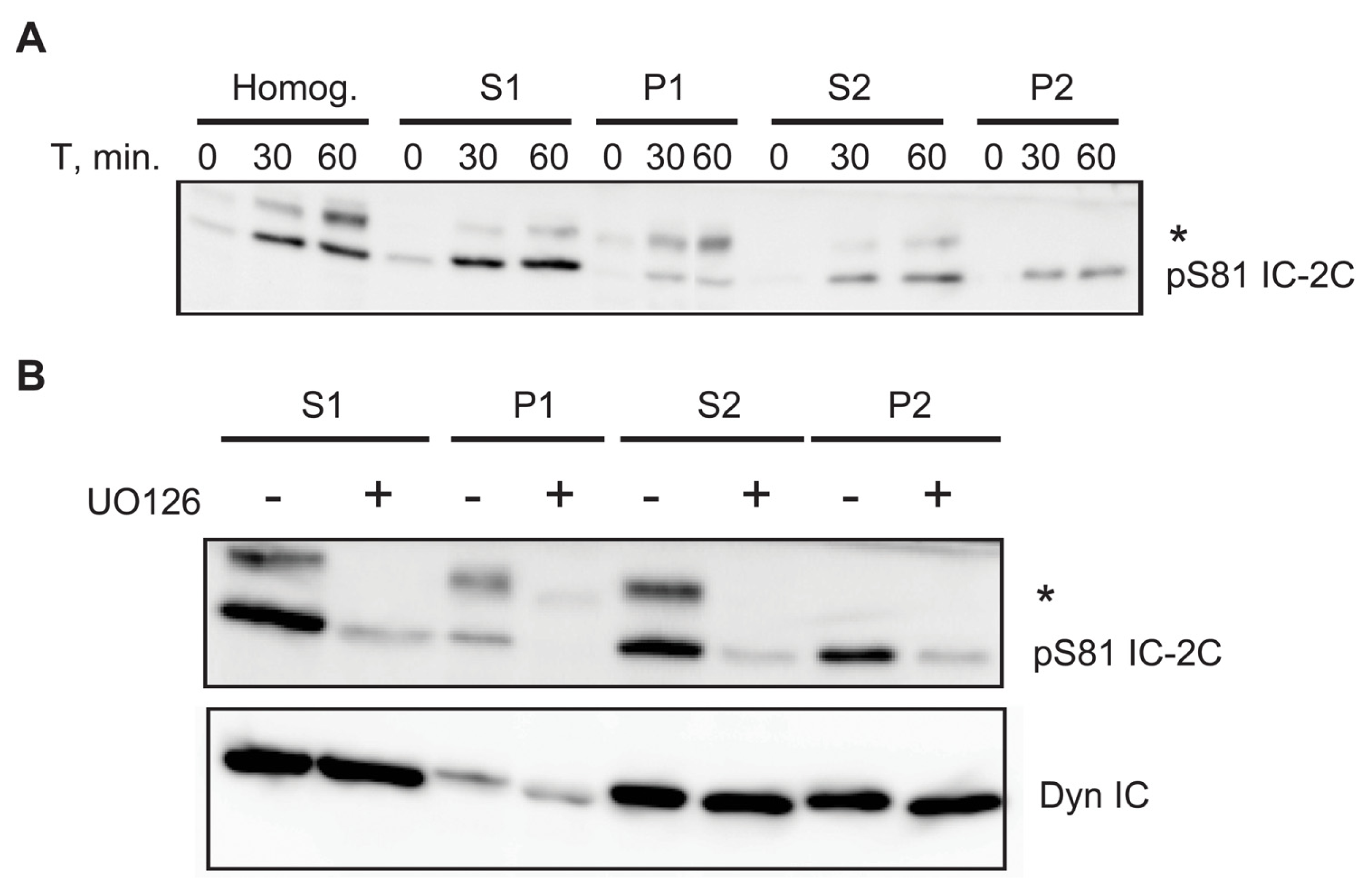
2.6. A Portion of Phospho Serine 81 Dynein Intermediate Chain Fractionates with Membrane Bound Organelles
3. Discussion
3.1. Identification of Dynein Intermediate Chain 2C as an ERK Substrate
3.2. Dynein Intermediate Chain 2C Is Phosphorylated on Serine 81
3.3. ERK Activity Is Necessary and Sufficient to Phosphorylate Serine 81
3.4. Association of Phospho-S81 IC-2C with Membrane Bounded Organelles
3.5. Serine 81 Phosphorylation Is without Effect on p150Glued Binding
3.6. Is Serine 81 Phosphorylation a Universal and Selective Mechanism for Receptor Tyrosine Kinase Trafficking?
4. Materials and Methods
4.1. Cells
4.2. Plasmids
4.3. Phospho-Specific Antisera
4.4. Anti-Phospho PXSP and PXTP Motif Immunoprecipitation
4.5. Identification of Candidate ERK Substrates
4.6. Identification of Phosphorylation Sites in Dynein Intermediate Chain 2C
4.7. Subcellular Fractionation
4.8. Recombinant Proteins and Pull down Assays
4.9. Co-Immunoprecipitation Assays
Acknowledgements
Conflict of Interest
References
- Haigler, H.; Ash, J.F.; Singer, S.J.; Cohen, S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc. Natl. Acad. Sci. USA 1978, 75, 3317–3321. [Google Scholar]
- Carpenter, G. Epidermal growth factor: Biology and receptor metabolism. J. Cell Sci 1985, 3, 1–9. [Google Scholar]
- Di, G.M.; Baass, P.C.; Ou, W.J.; Posner, B.I.; Bergeron, J.J. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J 1994, 13, 4269–4277. [Google Scholar]
- Baass, P.C.; di Guglielmo, G.M.; Authier, F.; Posner, B.I.; Bergeron, J.J. Compartmentalized signal transduction by receptor tyrosine kinases. Trends Cell Biol 1995, 5, 465–470. [Google Scholar]
- Bergeron, J.J.; di Guglielmo, G.M.; Baass, P.C.; Authier, F.; Posner, B.I. Endosomes, receptor tyrosine kinase internalization and signal transduction. Biosci. Rep 1995, 15, 411–418. [Google Scholar]
- Sorkin, A.; McClure, M.; Huang, F.; Carter, R. Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr. Biol 2000, 10, 1395–1398. [Google Scholar]
- Wang, Y.; Pennock, S.; Chen, X.; Wang, Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol. Cell Biol 2002, 22, 7279–7290. [Google Scholar]
- Pennock, S.; Wang, Z. Stimulation of cell proliferation by endosomal epidermal growth factor receptor as revealed through two distinct phases of signaling. Mol. Cell Biol 2003, 23, 5803–5815. [Google Scholar]
- Wang, Y.; Pennock, S.D.; Chen, X.; Kazlauskas, A.; Wang, Z. Platelet-derived growth factor receptor-mediated signal transduction from endosomes. J. Biol. Chem 2004, 279, 8038–8046. [Google Scholar]
- Heerssen, H.M.; Pazyra, M.F.; Segal, R.A. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat. Neurosci 2004, 7, 596–604. [Google Scholar]
- Vieira, A.V.; Lamaze, C.; Schmid, S.L. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 1996, 274, 2086–2089. [Google Scholar]
- Taub, N.; Teis, D.; Ebner, H.L.; Hess, M.W.; Huber, L.A. Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling. Mol. Biol. Cell 2007, 18, 4698–4710. [Google Scholar]
- Morishima-Kawashima, M.; Kosik, K.S. The pool of MAP kinase associated with microtubules is small but constitutively active. Mol. Biol. Cell 1996, 7, 893–905. [Google Scholar]
- Reszka, A.A.; Seger, R.; Diltz, C.D.; Krebs, E.G.; Fischer, E.H. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc. Natl. Acad. Sci. USA 1995, 92, 8881–8885. [Google Scholar]
- Reszka, A.A.; Bulinski, J.C.; Krebs, E.G.; Fischer, E.H. Mitogen-activated protein kinase/extracellular signal-regulated kinase 2 regulates cytoskeletal organization and chemotaxis via catalytic and microtubule-specific interactions. Mol. Biol. Cell 1997, 8, 1219–1232. [Google Scholar]
- Ray, L.B.; Sturgill, T.W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc. Natl. Acad. Sci. USA 1987, 84, 1502–1506. [Google Scholar]
- Deacon, S.W.; Nascimento, A.; Serpinskaya, A.S.; Gelfand, V.I. Regulation of bidirectional melanosome transport by organelle bound MAP kinase. Curr. Biol 2005, 15, 459–463. [Google Scholar]
- Andersson, T.P.; Svensson, S.P.; Karlsson, A.M. Regulation of melanosome movement by MAP kinase. Pigment Cell Res 2003, 16, 215–221. [Google Scholar]
- Andersson, L.; Bostrom, P.; Ericson, J.; Rutberg, M.; Magnusson, B.; Marchesan, D.; Ruiz, M.; Asp, L.; Huang, P.; Frohman, M.A.; et al. PLD1 and ERK2 regulate cytosolic lipid droplet formation. J. Cell Sci 2006, 119, 2246–2257. [Google Scholar]
- Teis, D.; Taub, N.; Kurzbauer, R.; Hilber, D.; de Araujo, M.E.; Erlacher, M.; Offterdinger, M.; Villunger, A.; Geley, S.; Bohn, G.; et al. p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J. Cell Biol 2006, 175, 861–868. [Google Scholar]
- Bohn, G.; Allroth, A.; Brandes, G.; Thiel, J.; Glocker, E.; Schaffer, A.A.; Rathinam, C.; Taub, N.; Teis, D.; Zeidler, C.; et al. A novel human primary immunodeficiency syndrome caused by deficiency of the endosomal adaptor protein p14. Nat. Med 2007, 13, 38–45. [Google Scholar]
- Pfister, K.K.; Fisher, E.M.; Gibbons, I.R.; Hays, T.S.; Holzbaur, E.L.; Mcintosh, J.R.; Porter, M.E.; Schroer, T.A.; Vaughan, K.T.; Witman, G.B.; et al. Cytoplasmic dynein nomenclature. J. Cell Biol 2005, 171, 411–413. [Google Scholar]
- Songyang, Z.; Blechner, S.; Hoagland, N.; Hoekstra, M.F.; Piwnica-Worms, H.; Cantley, L.C. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr. Biol 1994, 4, 973–982. [Google Scholar]
- Eblen, S.T.; Slack-Davis, J.K.; Tarcsafalvi, A.; Parsons, J.T.; Weber, M.J.; Catling, A.D. Mitogen-activated protein kinase feedback phosphorylation regulates MEK1 complex formation and activation during cellular adhesion. Mol. Cell Biol 2004, 24, 2308–2317. [Google Scholar]
- Catling, A.D.; Schaeffer, H.J.; Reuter, C.W.; Reddy, G.R.; Weber, M.J. A proline-rich sequence unique to MEK1 and MEK2 is required for Raf binding and regulates MEK function. Mol. Cell Biol 1995, 15, 5214–5225. [Google Scholar]
- Brunet, A.; Pages, G.; Pouyssegur, J. Growth factor-stimulated MAP kinase induces rapid retrophosphorylation and inhibition of MAP kinase kinase (MEK1). FEBS Lett 1994, 346, 299–303. [Google Scholar]
- Dillman, J.F., III; Pfister, K.K. Differential phosphorylation in vivo of cytoplasmic dynein associated with anterogradely moving organelles. J. Cell Biol. 1994, 127, 1671–1681. [Google Scholar]
- Vaughan, K.T.; Vallee, R.B. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J. Cell Biol 1995, 131, 1507–1516. [Google Scholar]
- Kuta, A.; Deng, W.; Morsi El-Kadi, A.; Banks, G.T.; Hafezparast, M.; Pfister, K.K.; Fisher, E.M. Mouse cytoplasmic dynein intermediate chains: Identification of new isoforms, alternative splicing and tissue distribution of transcripts. PLoS One 2010, 5, e11682. [Google Scholar]
- Pfister, K.K.; Salata, M.W.; Dillman, J.F., III; Vaughan, K.T.; Vallee, R.B.; Torre, E.; Lye, R.J. Differential expression and phosphorylation of the 74-kDa intermediate chains of cytoplasmic dynein in cultured neurons and glia. J. Biol. Chem. 1996, 271, 1687–1694. [Google Scholar]
- Pfister, K.K.; Salata, M.W.; Dillman, J.F., III; Torre, E.; Lye, R.J. Identification and developmental regulation of a neuron-specific subunit of cytoplasmic dynein. Mol. Biol. Cell 1996, 7, 331–343. [Google Scholar]
- Vaughan, P.S.; Leszyk, J.D.; Vaughan, K.T. Cytoplasmic dynein intermediate chain phosphorylation regulates binding to dynactin. J. Biol. Chem 2001, 276, 26171–26179. [Google Scholar]
- Whyte, J.; Bader, J.R.; Tauhata, S.B.; Raycroft, M.; Hornick, J.; Pfister, K.K.; Lane, W.S.; Chan, G.K.; Hinchcliffe, E.H.; Vaughan, P.S.; et al. Phosphorylation regulates targeting of cytoplasmic dynein to kinetochores during mitosis. J. Cell Biol 2008, 183, 819–834. [Google Scholar]
- Salata, M.W.; Dillman, J.F., III; Lye, R.J.; Pfister, K.K. Growth factor regulation of cytoplasmic dynein intermediate chain subunit expression preceding neurite extension. J. Neurosci. Res. 2001, 65, 408–416. [Google Scholar]
- Crackower, M.A.; Sinasac, D.S.; Xia, J.; Motoyama, J.; Prochazka, M.; Rommens, J.M.; Scherer, S.W.; Tsui, L.C. Cloning and characterization of two cytoplasmic dynein intermediate chain genes in mouse and human. Genomics 1999, 55, 257–267. [Google Scholar]
- Dillman, J.F., III; Dabney, L.P.; Karki, S.; Paschal, B.M.; Holzbaur, E.L.; Pfister, K.K. Functional analysis of dynactin and cytoplasmic dynein in slow axonal transport. J. Neurosci. 1996, 16, 6742–6752. [Google Scholar]
- Pfister, K.K.; Shah, P.R.; Hummerich, H.; Russ, A.; Cotton, J.; Annuar, A.A.; King, S.M.; Fisher, E.M. Genetic analysis of the cytoplasmic dynein subunit families. PLoS Genet 2006, 2, e1. [Google Scholar]
- Mitchell, D.J.; Blasier, K.R.; Jeffery, E.D.; Ross, M.W.; Pullikuth, A.K.; Suo, D.; Park, J.; Smiley, W.R.; Lo, K.W.; Shabanowitz, J.; et al. Trk activation of the ERK1/2 kinase pathway stimulates intermediate chain phosphorylation and recruits cytoplasmic dynein to signaling endosomes for retrograde axonal transport. J. Neurosci 2012, 32, 15495–15510. [Google Scholar]
- Tokito, M.K.; Howland, D.S.; Lee, V.M.; Holzbaur, E.L. Functionally distinct isoforms of dynactin are expressed in human neurons. Mol. Biol. Cell 1996, 7, 1167–1180. [Google Scholar]
- Rome, P.; Montembault, E.; Franck, N.; Pascal, A.; Glover, D.M.; Giet, R. Aurora A contributes to p150(glued) phosphorylation and function during mitosis. J. Cell Biol 2010, 189, 651–659. [Google Scholar]
- Vaughan, P.S.; Miura, P.; Henderson, M.; Byrne, B.; Vaughan, K.T. A role for regulated binding of p150(Glued) to microtubule plus ends in organelle transport. J. Cell Biol 2002, 158, 305–319. [Google Scholar]
- Feinmesser, R.L.; Wicks, S.J.; Taverner, C.J.; Chantry, A. Ca2+/calmodulin-dependent kinase II phosphorylates the epidermal growth factor receptor on multiple sites in the cytoplasmic tail and serine 744 within the kinase domain to regulate signal generation. J. Biol. Chem 1999, 274, 16168–16173. [Google Scholar]
- Hoeflich, K.P.; Gray, D.C.; Eby, M.T.; Tien, J.Y.; Wong, L.; Bower, J.; Gogineni, A.; Zha, J.; Cole, M.J.; Stern, H.M.; et al. Oncogenic BRAF is required for tumor growth and maintenance in melanoma models. Cancer Res 2006, 66, 999–1006. [Google Scholar]
- Ha, J.; Lo, K.W.; Myers, K.R.; Carr, T.M.; Humsi, M.K.; Rasoul, B.A.; Segal, R.A.; Pfister, K.K. A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes. J. Cell Biol 2008, 181, 1027–1039. [Google Scholar]
- Grimes, M.L.; Zhou, J.; Beattie, E.C.; Yuen, E.C.; Hall, D.E.; Valletta, J.S.; Topp, K.S.; LaVail, J.H.; Bunnett, N.W.; Mobley, W.C. Endocytosis of activated TrkA: Evidence that nerve growth factor induces formation of signaling endosomes. J. Neurosci 1996, 16, 7950–7964. [Google Scholar]
- Ross, J.L.; Ali, M.Y.; Warshaw, D.M. Cargo transport: Molecular motors navigate a complex cytoskeleton. Curr. Opin. Cell Biol 2008, 20, 41–47. [Google Scholar]
- Howe, C.L.; Mobley, W.C. Long-distance retrograde neurotrophic signaling. Curr. Opin. Neurobiol 2005, 15, 40–48. [Google Scholar]
- Vallee, R.B.; Williams, J.C.; Varma, D.; Barnhart, L.E. Dynein: An ancient motor protein involved in multiple modes of transport. J. Neurobiol 2004, 58, 189–200. [Google Scholar]
- Levy, J.R.; Holzbaur, E.L. Cytoplasmic dynein/dynactin function and dysfunction in motor neurons. Int. J. Dev. Neurosci 2006, 24, 103–111. [Google Scholar]
- Iyadurai, S.J.; Robinson, J.T.; Ma, L.; He, Y.; Mische, S.; Li, M.G.; Brown, W.; Guichard, A.; Bier, E.; Hays, T.S. Dynein and Star interact in EGFR signaling and ligand trafficking. J. Cell Sci 2008, 121, 2643–2651. [Google Scholar]
- Driskell, O.J.; Mironov, A.; Allan, V.J.; Woodman, P.G. Dynein is required for receptor sorting and the morphogenesis of early endosomes. Nat. Cell Biol 2007, 9, 113–120. [Google Scholar]
- Burkhardt, J.K.; Echeverri, C.J.; Nilsson, T.; Vallee, R.B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol 1997, 139, 469–484. [Google Scholar]
- Nurminsky, D.I.; Nurminskaya, M.V.; Benevolenskaya, E.V.; Shevelyov, Y.Y.; Hartl, D.L.; Gvozdev, V.A. Cytoplasmic dynein intermediate-chain isoforms with different targeting properties created by tissue-specific alternative splicing. Mol. Cell Biol 1998, 18, 6816–6825. [Google Scholar]
- Wilson, M.J.; Salata, M.W.; Susalka, S.J.; Pfister, K.K. Light chains of mammalian cytoplasmic dynein: Identification and characterization of a family of LC8 light chains. Cell Motil. Cytoskeleton 2001, 49, 229–240. [Google Scholar]
- Songyang, Z.; Lu, K.P.; Kwon, Y.T.; Tsai, L.H.; Filhol, O.; Cochet, C.; Brickey, D.A.; Soderling, T.R.; Bartleson, C.; Graves, D.J.; et al. A structural basis for substrate specificities of protein Ser/Thr kinases: Primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and ERK1. Mol. Cell Biol 1996, 16, 6486–6493. [Google Scholar]
- Karki, S.; Tokito, M.K.; Holzbaur, E.L. Casein kinase II binds to and phosphorylates cytoplasmic dynein. J. Biol. Chem 1997, 272, 5887–5891. [Google Scholar]
- Ikeda, K.; Zhapparova, O.; Brodsky, I.; Semenova, I.; Tirnauer, J.S.; Zaliapin, I.; Rodionov, V. CK1 activates minus-end-directed transport of membrane organelles along microtubules. Mol. Biol. Cell 2011, 22, 1321–1329. [Google Scholar]
- Hanson, P.I.; Schulman, H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu. Rev. Biochem 1992, 61, 559–601. [Google Scholar]
- Ando, S.; Tokui, T.; Yamauchi, T.; Sugiura, H.; Tanabe, K.; Inagaki, M. Evidence that Ser-82 is a unique phosphorylation site on vimentin for Ca2+-calmodulin-dependent protein kinase II. Biochem. Biophys. Res. Commun 1991, 175, 955–962. [Google Scholar]
- Karki, S.; Holzbaur, E.L. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol 1999, 11, 45–53. [Google Scholar]
- King, S.J.; Brown, C.L.; Maier, K.C.; Quintyne, N.J.; Schroer, T.A. Analysis of the dynein-dynactin interaction in vitro and in vivo. Mol. Biol. Cell 2003, 14, 5089–5097. [Google Scholar]
- Marshall, C.J. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell 1995, 80, 179–185. [Google Scholar]
- Giroux, S.; Tremblay, M.; Bernard, D.; Cardin-Girard, J.F.; Aubry, S.; Larouche, L.; Huot, J.; Landry, J.; Jeannotte, L.; Charron, J. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol 1999, 9, 369–372. [Google Scholar]
- Pullikuth, A.; McKinnon, E.; Schaeffer, H.J.; Catling, A.D. The MEK1 scaffolding protein MP1 regulates cell spreading by integrating PAK1 and Rho signals. Mol. Cell Biol 2005, 25, 5119–5133. [Google Scholar]
- Immunoaffinity Isolation of Modified Peptides from Complex Mixtures. U.S. Patent 7198896, 3 April 2007.
- Immunoaffinity Isolation of Modified Peptides from Complex Mixtures. U.S. Patent 7300753, 27 November 2007.
- Schaeffer, H.J.; Catling, A.D.; Eblen, S.T.; Collier, L.S.; Krauss, A.; Weber, M.J. MP1: A MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 1998, 281, 1668–1671. [Google Scholar]


| Protease Digest | IC-2C peptide | +P/−P ratio, EGF 30 min |
|---|---|---|
| Trypsin | EAEALLQSMGLTTDSPIVPPPMSPSSK (S81) | 4.873 |
| Endo AspN | DSPIVPPPMSPSSKSVSTPSEAGSQ (S81) | 3.318 |
| Trypsin | SVSTPSEAGSQDSGDGAVGSR (T89/S95/S98 *) | 0.006 |
| Endo AspN | DDVATPKPPVEPEEEKTLKKDEEN (T154) | 0.080 |
| Endo GluC | TQTPVTAQPKEDEEEEDDVATPKPPVEPEEE (T154) | 0.019 |
| Endo GluC | AAVSVQE (S51) | 0.005 |
| Protease | Digest IC-2C S81 peptide | Control | EGF | |
|---|---|---|---|---|
| Trypsin | ||||
| EAE…. | 1.74 × 106 | 1.45 × 106 | ||
| EAEmM…. | 1.39 × 106 | 8.57 × 106 | ||
| EAEMm…. | 2.68 × 106 | 2.58 × 106 | ||
| EAEmm…. | 5.62 × 106 | 4.75 × 106 | ||
| Total | 1.14 × 107 | 9.64 × 106 | ||
| EAE p…. | 1.60 × 106 | 8.66 × 106 | ||
| EAEmM p…. | 1.21 × 106 | 5.50 × 106 | ||
| EAEMm p…. | 2.35 × 106 | 1.22 × 106 | ||
| EAEmm p…. | 4.10 × 106 | 2.06 × 106 | ||
| Total | 9.26 × 106 | 4.70 × 107 | ||
| +P/−P | 0.81 | 4.87 | ||
| Fold change | 1 | 6.01 | ||
| Endo Asp-N | ||||
| DSP…. | 1.67 × 107 | 1.61 × 106 | ||
| DSPm…. | 2.98 × 107 | 9.66 × 106 | ||
| Total | 4.65 × 107 | 1.13 × 107 | ||
| DSP p…. | 8.27 × 106 | 7.69 × 106 | ||
| DSPm p…. | 1.97 × 107 | 2.97 × 107 | ||
| Total | 2.80 × 107 | 3.74 × 107 | ||
| +P/−P | 0.60 | 3.32 | ||
| Fold change | 1 | 5.52 |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pullikuth, A.K.; Ozdemir, A.; Cardenas, D.; Bailey, E.; Sherman, N.E.; Pfister, K.K.; Catling, A.D. Epidermal Growth Factor Stimulates Extracellular-Signal Regulated Kinase Phosphorylation of a Novel Site on Cytoplasmic Dynein Intermediate Chain 2. Int. J. Mol. Sci. 2013, 14, 3595-3620. https://doi.org/10.3390/ijms14023595
Pullikuth AK, Ozdemir A, Cardenas D, Bailey E, Sherman NE, Pfister KK, Catling AD. Epidermal Growth Factor Stimulates Extracellular-Signal Regulated Kinase Phosphorylation of a Novel Site on Cytoplasmic Dynein Intermediate Chain 2. International Journal of Molecular Sciences. 2013; 14(2):3595-3620. https://doi.org/10.3390/ijms14023595
Chicago/Turabian StylePullikuth, Ashok K., Aysun Ozdemir, Daviel Cardenas, Evangeline Bailey, Nicholas E. Sherman, K. Kevin Pfister, and Andrew D. Catling. 2013. "Epidermal Growth Factor Stimulates Extracellular-Signal Regulated Kinase Phosphorylation of a Novel Site on Cytoplasmic Dynein Intermediate Chain 2" International Journal of Molecular Sciences 14, no. 2: 3595-3620. https://doi.org/10.3390/ijms14023595




