2.1. DSC of RALA-CD Complexes
Complex formation of RALA and the different CDs can be evaluated using DSC, since the melting, boiling or sublimation points of these complexes usually shift to a different temperature compared to the melting or boiling point of free lipoic acid.
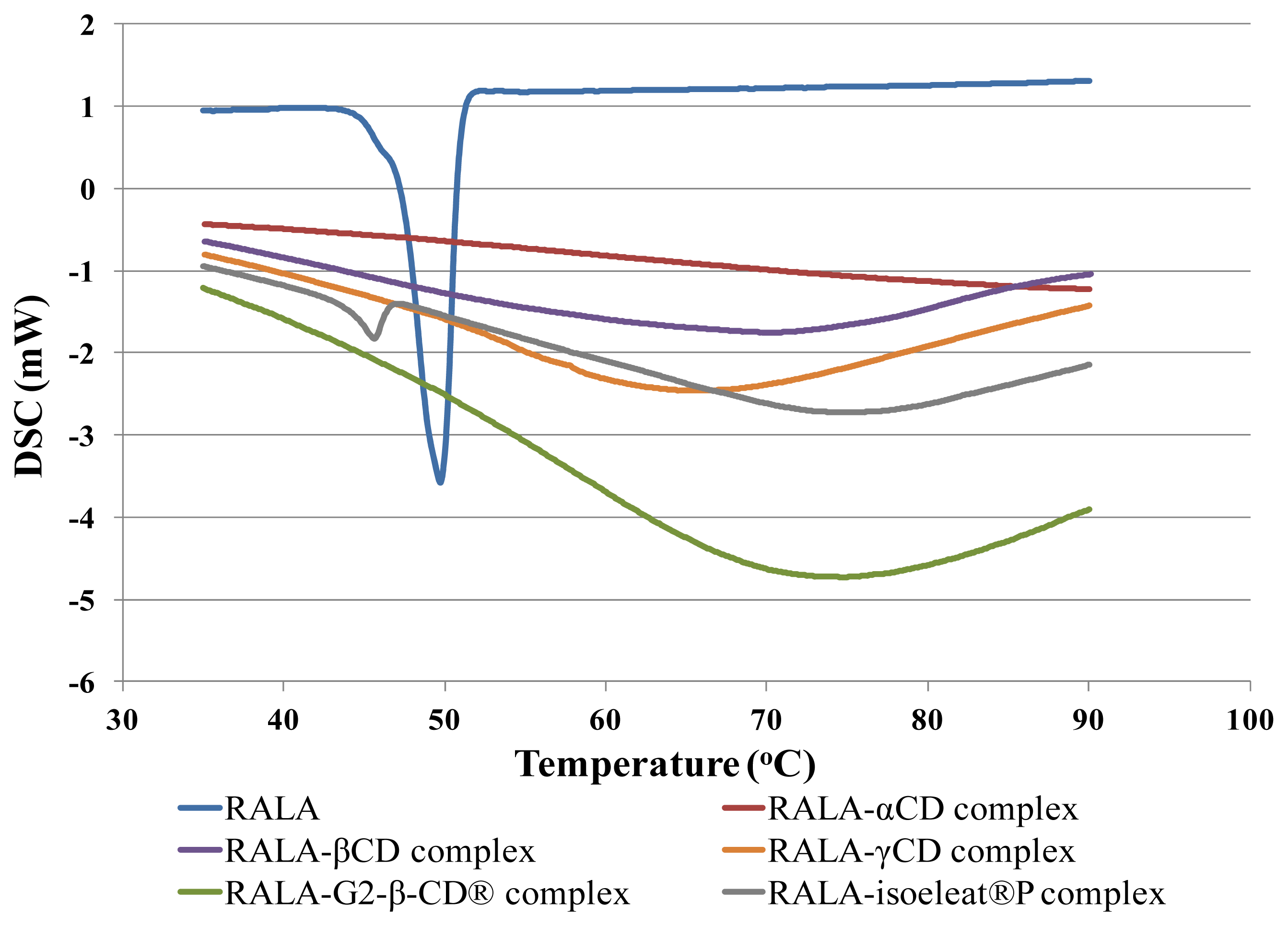
The DSC thermograms of RALA and the RALA-CD complexes are presented in
Figure 1. As can be seen in this figure, RALA shows a characteristic endothermic fusion peak around 50 °C, while RALA-αCD, RALA-βCD, RALA-γCD and RALA-G2-β-CD
® did not show any clear peaks within the analyzed temperature range from 30 °C to 100 °C. However, the RALA-isoeleat
®P complex showed a peak at 45.6 °C, indicating that isoeleat
®P could not stabilize RALA. We calculated the uncomplexed RALA from the peak area ratio (RALA-isoeleat
®P/RALA), and it was found that
ca. 10% of the RALA contained in RALA-isoeleat
®P was not stabilized. Based upon these results, it was deduced that complex formation of RALA with α-CD, β-CD, γ-CD or G2-β-CD
® had occurred and that the complexes led to an improved stability of their host molecule RALA.
Similar results were found in a previous study [
13] in which racemic ALA complex formation with G2-β-CD
® and β-CD led to the disappearance of the endothermic DSC peak. Interestingly, ALA complex formation with α-CD, γ-CD and isoeleat
®P by Mikuni and colleagues led only to a reduction of the peak sizes in the DSC thermogram. Since in our study the RALA-α-CD and RALA-γ-CD complexes did not exhibit a peak in the DSC scans, it appears that we have produced more stable RALA-CD complexes than Mikuni and colleagues. Regarding the complex formation with isoeleat
®P, it seems that this RALA complex differed from the other RALA-CD complexes, as RALA-isoeleat
®P shows a DSC peak at an even lower temperature than free RALA. This result indicates that decomposition or polymerization of RALA in the isoeleat
®P-complex is likely to happen at even lower temperatures than would happen without complex formation. Therefore, it seems that this type of complex does not favor the stability of RALA.
A DSC scan of a polymerized RALA sample showed no peak within the analyzed temperature range (data not shown).
2.2. HPLC Analysis of the RALA Content in the RALA-CD Complexes
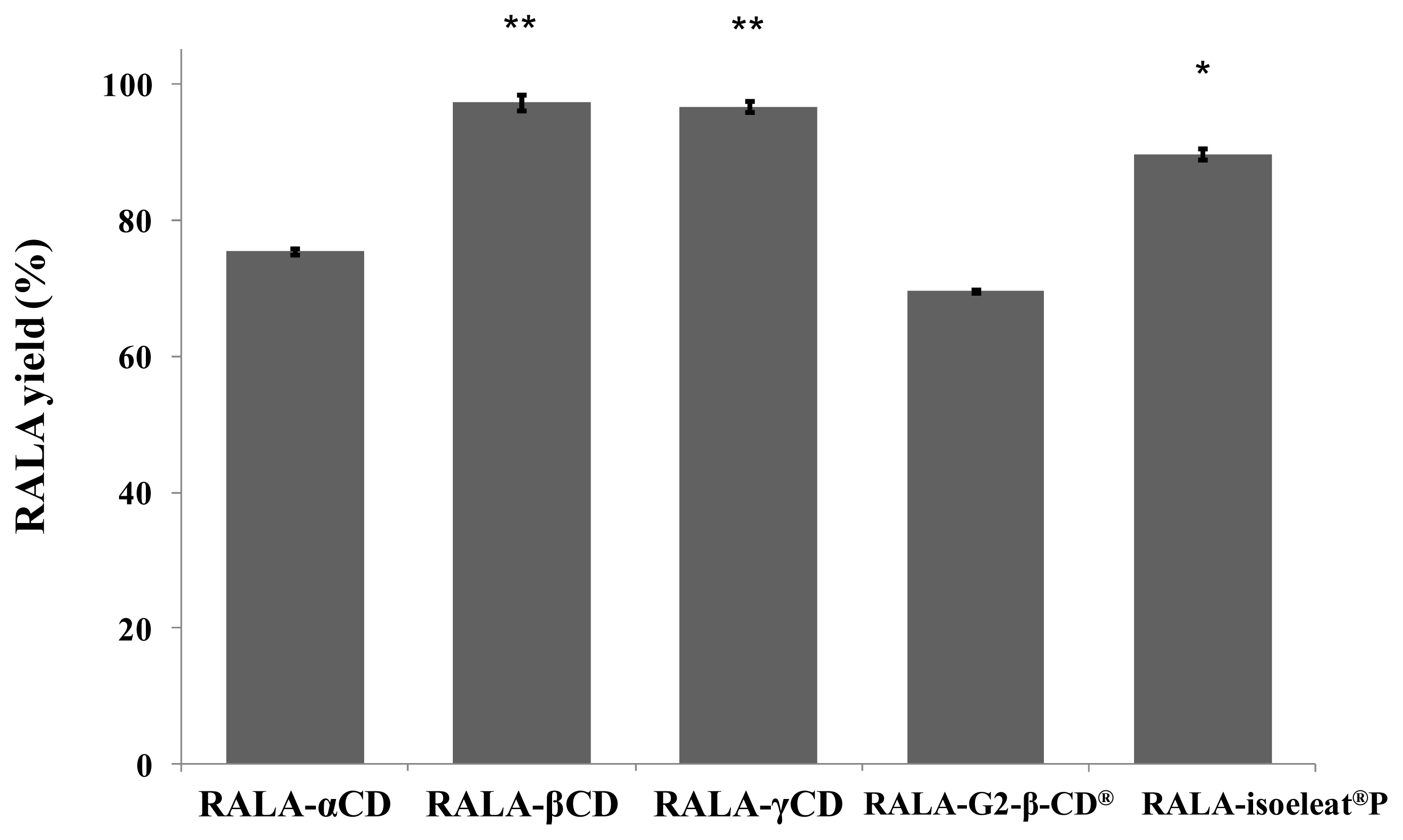
The amount of RALA in the different RALA-CD complex preparations was measured using HPLC, and the results are shown in
Figure 2. The yields are expressed as the percentages of RALA found in the complexes when compared to the amount of RALA used for the preparation of these complexes.
In the case of RALA-βCD and RALA-γCD, the yield was almost 90%, whereas the RALA-αCD and RALA-G2-β-CD
® complexes had a significantly smaller RALA yield of around 75% (
Figure 2).
As mentioned in section 2.1, the RALA polymer shows no peak in the DSC scan, and therefore, polymerized RALA that could have been generated in the manufacturing process of the complex cannot be detected by the analytical methods used. However, it is likely that the missing RALA has been polymerized during complex generation.
Our results show that β-CD and γ-CD are more suitable for preparing RALA complexes than α-CD, G2-β-CD® and isoeleat®P and that only a small amount of RALA is lost during the preparation of these complexes. There was a statistically significant difference between β-CD or γ-CD and the other CD complexes. Isoeleat®P also showed a significantly higher RALA yield than α-CD and G2-β-CD®. However, complex formation with isoeleat®P, α-CD or G2-β-CD® is less efficient than β-CD and γ-CD, and a substantial amount of RALA seems to get lost in the manufacturing process of these complexes. This could be caused by RALA polymerization during complex preparation or by other factors that hinder a complete and stable complex formation of RALA with α-CD, G2-β-CD® or isoeleat®P.
In these experiments, we have shown that the percentage of lost RALA raw material during the process of complex formation is smallest in the case of RALA-βCD and RALA-γCD.
2.3. Thermal Stability of RALA-CD Complexes
As mentioned in the introduction, RALA is unstable and easily polymerized when heated or exposed to light [
7].
In this thermal stability test, RALA, NaRALA and RALA-CD complexes were exposed to 100% relative humidity for 30 min, 1 h, 2 h, 5 h, 24 h or 48 h at 25 °C, which is below the melting point of RALA, or 70 °C, which is above the melting point of RALA. After incubation, the remaining RALA was measured by HPLC.
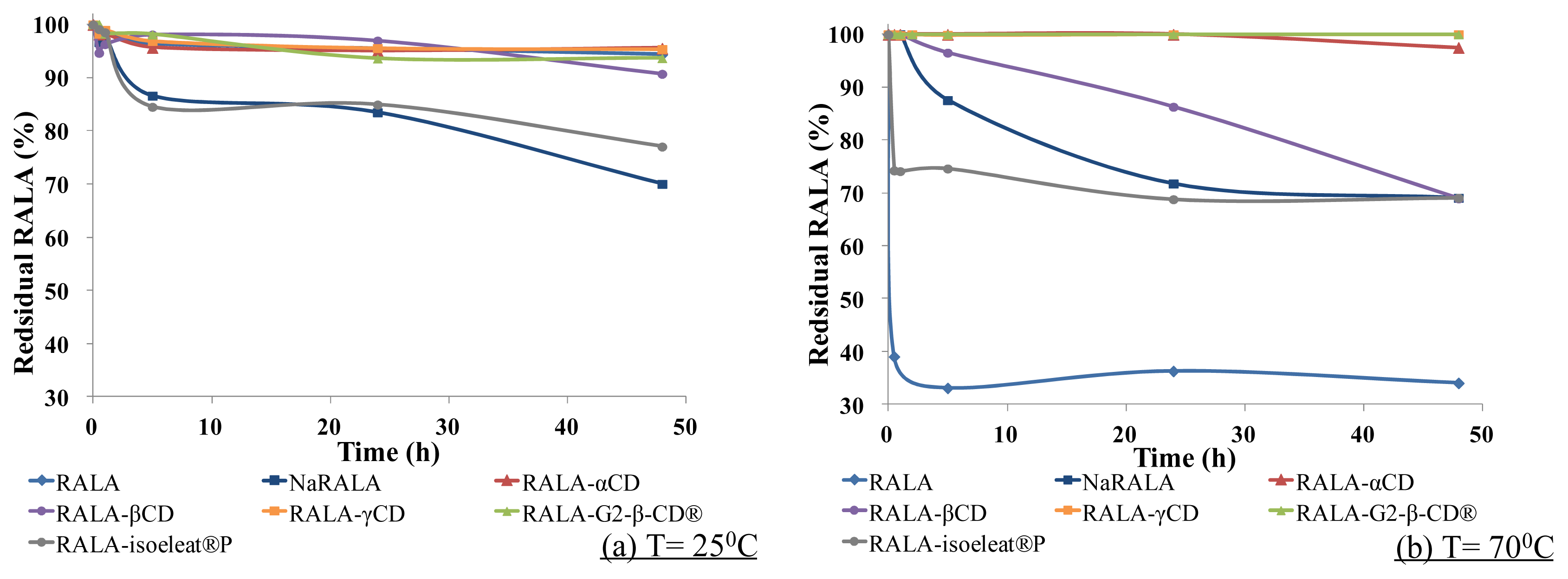
As shown in
Figure 3a, at 25 °C (<melting point), free RALA was stable even under the humidity conditions. However, NaRALA, which is called “stabilized RALA” in the U.S., decomposed faster than free RALA, and the residual NaRALA was ca. 70% after 48 h.
The RALA-isoeleat®P complex was only slightly more stable than NaRALA, but the other complexes, RALA-αCD, RALA-βCD, RALA-γCD and RALA-G2-β-CD®, stabilized RALA, and more than 95% of the RALA remained in these complexes after 48 h at 25 °C/100% relative humidity.
As shown in
Figure 3b, at 70 °C (>melting point)/100% relative humidity for 5 h, almost 70% of the free RALA had decomposed. Although NaRALA was more stable than free RALA under these conditions, a 13% loss of NaRALA was observed after 5 h and a 30% loss after 24 h. While the complex formation with α-CD, γ-CD and G2-β-CD
® improved RALA stability greatly with the recovery rates ranging from 97% (RALA-αCD) to almost 100% (RALA-γCD and RALA-G2-β-CD
®) after 48 h, β-CD also improved RALA stability, albeit to a lesser extent. After 5 h, the RALA recovery was 96% for the RALA-β-CD complex, but the residual RALA decreased gradually over time, and after 48 h, barely 70% of the RALA was recovered (
Figure 3b). However, although the residual percentage of RALA was higher after complex formation with isoeleat
®P than in the non-complexed condition, the isoeleat
®P complex does not withstand heat and humidity any better than the sodium salt. Over 5 h, the residual RALA in the RALA-αCD, RALA-γCD and RALA-G2-β-CD
® complexes did not change. However, the amount of remaining RALA in the RALA-βCD and RALA-isoeleat
®P complexes decreased.
A possible explanation for the lower amount of remaining RALA in the RALA-isoeleat®P complex could be that this complex is less thermostable than the other cyclodextrin complexes. In the DSC (see Section 2.1), RALA-isoeleat®P was the only complex that showed a peak within the analyzed temperature range, indicating instability towards heat. In turn, the complexes without a peak in their DSC scans (RALA-αCD, RALA-βCD, RALA-γCD and RALA-G2-β-CD®) ameliorate the RALA yield after heat and humidity exposure, also when compared to NaRALA.
These results suggest that complex formation of RALA with the cyclodextrins α-CD, β-CD, γ-CD and G2-β-CD® improves its thermal stability and its stability towards humidity.
These findings are in line with prior reports on improving racemic ALA stability regarding heat exposure through complex formation with cyclodextrins [
13]. However, we were able to specifically analyze the remaining enantiomer RALA after exposure to high temperatures and have intensified the destabilizing treatment by incubating at 100% relative humidity.
Polymerization of RALA occurs via the S–S bond in the 1,2-dithiolane ring [
7], and it is possible that the complex formation of RALA by cyclodextrins protects this S–S bond, thereby making the acid more stable.
Due to the poor stability of enantiopure RALA, the racemate containing SALA is sold, although RALA is the bioactive lipoic acid. Attempts to stabilize commercially available RALA have been fairly successful, as in the case of NaRALA, which is somewhat more stable than free RALA [
10], although we have shown that complex formation with CDs, in terms of stability, is superior to the salt formulation.
As there seems to be a method to stabilize enantiopure RALA, further studies investigating the bioavailability of pure RALA in comparison to the racemic ALA and SALA would be possible. This is of especially high interest, as it is known that other racemic bioactive compounds, as for example vitamin E, differ in their kinetic, toxicological and biological properties [
14]. It has been demonstrated that the naturally occurring RRR-α-tocopherol has a higher biopotency than the synthetically produced all-rac tocopherol and that the other α-tocopherol stereoisomers are bioactive, albeit to a lower extent than RRR-α-tocopherol [
15,
16]. To our knowledge, there is no data on the different RALA/SALA bioavailabilities and bioactivities. However, considering the widespread use of α-lipoic acid as a drug and food supplement [
4], it seems very important to gain such knowledge and carry out relevant studies.
2.4. Stability of RALA-CD Complexes towards Low pH
Apart from studying the stability of the RALA-CD complexes towards heat exposure, where α-CD, β-CD, γ-CD and G2-β-CD® provided better protection against RALA degradation than isoeleat®P, we additionally analyzed their stability under acidic conditions. Similar to heat and humidity, low pH also favors RALA polymerization, therefore leading to poor absorption in the stomach. In order to imitate the acidic environment in the stomach, we exposed free RALA and the RALA-CD complexes to pH 1.2 and incubated the samples at 37 °C for 1 h.
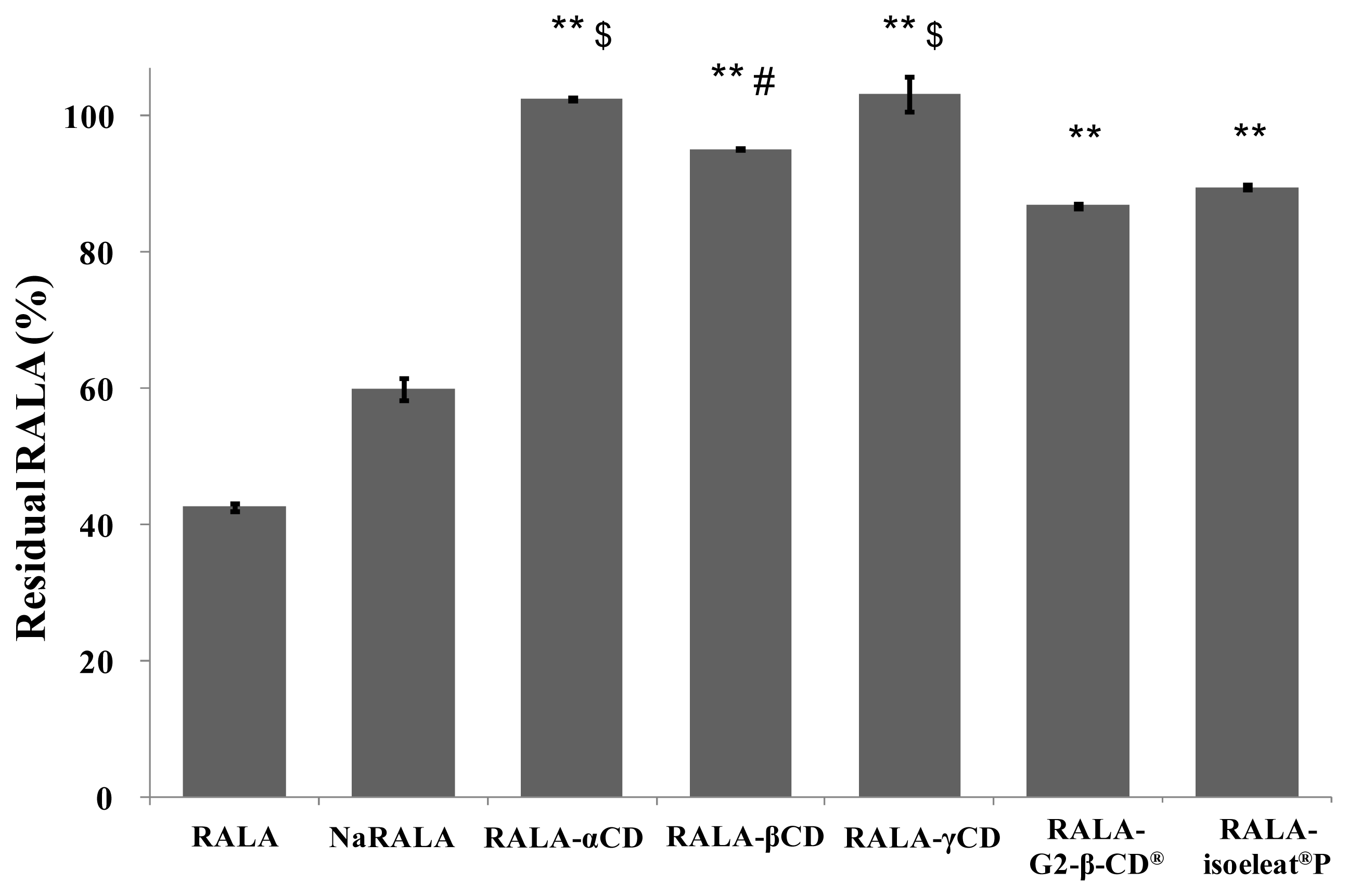
Figure 4 shows the remaining RALA percentages of free RALA, NaRALA and the RALA-CD complexes after incubation with the stomach acid imitation. While free RALA was very unstable (43% of the incubated RALA remained) and NaRALA was only slightly more stable (60% of the incubated RALA resisted degradation) than RALA, complex formation with any of the tested cyclodextrins improved the RALA yield significantly. However, RALA-αCD and RALA-γCD showed the highest stabilization of the incorporated RALA, which was almost 100%. The differences between RALA-αCD, RALA-βCD and RALA-γCD compared with RALA-G2-β-CD
® and RALA-isoeleat
®P were statistically significant, whereby RALA-αCD and RALA-γCD were also more stable than RALA-βCD (statistically significant). Additionally, these results indicate that G2-β-CD
®, maltosyl-β-CD and isoeleat
®P (a mixture of α-, β- and γ-CDs, maltosyl-α-, -β- and -γ-CDs) are water-soluble cyclodextrins, and their decreased stability under acidic conditions could be due to being dissolved as opposed to being in suspension.
Interestingly, the imitated stomach acid samples had a different physical appearance depending on the RALA formulation they contained. While non-complexed RALA polymerizes in the acidic environment, the RALA-γCD complex remains suspended and no signs of polymerization are observed. Similar effects are seen for the other RALA-CD complexes (data not shown).
In these experiments, we have been able to show that complex formation with cyclodextrins, and in particular with α-CD and γ-CD, stabilizes RALA under acidic conditions. Similar to what we have demonstrated regarding the NaRALA heat and humidity stability that is inferior to RALA-CD complexes, RALA in RALA-CD complexes are significantly more stable towards low pH than the sodium salt.
2.5. Scanning Electron Microscopy of the Different RALA-CD Complexes
Having found differences regarding RALA stability in the studied RALA-cyclodextrin complexes, we also analyzed the different complexes using SEM. These experiments showed that the shape and aspect of the complex particles differed considerably and depended on the cyclodextrin used for complex formation.
Figure 5 shows SEM images of CDs, RALA + CD physical mixtures and RALA-CD complexes. CDs and RALA + CD physical mixtures contain particles that appear cracked and wrinkled, their shapes are uneven and particle sizes vary considerably in the physical mixtures, with the maximum RALA + CD physical mixture particle size exceeding 50 μm. There was no considerable change observed between the CD and its physical mixture with RALA in SEM analysis.
This observation was confirmed in three different fields within each sample of which images with 300-, 500-, 1000- and 5000-times magnification were taken.
Figure 5 shows the highest magnification (5000). Contrarily, the particle size distribution of RALA-αCD, RALA-βCD and RALA-γCD complexes appears more homogenous than in the RALA + CD physical mixtures. Particles of RALA-CD complexes form larger aggregates, which seem to pile up.
Additionally, the RALA-βCD and RALA-γCD complex crystals are shaped like prisms with parallel sides, and in the case of the rod-shaped RALA-γCD particles, there are tetragonal and orthorhombic crystals to be seen in the SEM images. While the RALA-βCD and RALA-γCD complex particles have rather smooth surfaces, the particles that constitute the RALA-αCD complex are rougher, and the shapes formed by these crystals seldom show square and parallel surfaces. These results show that the morphological characteristics of RALA-CD complexes are different from CDs or RALA + CD physical mixtures, and it appears that RALA-CD complex particles form crystals during their manufacturing process.
The RALA complexes with G2-β-CD
® and isoeleat
®P form particles with smooth and flat surfaces, although their shapes are not symmetrical, nor do they have parallel surfaces. The particle sizes are also much bigger for RALA-G2-β-CD
® and RALA-isoeleat
®P complexes than for RALA-αCD, RALA-βCD and RALA-γCD complexes (
Figure 5).
To further examine the particle morphology, we conducted a particle size distribution analysis of the CDs, physical mixtures and RALA-CD complexes using a particle size distribution analyzer (
Figure 6). In this measurement, α-CD showed a single peak, and the mean median diameter was 43 μm. The RALA + αCD physical mixture also showed only one peak, and the mean median diameter was 40 μm. Contrarily, RALA-αCD showed two peaks, one at a smaller diameter (13 μm) and one at a larger diameter (77 μm) than the peak of α-CD.
RALA-βCD showed one sharp peak at a smaller diameter than the peak of β-CD itself. On complexing RALA, the particle size peak for β-CD shifted from 152 μm to 6 μm for the RALA-βCD complex, thereby differing considerably from the peaks and type of peak shifts observed for complexation with α-CD. The RALA + γCD physical mixture showed one peak with an average mean diameter of 52 μm. This average mean diameter is almost the same as for γ-CD on its own (55 μm). The mean median diameter of the RALA-γCD complex was 76 μm. In the case of γ-CD, there did not seem to be great differences between the cyclodextrin on its own, the physical mixture or the complex concerning the particle size distribution pattern, although the SEM images showed considerable morphology changes upon physical mixing and complexation.
In order to confirm the difference of the particle size distributions of RALA-γCD and γ-CD or RALA + γCD physical mixture, we carried out an F-test and t-test. The F-test results revealed that the particle size distributions of RALA-γCD and γ-CD or the physical mixture were not significantly different. Next, a t-test clarified that the mean particle size of RALA-γCD and γ-CD or the physical mixture were completely different. The SEM images of RALA-γCD showed a characteristic rod shape, which was not observed in the cases of γ-CD or the RALA + γCD physical mixture. These results can be explained by considering the difference of the mean particle size of RALA-γCD, RALA + γCD physical mixture and γ-CD itself.
The particle size distribution measurement showed different results for RALA-γCD compared to RALA-αCD and RALA-βCD. On the one hand, in the case of the αCD- and βCD-complexes, the particle size distributions changed when the cyclodextrin complexed RALA. This change is consistent with differences observed between the complexes and the physical mixtures in the SEM images. On the other hand, in the case of γ-CD, the particle size distributions seem to remain unchanged upon complexation, although differences between the mean particle size and the SEM images can be clearly observed. These results suggest that the manufacturing process affected the particle size distributions of RALA-αCD and RALA-βCD, but did not affect that of RALA-γCD. Nevertheless, the manufacturing process influenced the particle shapes for all CD complexes and affected the stability in the DSC analysis.
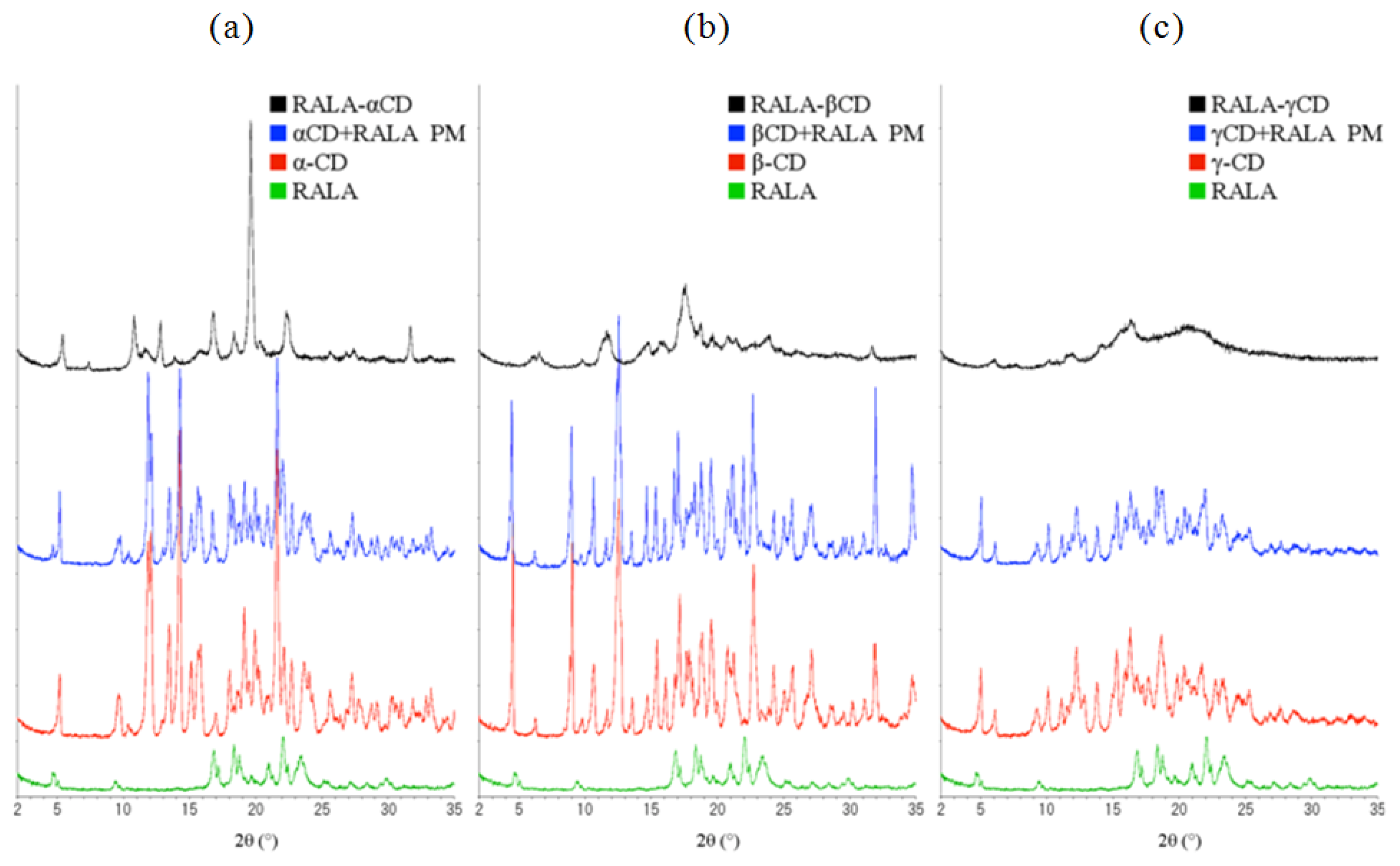
Figure 7 shows the X-ray diffraction patterns of α-, β- and γ-CD compared with free RALA, the physical mixture of RALA with the different CDs and the RALA-CD complexes.
As can be seen in
Figure 7, there are four characteristic diffraction peaks for RALA at 2θ = 17, 18.5, 21 and 22. α-, β- and γ-CD differed from each other in their diffraction patterns, and the physical mixture diffraction patterns appeared as the addition of CD and RALA patterns.
Contrarily, complexation of RALA with the CDs changed the XRD patterns when compared to the physical mixtures. The RALA-αCD complex showed one large peak at 2θ = 19.6 (d = 4.53), which was not observed in the physical mixture. RALA-βCD showed fewer and smaller peaks than its physical mixture. RALA-γCD showed a broad diffraction profile, and the peaks observed for free RALA, CD or its physical mixture was absent.
The DSC, SEM studies and XRD analysis have shown that RALA forms complexes with all tested cyclodextrins and that these complexes have different physicochemical properties.
Cyclodextrins have been used for complex formation of various bioactive or pharmacologically active compounds, such as drugs and vitamin-like substances, thereby enhancing the bioavailability of these molecules [
17–
21]. Some of these studies also found that the physicochemical properties depended on the method used for complex formation [
20]. In further experiments, it would be interesting to investigate whether different complex morphologies lead to different bioavailabilities
in vivo.