Stabilized Conversion Efficiency and Dye-Sensitized Solar Cells from Beta vulgaris Pigment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Absorption Spectra
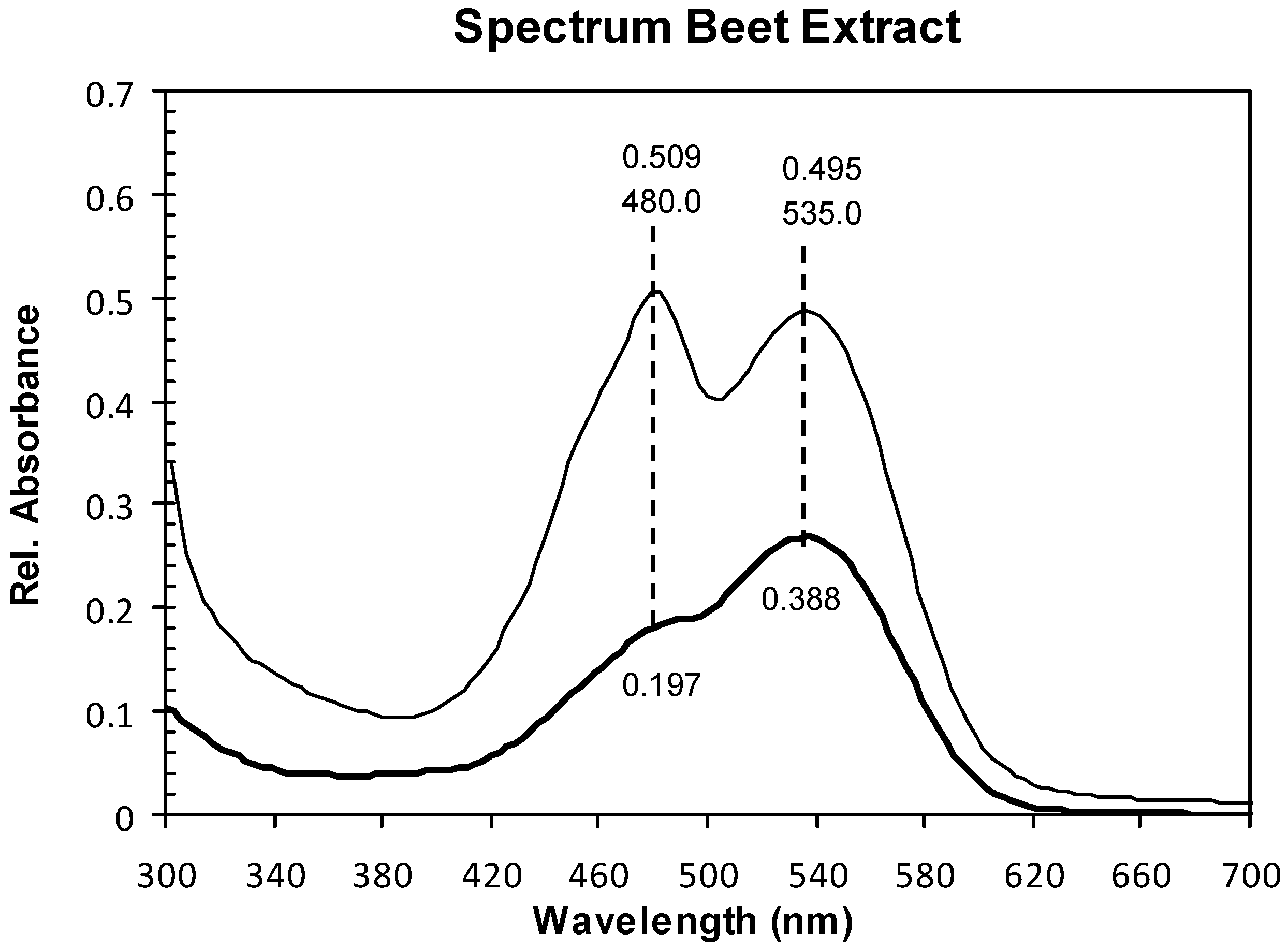
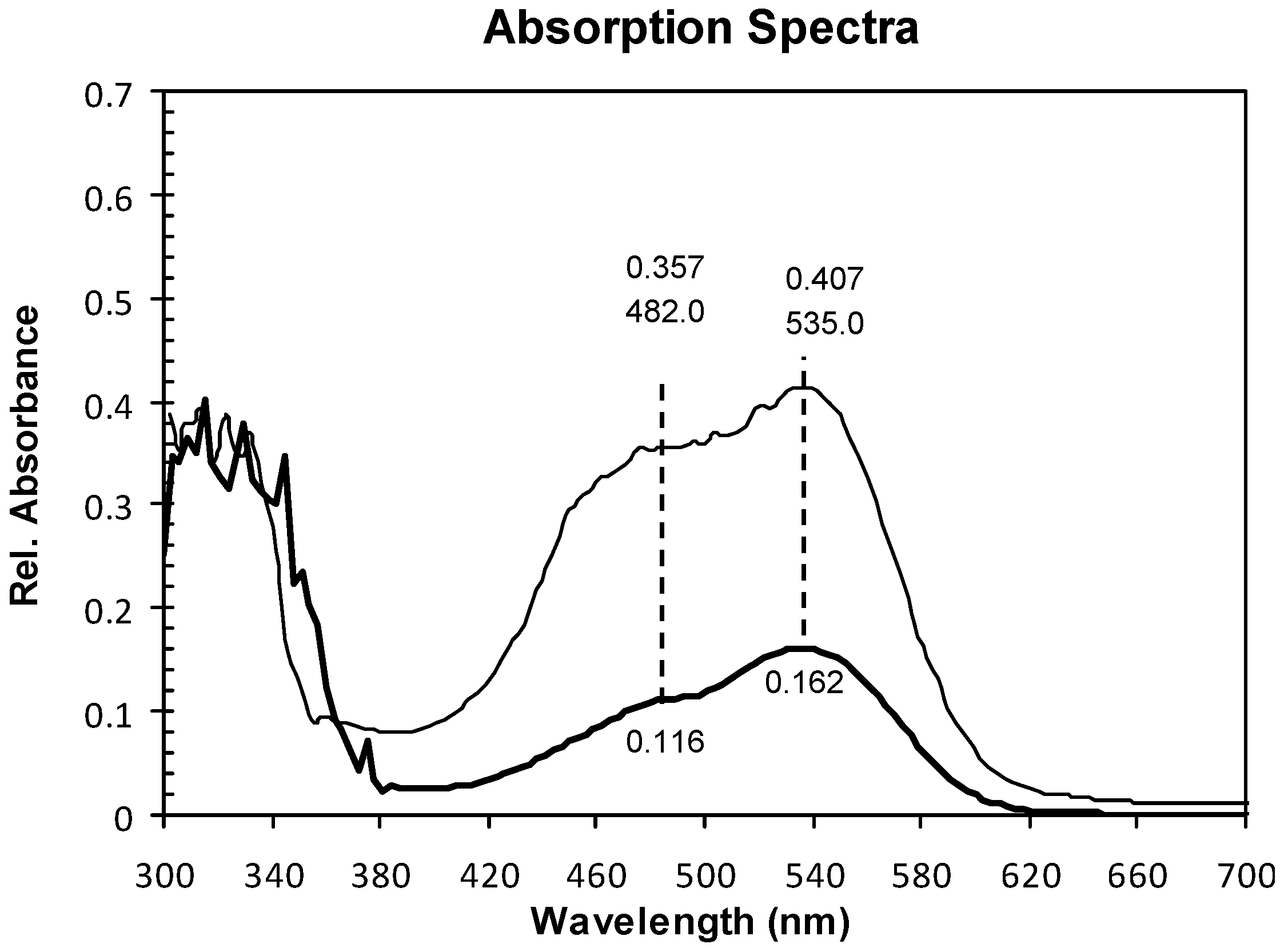
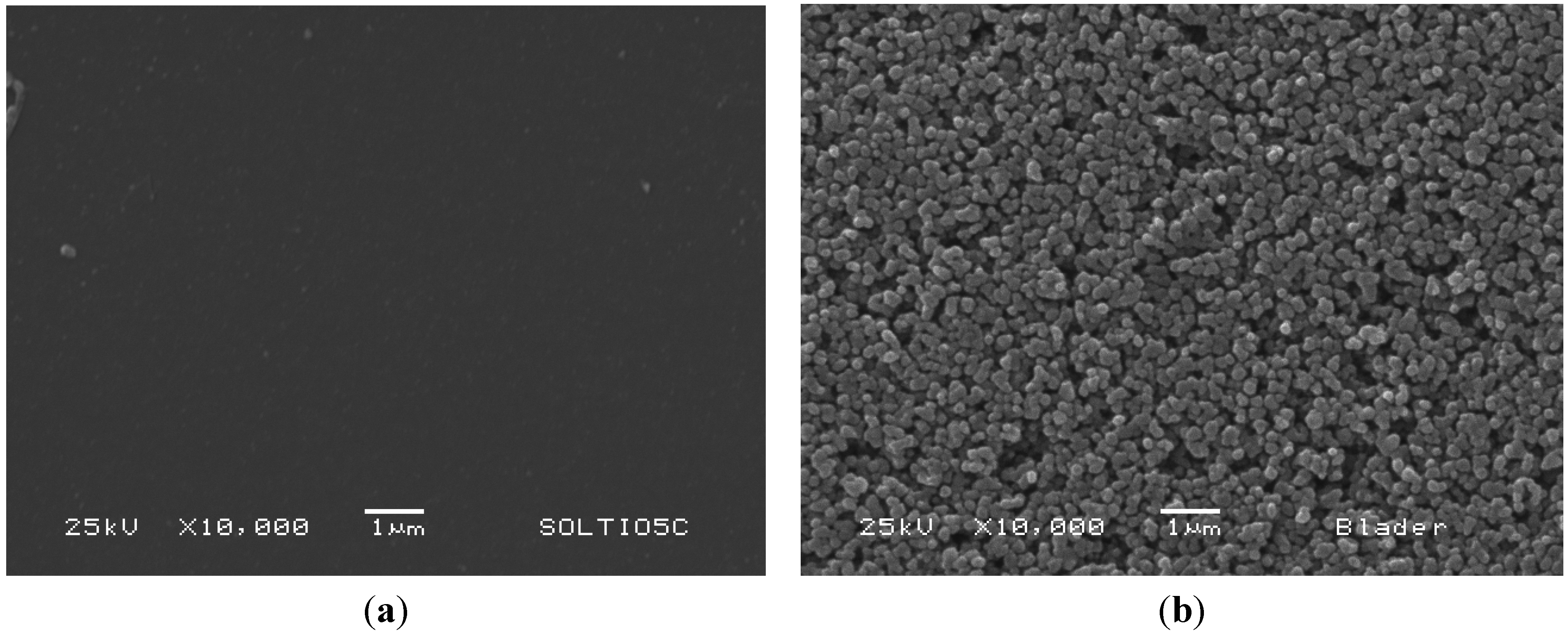
2.2. Structure and Surface Characterization
2.3. Photoelectrochemistry
| Jsc (mA/cm2) | JMP (mA/cm2) | VOC (V) | VMP (V) | PM (mW) | PT (mW) | FF | η (%) | |
|---|---|---|---|---|---|---|---|---|
| BVE | 2.71 ± 0.003 | 1.98 ± 0.003 | 0.576 ± 0.0002 | 0.451 ± 0.0002 | 0.89 ± 0.006 | 1.561 ± 0.007 | 0.572 ± 0.003 | 0.89 ± 0.006 |
| BVE/TEOS | 2.08 ± 0.003 | 1.61 ± 0.003 | 0.609 ± 0.0002 | 0.422 ± 0.0002 | 0.68 ± 0.005 | 1.266 ± 0.006 | 0.537 ± 0.003 | 0.68 ± 0.005 |




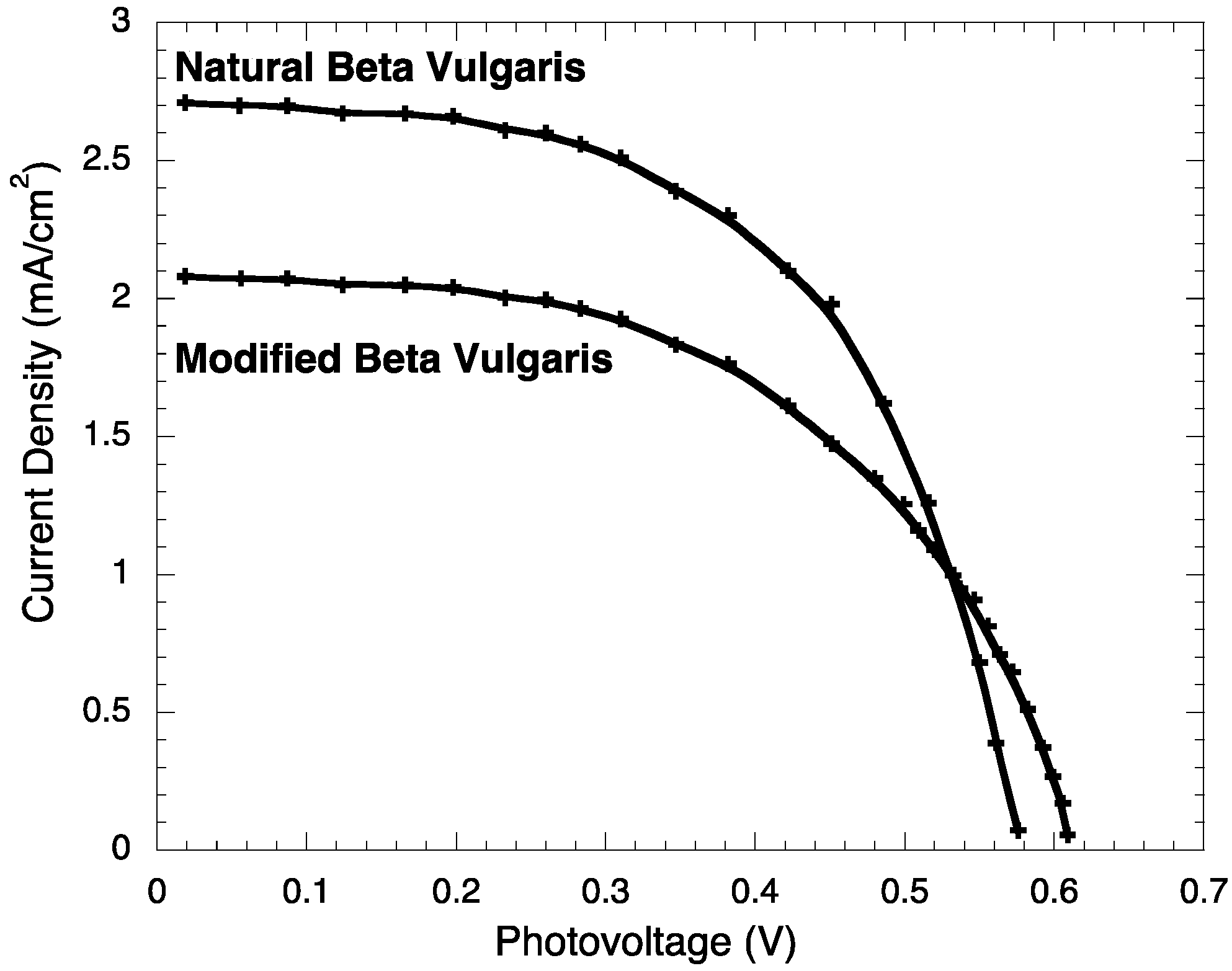
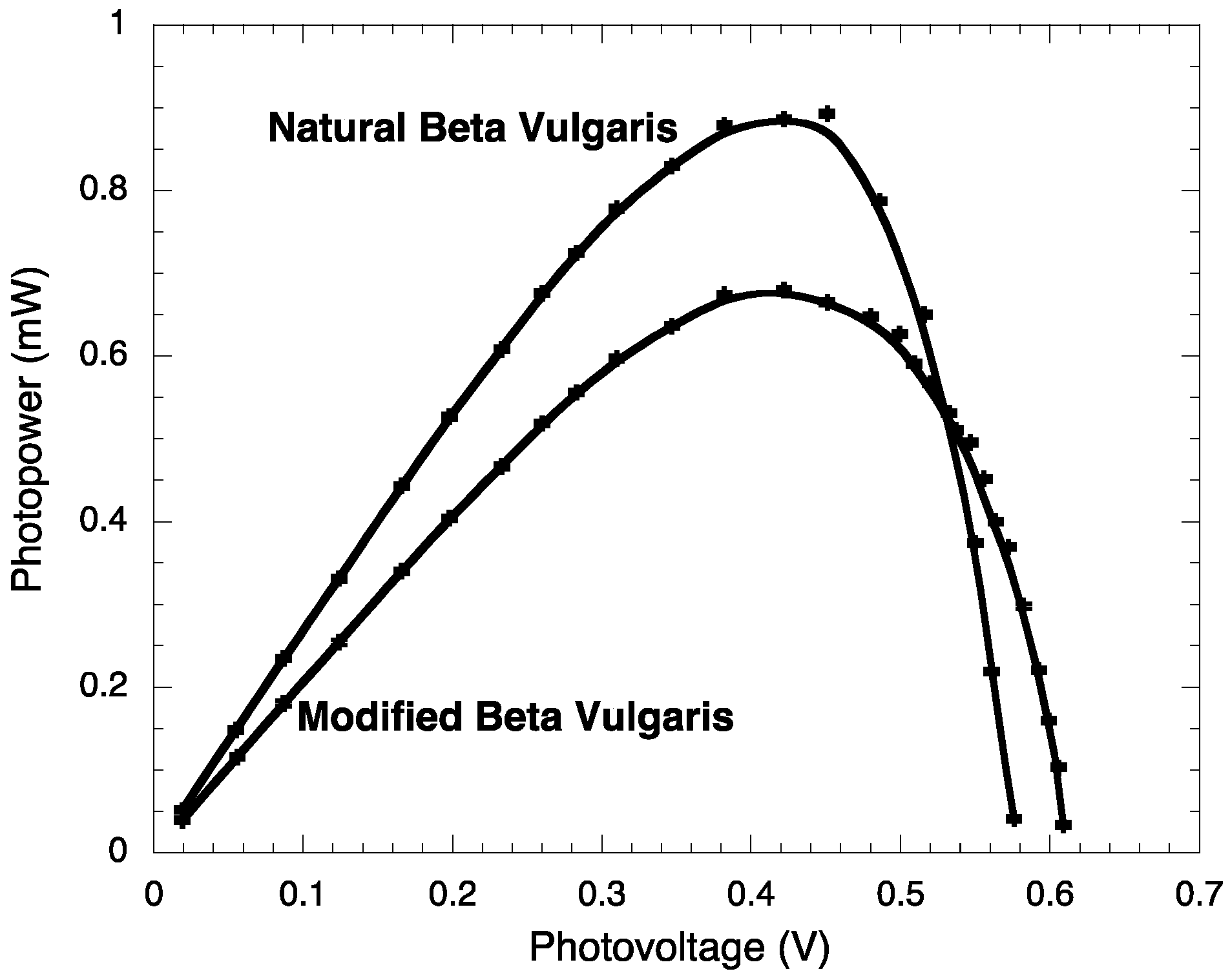
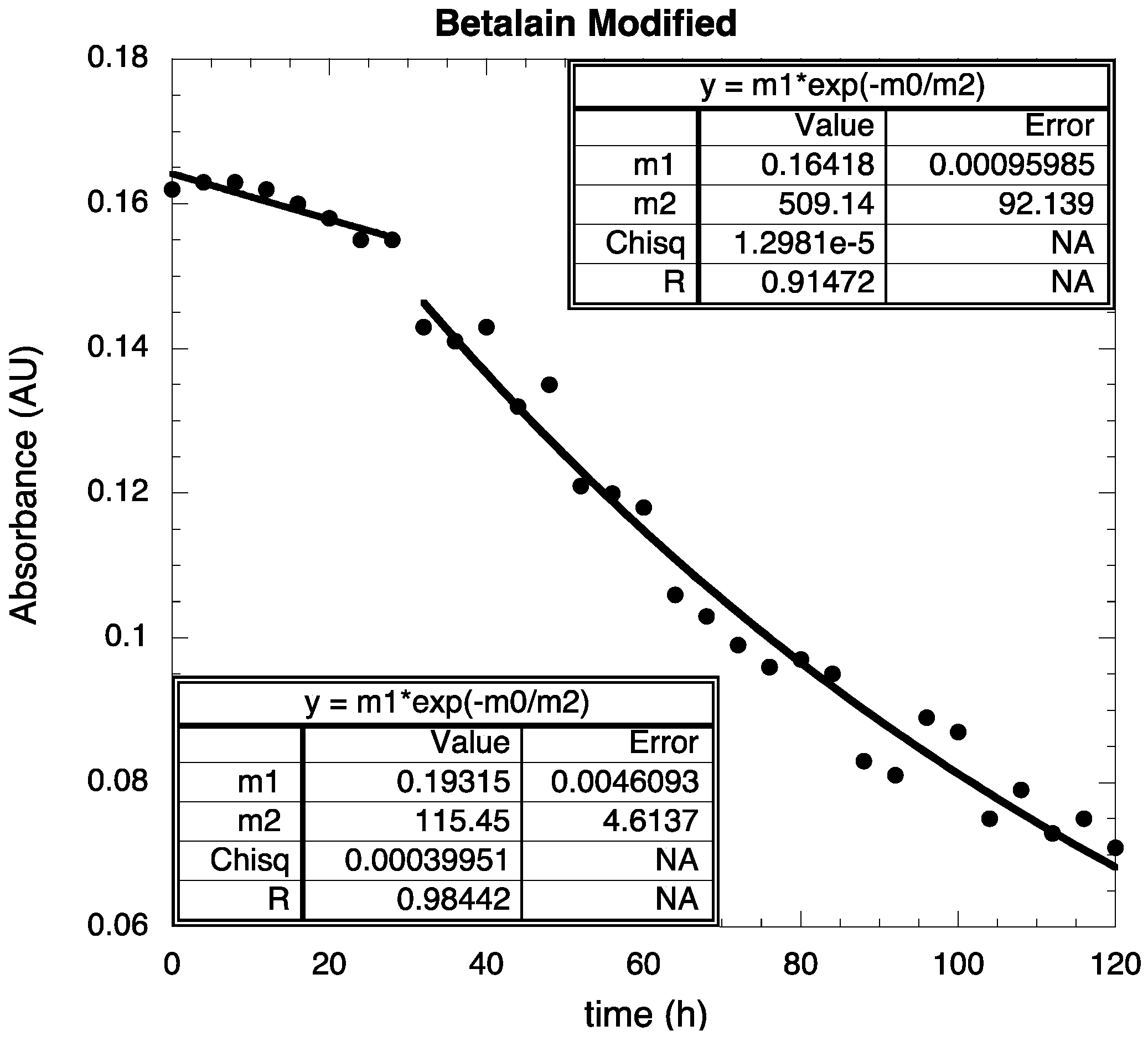
2.4. Stability Test
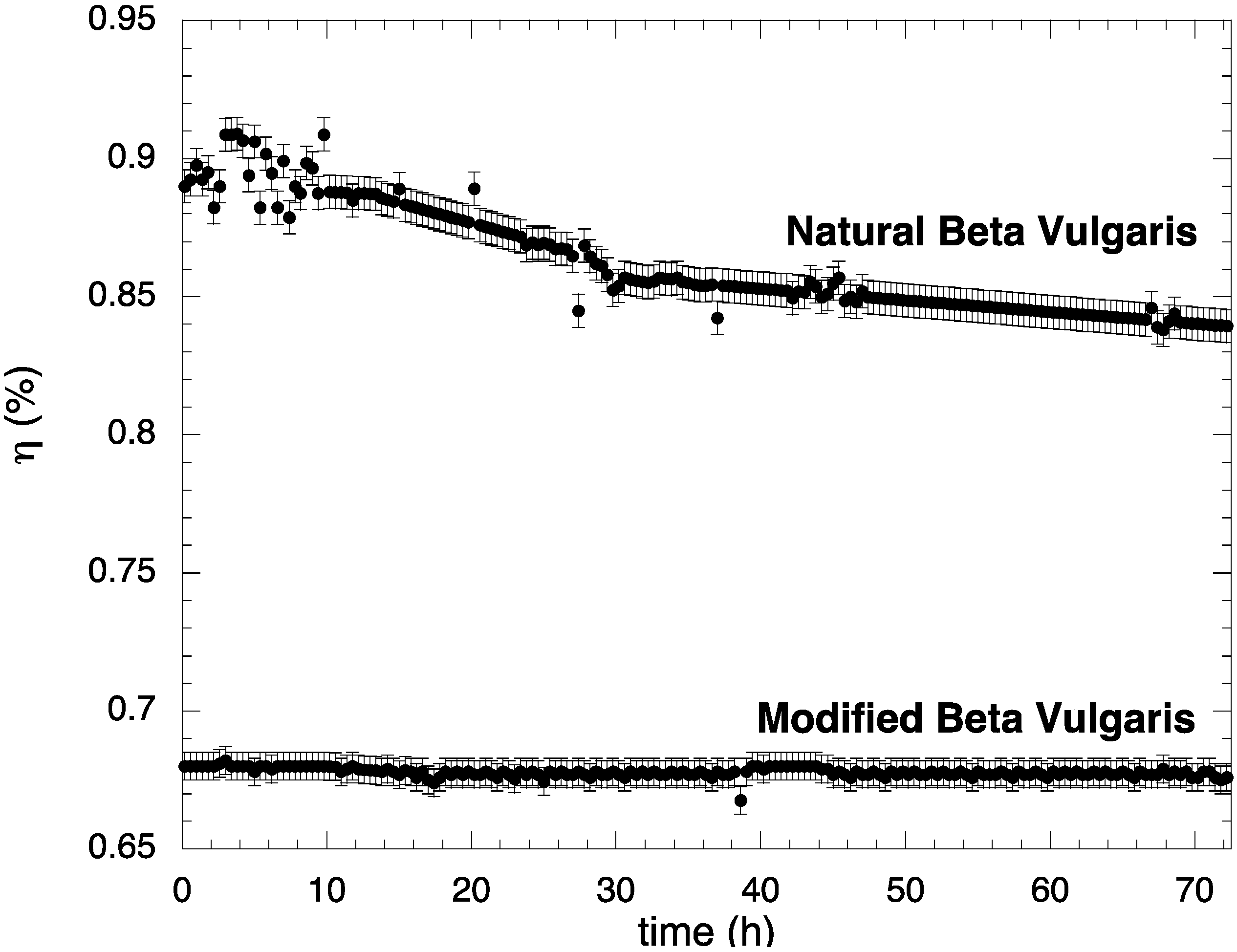
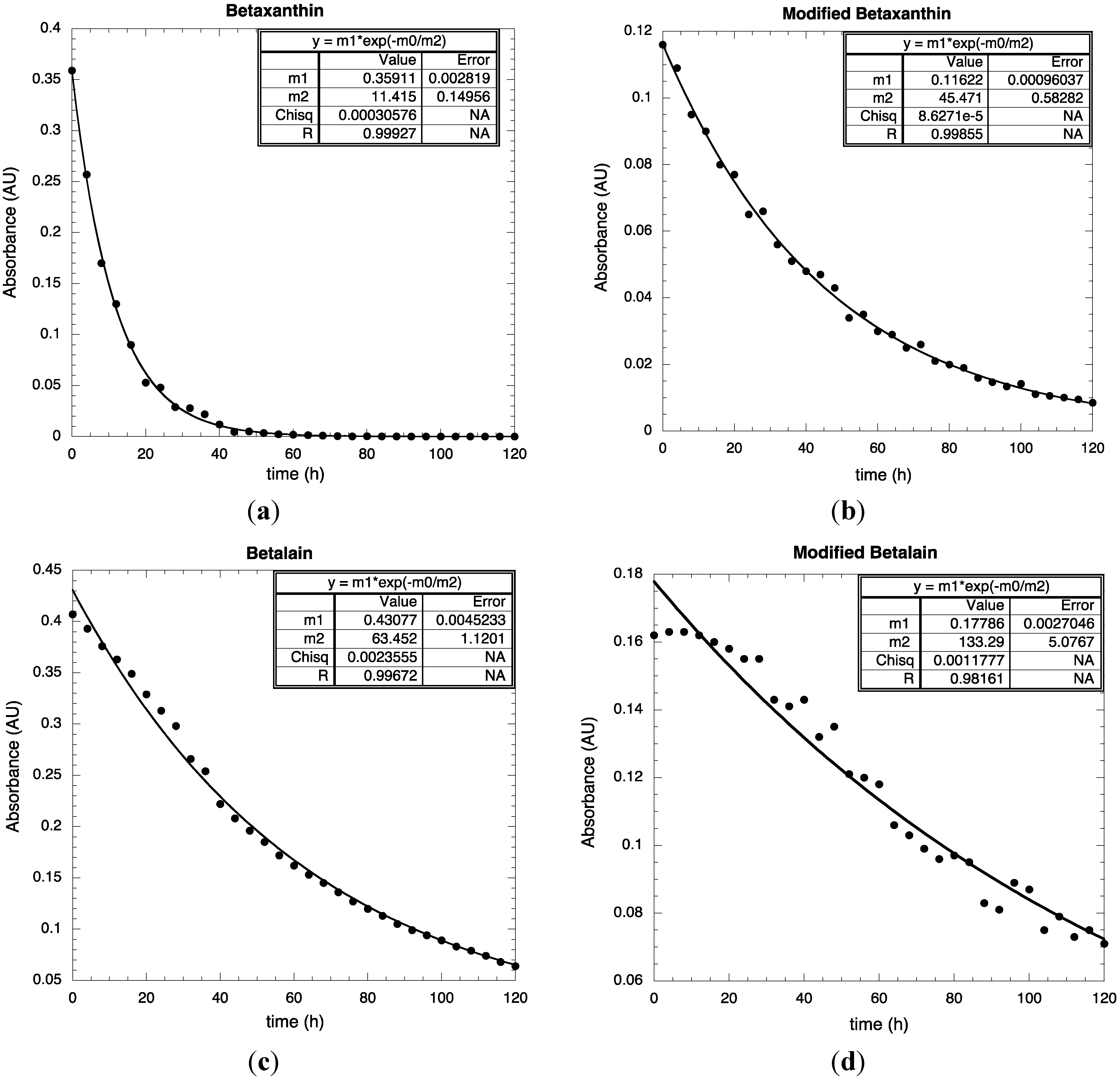
3. Experimental Section
3.1. Materials
3.2. Preparation of BVE/TEOS
3.3. Electrodes Preparation
3.4. DSSC Assembling
3.5. Characterization Techniques
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Calogero, G.; Di Marco, G.; Cazzanti, S.; Caramori, S.; Argazzi, R.; di Carlo, A.; Bignozzi, C.A. Efficient dye-sensitized solar cells using red turnip and purple wild sicilian prickly pear fruits. Int. J. Mol. Sci. 2010, 11, 254–267. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Mueller, E.; Liska, P.; Vlachopoulos, N.; Graetzel, M. Conversion of light to electricity by cis-X2bis(2,2’-bipyridyl-4,4’-dicarboxylate) ruthenium(II) charge-transfer sensitizers (X = Cl−, Br−, I−, CN−, and SCN−) on nanocrystalline TiO2 electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar]
- Castellar, R.; Obon, J.M.; Alacid, M.; Fernandez-lopez, J.A. Color properties and stability of betacyanins from Opuntia fruits. J. Agric. Food Chem. 2003, 51, 2772–2776. [Google Scholar] [CrossRef]
- Hao, S.; Wu, J.; Huang, Y.; Lin, J. Natural dyes as photosensitizers for dye-sensitized solar cell. Solar Energy 2006, 80, 209–214. [Google Scholar] [CrossRef]
- Tesoriere, L.; Allegra, M.; Butera, D.; Livrea, M.A. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. Am. J. Clin. Nutr. 2004, 80, 941–945. [Google Scholar]
- Zhang, D.; Lanier, S.M.; Downing, J.A.; Avent, J.L.; Lum, J.; McHale, J.L. Betalain pigments for dye-sensitized solar cells. J. Photochem. Photobiol. A 2008, 195, 72–80. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Current Protocols in Food analytical Chemistry; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Zhou, H.; Wu, L.; Gao, Y.; Ma, T. Dye-sensitized solar cells using 20 natural dyes as sensitizers. J. Photochem. Photobiol. A 2011, 219, 188–194. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Smestad, G.P.; Grätzel, M.; Zhang, J.Z. Ultrafast electron injection: Implications for a photoelectrochemical cell utilizing an anthocyanin dye-sensitized TiO2 nanocystalline electrode. J. Phys. Chem. B 1997, 101, 9342–9351. [Google Scholar] [CrossRef]
- Hernandez-Martinez, A.R.; Estevez, M.; Vargas, S.; Quintanilla, F.; Rodriguez, R. New dye-sensitized solar cells obtained from extracted bracts of bougainvillea glabra and spectabilis betalain pigments by different purification processes. Int. J. Mol. Sci. 2011, 12, 5565–5576. [Google Scholar] [CrossRef]
- Hernandez-Martinez, A.R.; Estevez, M.; Vargas, S.; Quintanilla, F.; Rodriguez, R. Natral pigment-based dye-sensitised solar cells. J. Appl. Res. Technol. 2012, 10, 38–47. [Google Scholar]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef]
- Mabry, T.J.; Taylor, A.; Turner, B.L. The betacyanins and their distribution. Phytochemistry 1963, 2, 61–64. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Zhang, D.; Yamamoto, N.; Yoshida, T.; Minoura, H. Natural dye sensitized solar cells. Trans. Mater. Res. Soc. Jpn. 2002, 27, 811–814. [Google Scholar]
- Argazzi, R.; Iha, N.Y.M.; Zabri, H.; Odobel, F.; Bignozzi, C.A. Design of molecular dyes for application in photoelectrochemical and electrochromic devices based on nanocrystalline metal oxide semiconductors. Coord. Chem. Rev. 2004, 248, 1299–1316. [Google Scholar] [CrossRef]
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Polo, A.S.; Itokazu, M.K.; Iha, N.Y.M. Metal complex sensitizers in dye-sensitized solar cells. Coord. Chem. Rev. 2004, 248, 1343–1361. [Google Scholar] [CrossRef]
- Hao, S.; Wu, J.; Fan, L.; Huang, Y.; Lin, J.; Wei, Y. The influence of acid treatment of TiO2 porous film electrode on photoelectric performance of dye-sensitized solar cell. Solar Energy 2004, 76, 745–750. [Google Scholar] [CrossRef]
- Huang, S.Y.; Schlichthörl, G.; Nozik, A.J.; Grätzel, M.; Frank, A.J. Charge recombination in dye-sensitized nanocrystalline TiO2 solar cells. J. Phys. Chem. B 1997, 101, 2576–2582. [Google Scholar] [CrossRef]
- Calogero, G.; Marco, G.D. Red sicilian orange and purple eggplant fruits as natural sensitizers for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 1341–1346. [Google Scholar] [CrossRef]
- Su, C.; Sheu, T.K.; Chang, Y.T.; Wan, M.A.; Feng, M.C.; Hung, W.C. Preparation of ITO thin films by sol-gel process and their characterizations. Synth. Met. 2005, 153, 9–12. [Google Scholar] [CrossRef]
- Islam, A.; Sugihara, H.; Hara, K.; Singh, L.P.; Katoh, R.; Yanagida, M.; Takahashi, Y.; Murata, S.; Arakawa, H. Dye sensitization of nanocrystalline titanium dioxide with square planar Platinum(II) diimine dithiolate complexes. Inorg. Chem. 2001, 40, 5371–5380. [Google Scholar]
- Garcia, C.G.; Polo, A.S.; Iha, N.Y.M. A one-pot diastereoselective synthesis of cis-3-Hexene-1,6-diols via an unusually reactive organozinc intermediate. J. Photochem. Photobiol. A 2003, 160, 87–91. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hernández-Martínez, A.R.; Estévez, M.; Vargas, S.; Rodríguez, R. Stabilized Conversion Efficiency and Dye-Sensitized Solar Cells from Beta vulgaris Pigment. Int. J. Mol. Sci. 2013, 14, 4081-4093. https://doi.org/10.3390/ijms14024081
Hernández-Martínez AR, Estévez M, Vargas S, Rodríguez R. Stabilized Conversion Efficiency and Dye-Sensitized Solar Cells from Beta vulgaris Pigment. International Journal of Molecular Sciences. 2013; 14(2):4081-4093. https://doi.org/10.3390/ijms14024081
Chicago/Turabian StyleHernández-Martínez, Angel Ramon, Miriam Estévez, Susana Vargas, and Rogelio Rodríguez. 2013. "Stabilized Conversion Efficiency and Dye-Sensitized Solar Cells from Beta vulgaris Pigment" International Journal of Molecular Sciences 14, no. 2: 4081-4093. https://doi.org/10.3390/ijms14024081





