Effect of Nanoencapsulated Vitamin B1 Derivative on Inhibition of Both Mycelial Growth and Spore Germination of Fusarium oxysporum f. sp. raphani
Abstract
:1. Introduction
2. Results and Discussion
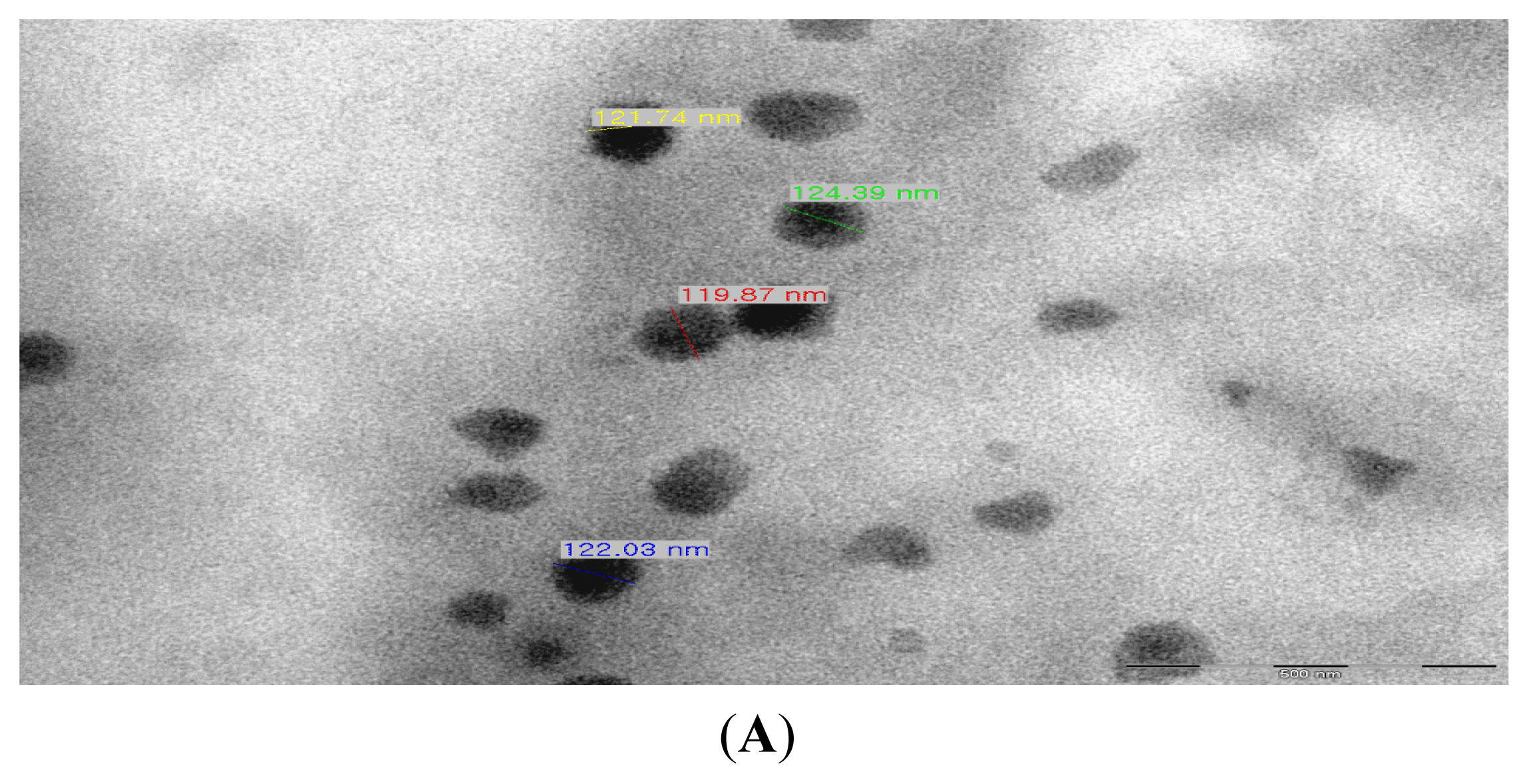
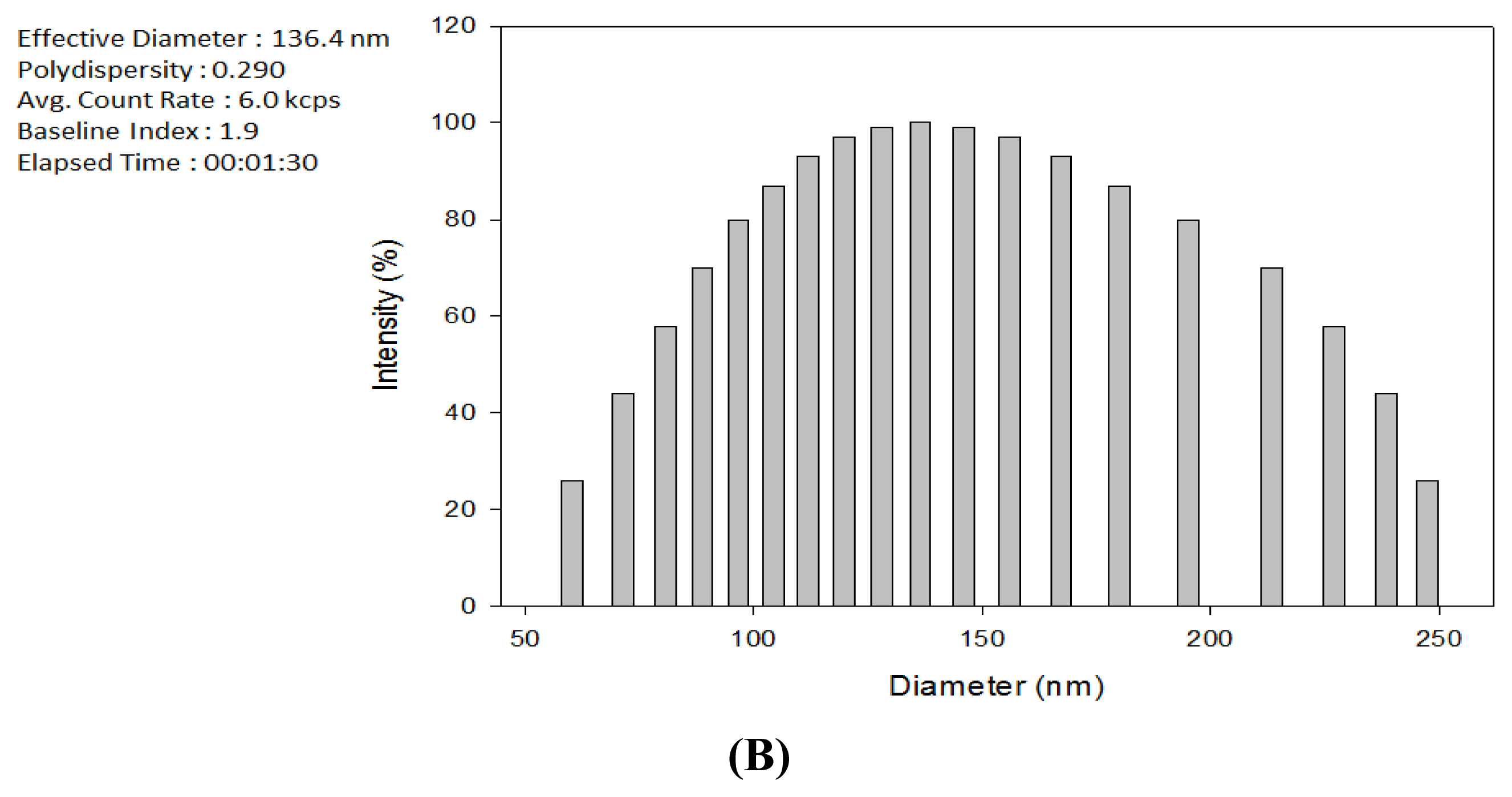
2.1. Production of TDS Nanoparticles and Identification of Their Characteristics
2.2. Encapsulation Efficiency of the TDS Nanoparticle
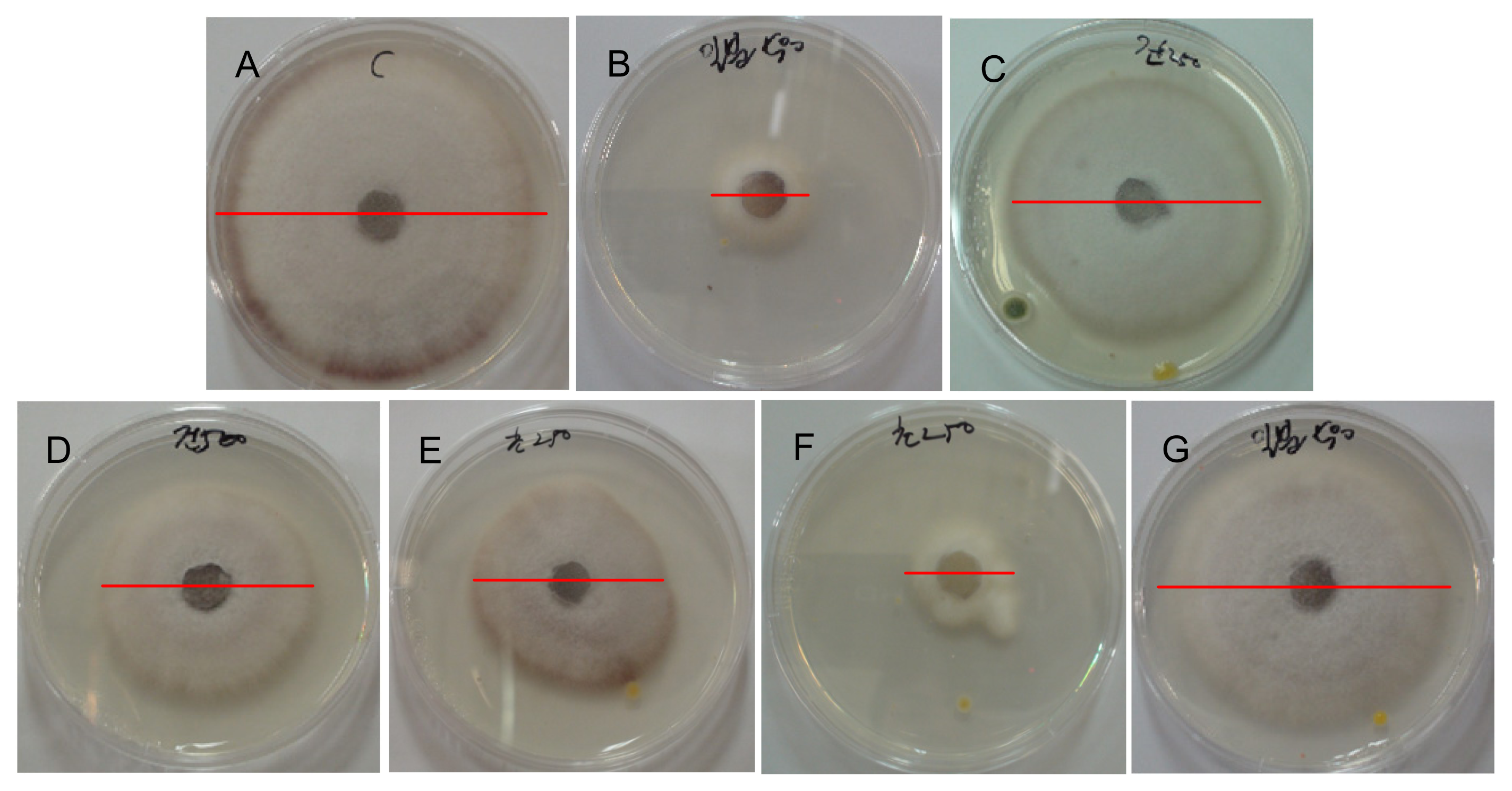
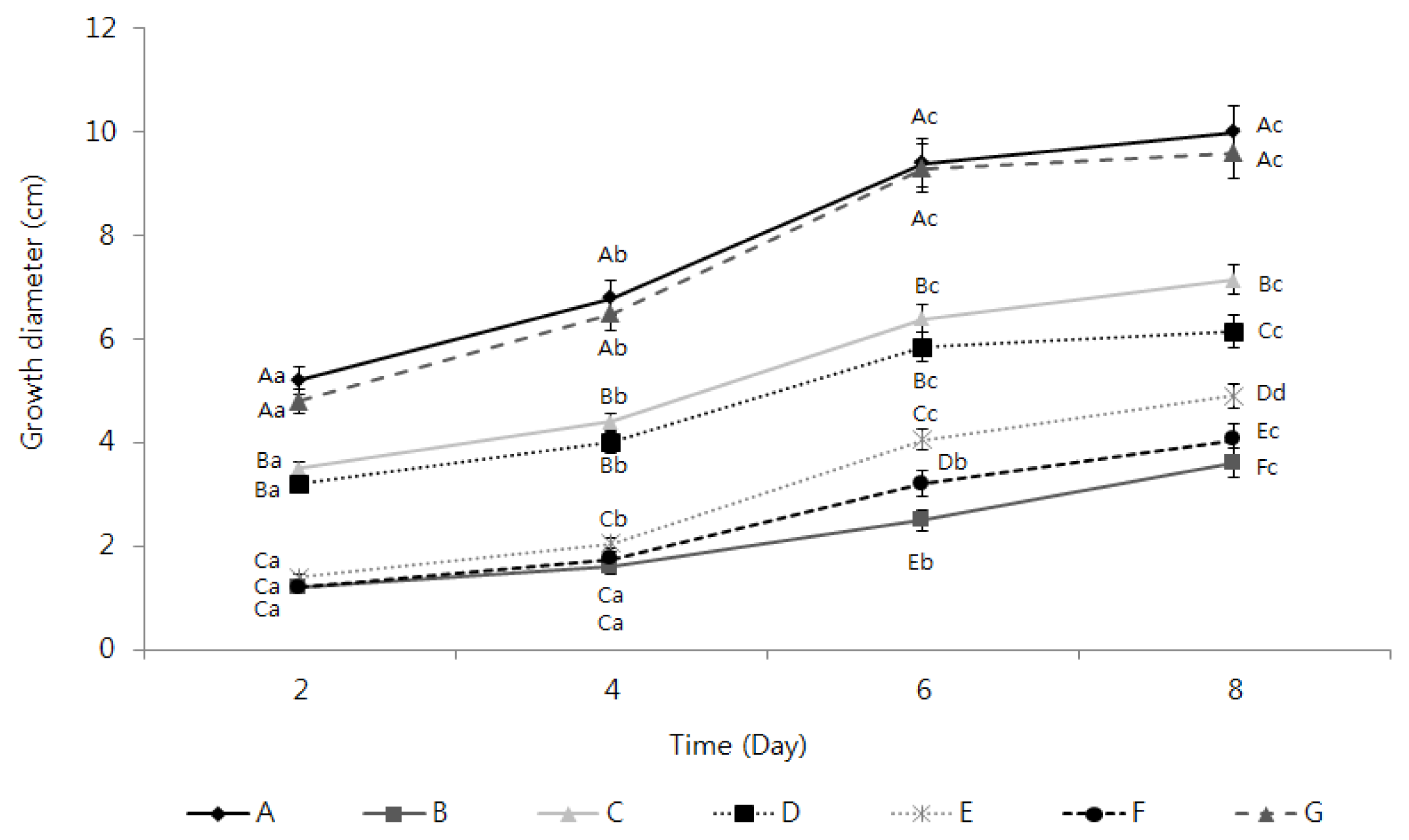
2.3. Activation of the Inhibition of Fusarium oxysporum Mycelia Growth by the TDS Nanoparticle Solution
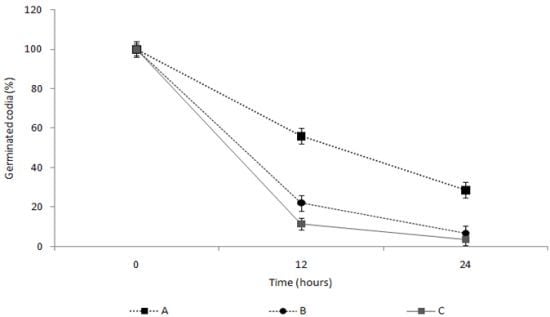
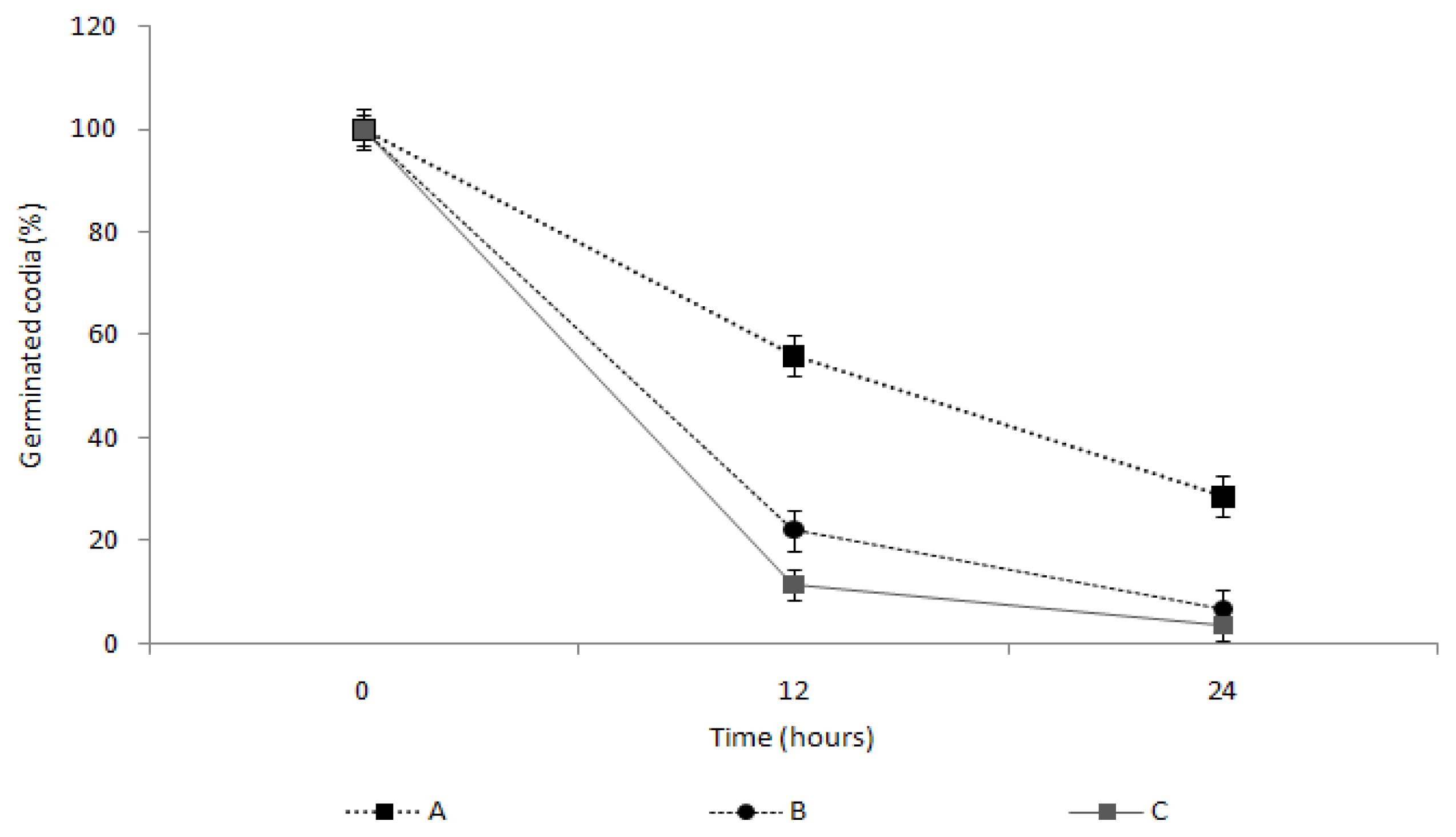
2.4. Measurement of the Spore Germination Inhibition Effect of the TDS Nanoparticle Solution
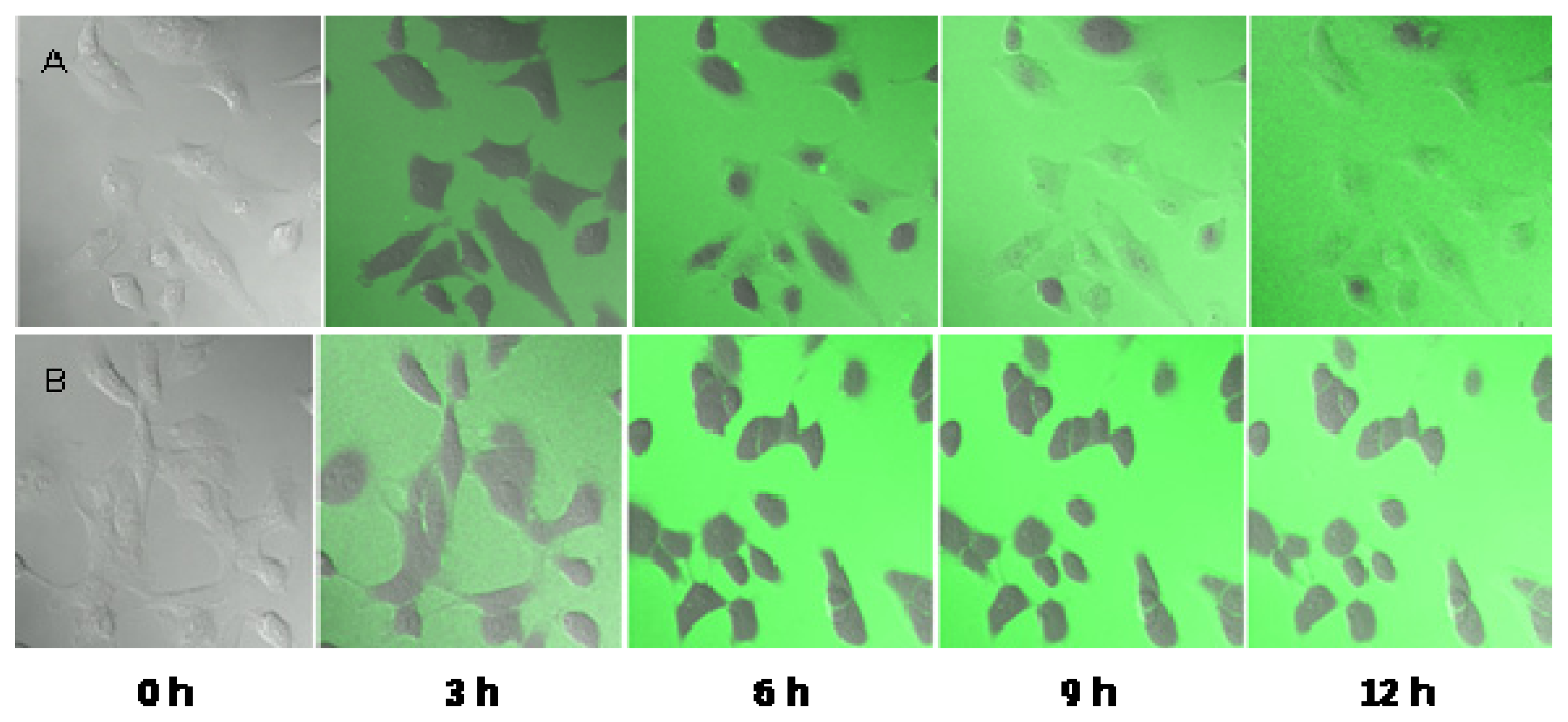
2.5. Observation of the Spore Extinction Effect of the TDS Nanoparticle Solution
3. Experimental Section
3.1. Cultivation of White Radish
3.2. Fusarium oxysporum f. sp. raphani Spores
3.3. Production of TDS Nanoparticle Solution
3.4. Observation of the TDS Nanoparticles
3.5. Measurement of Encapsulation Efficiency of the TDS Nanoparticle
3.6. Measurement of the F. oxysporum Mycelia Inhibition Effect of the TDS Nanoparticle Solution
3.7. Measurement of the Spore Germination Inhibition Effect of the TDS Nanoparticle Solution
3.8. Observation of the Spore Extinction Effect of the TDS Nanoparticle Solution
3.9. Statistical Processing
4. Conclusions
Acknowledgements
References
- Ku, K.H.; Lee, K.A.; Kim, Y.L.; Lee, Y.W. Quality characteristics of hat-air dried radish (Raphanus sativus L.) leaves. J. Korea Soc. Food Sci. Nutr 2006, 35, 780–785. [Google Scholar]
- Baik, S.Y.; Kim, J.C.; Jang, K.S.; Choi, Y.H.; Choi, G.J. Development of effective screening method and evaluation of radish cultivars for resistance to Fusarium oxysporum f. sp. raphani. Res. Plant Dis 2010, 16, 148–152. [Google Scholar]
- Pound, G.S. Red prince is new radish. Annu. Rep. Agric. Exp. Stn 1959, 538, 93–97. [Google Scholar]
- Pound, G.S.; Fowler, D.L. Fusarium wilt of radish in Wisconsin. Phytopathology 1953, 43, 277–280. [Google Scholar]
- Du Toit, L.J.; Inglis, D.A.; Pelter, G.Q. Fusarium proliferatum pathogenic on onion bulbs in Washington. Plant Dis 2003, 87, 750–756. [Google Scholar]
- Moon, Y.G.; Kim, W.G.; Cho, W.D.; Sung, J.M. Occurrence of Fusarium wilt on cruciferous vegetable crops and pathogenic differentiation of the causal fungus. Res. Plant Dis 2001, 7, 93–101. [Google Scholar]
- Van Peer, R.; Xu, T.; Rattink, H.; Schippers, B. Biological control of carnation wilt caused by Fusarium oxysporum f. sp. dianthi in hydroponic system. ISOSC Proc. 1988, 361–373. [Google Scholar]
- Alabouvette, C.; Schippers, B.; Lemanceau, P.; Bakker, P.A.H.M. Biological Control of Fusarium wilts. Plant-Microbe Interactions and Biological Control; Marcel Dekker: New York, NY, USA, 1998; pp. 15–36. [Google Scholar]
- Seo, Y.C.; Cho, J.S.; Jeong, H.Y.; Yim, T.B.; Cho, K.S.; Lee, T.W.; Jeong, M.H.; Lee, G.H.; Kin, S.I.; Yoon, W.B.; et al. Enhancement of antifungal activity of anthracnose in pepper by nanopaticles of thiamine dilauryl sulfate. Korean J. Med. Crop Sci 2011, 19, 198–240. [Google Scholar]
- Seo, D.S.; Kim, J.C.; Sohn, H.H.; Cho, W.G.; Lee, S.U.; Kim, E.Y.; Tae, G.Y.; Kim, J.D.; Lee, S.Y.; et al. Preparation and characterization of chitosan/gelatin microcapsules containing triclosan. J. Colloid Interface Sci 2004, 273, 596–603. [Google Scholar]
- Kim, J.C.; Lee, H.Y.; Kim, M.H.; Lee, H.J.; Kang, H.Y.; Kim, S.M. Preparation and characterization of chitosan/gelatin microcapsules containing triclosan. Colloids Surf. B 2006, 52, 52–56. [Google Scholar]
- Li, D.-C.; Zhong, X.-K.; Zeng, Z.-P.; Jiang, J.-G.; Li, L.; Zhao, M.-M.; Yang, X.-Q.; Chen, J.; Zhang, B.-S.; Zhao, Q.-Z.; et al. Application of targeted drug delivery system in Chinese medicine. J. Control. Release 2009, 138, 103–112. [Google Scholar]
- Seo, Y.C.; Jeong, M.H.; Jeong, H.S.; Kim, J.S.; Zou, Y.Y.; Ahn, J.H.; Shin, I.S.; Lee, H.Y. Enhancement of antimicrobial activity of nano-encapsulated horseradish aqueous extracts against food-borne pathogens. Korean J. Med. Crop Sci. 2010, 18, 389–397. [Google Scholar]
- Seo, Y.C.; Choi, Y.W.; Lee, C.G.; Cho, J.S.; Yim, T.B.; Jeong, M.H.; Kim, S.I.; Yoon, W.B.; Lee, H.Y. Effect of solubility of thiamine dilauryl sulfate solution through the manufacture of the nano paticles on antifungal activity. Korean J. Med. Crop Sci 2011, 19, 464–471. [Google Scholar]
- Kim, C.H.; Yang, S.S.; Hahn, K.D. Effects of soil disinfection, fungicide application, and narrow ridge cultivation on development of ginger rhizome rot caused by Pythium myriothlum in fields. Korean J. Plant Pathol 1998, 14, 253–259. [Google Scholar]
- Vasir, J.K.; Reddy, M.K.; Labhasetwar, V.D. Nanosystems in drug targeting: Opportunities and challenges. Curr. Nanosci 2005, 1, 47–64. [Google Scholar]
- Nakanishi, T.; Oku, H. Metabolism and accumulation of pentachloronitrobenzene by phytopathogenic fungi in relation to selective toxicity. Phytopathology 1969, 59, 1761–1765. [Google Scholar]
- Dekker, J. Acquired resistance to fungicides. Annu. Rev. Phytopathol 1976, 14, 405–428. [Google Scholar]
- Fallouh, N.A.; Treupel, L.R.; Fessi, H.; Devissaguet, J.; Puisieux, F. Development of a new process for the manufacture of polyisobutylcyanoacrylate nanocapsules. Int. J. Pharm. 1986, 28, 125–132.18. [Google Scholar]
- Saxena, A.; Sachin, K.; Bohidar, H.B.; Verma, A.K. Effect of molecular weight heterogeneity on drug encapsulation efficiency of gelatin nano-particles. Colloids Surf. B 2005, 45, 42–48. [Google Scholar]
- Ramachandran, R.; Shanmughavel, P. Preparation and characterization of biopolymeric nanoparticles used in drug delivery. Indian J. Biochem. Biophys 2010, 47, 56–59. [Google Scholar]
- Ko, S.W.; So, I.S.; Huh, M.R. Study on antifungal activity of Aloe arborescens M. for a potential bio-pestcide. J. Agric. Life Sci 2009, 43, 35–44. [Google Scholar]
- Savini, G.; Dunsmore, J.D.; Robertson, I.D. Studies on pathogenesis, tissue infection and congenital transmission in cows experimentally infected with Sarcocystis cruzi by various routes. Vet. Parasitol 1996, 64, 319–327. [Google Scholar]
- Han, J.G.; Kwon, M.C.; Ha, J.H.; Jeong, H.S.; Kim, Y.; Jeong, M.H.; Kim, J.C.; Lee, H.Y. Enhancement of immuno modulatory activities of Rubus coreanus miquel extracts by nano-encapsulation process. Korean J. Med. Crop Sci 2009, 17, 54–60. [Google Scholar]
- Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, Y.S.; Sim, S.J.; Kim, H.S.; Chang, S.J.; Kim, J.K.; Kim, K.S.; Lee, Y.S. Control efficacy of nano-silver liquid on Oak wilt caused by Raffaelea sp. in the field. Res. Plant Dis. 2011, 17, 136–141. [Google Scholar]
- Lee, Y.H. Use of Vitamin B1 as Agents for Controlling Plantdiseases. Korea Patent 7,011,615, 2002. [Google Scholar]
- Rhaese, S.; Vonbriesen, H.; Rubsamen-Waigmann, H.; Kreuter, J.; Langer, K. Human serum albumin-polyethylenimine nanoparticles for gene delivery. J. Control. Release 2003, 92, 199–208. [Google Scholar]
- Jochen, W.; Paul, T.; Julian, M. Functional materials in food nanotechnology. J. Food Sci 2006, 71, 107–116. [Google Scholar]
- Leyva, M.O.; Vicedo, B.; Finiti, I.; Flors, V.; Del Amo, G.; Real, M.D.; García-Agustín, P.; González-Bosch, C. Preventive and post-infection control of Botrytis cinerea in tomato plants by hexanoic acid. Plant Pathol. 2008, 57, 1038–1046. [Google Scholar]



© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cho, J.S.; Seo, Y.C.; Yim, T.B.; Lee, H.Y. Effect of Nanoencapsulated Vitamin B1 Derivative on Inhibition of Both Mycelial Growth and Spore Germination of Fusarium oxysporum f. sp. raphani. Int. J. Mol. Sci. 2013, 14, 4283-4297. https://doi.org/10.3390/ijms14024283
Cho JS, Seo YC, Yim TB, Lee HY. Effect of Nanoencapsulated Vitamin B1 Derivative on Inhibition of Both Mycelial Growth and Spore Germination of Fusarium oxysporum f. sp. raphani. International Journal of Molecular Sciences. 2013; 14(2):4283-4297. https://doi.org/10.3390/ijms14024283
Chicago/Turabian StyleCho, Jeong Sub, Yong Chang Seo, Tae Bin Yim, and Hyeon Yong Lee. 2013. "Effect of Nanoencapsulated Vitamin B1 Derivative on Inhibition of Both Mycelial Growth and Spore Germination of Fusarium oxysporum f. sp. raphani" International Journal of Molecular Sciences 14, no. 2: 4283-4297. https://doi.org/10.3390/ijms14024283