Incidence of Bacteriocins Produced by Food-Related Lactic Acid Bacteria Active towards Oral Pathogens
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening LAB Food Isolates for the Production of Bacteriocins Active against Oral Pathogens
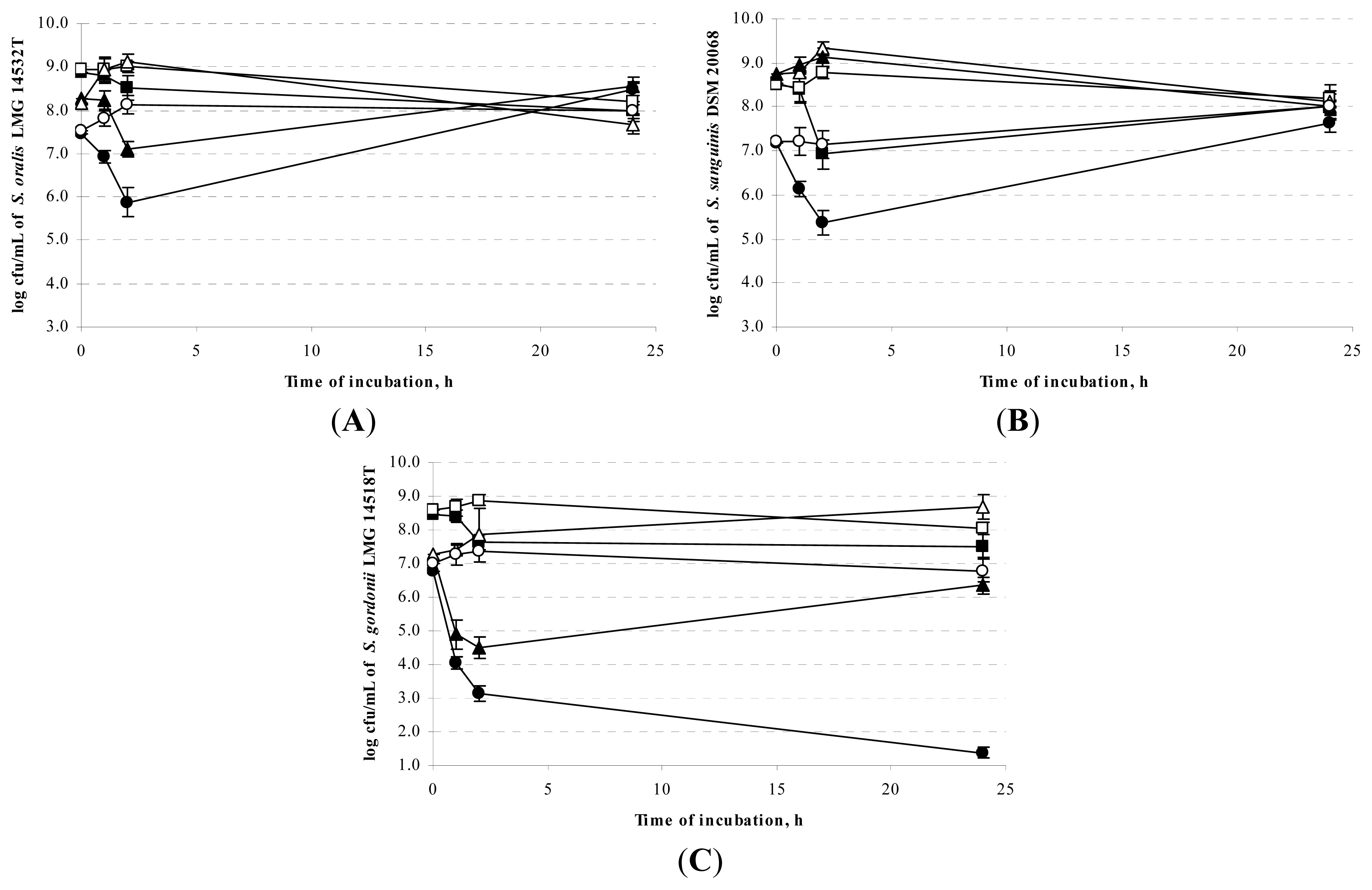
2.2. Sensitivity of Oral Streptococci towards the Antimicrobial Activity of the Food LAB Strains
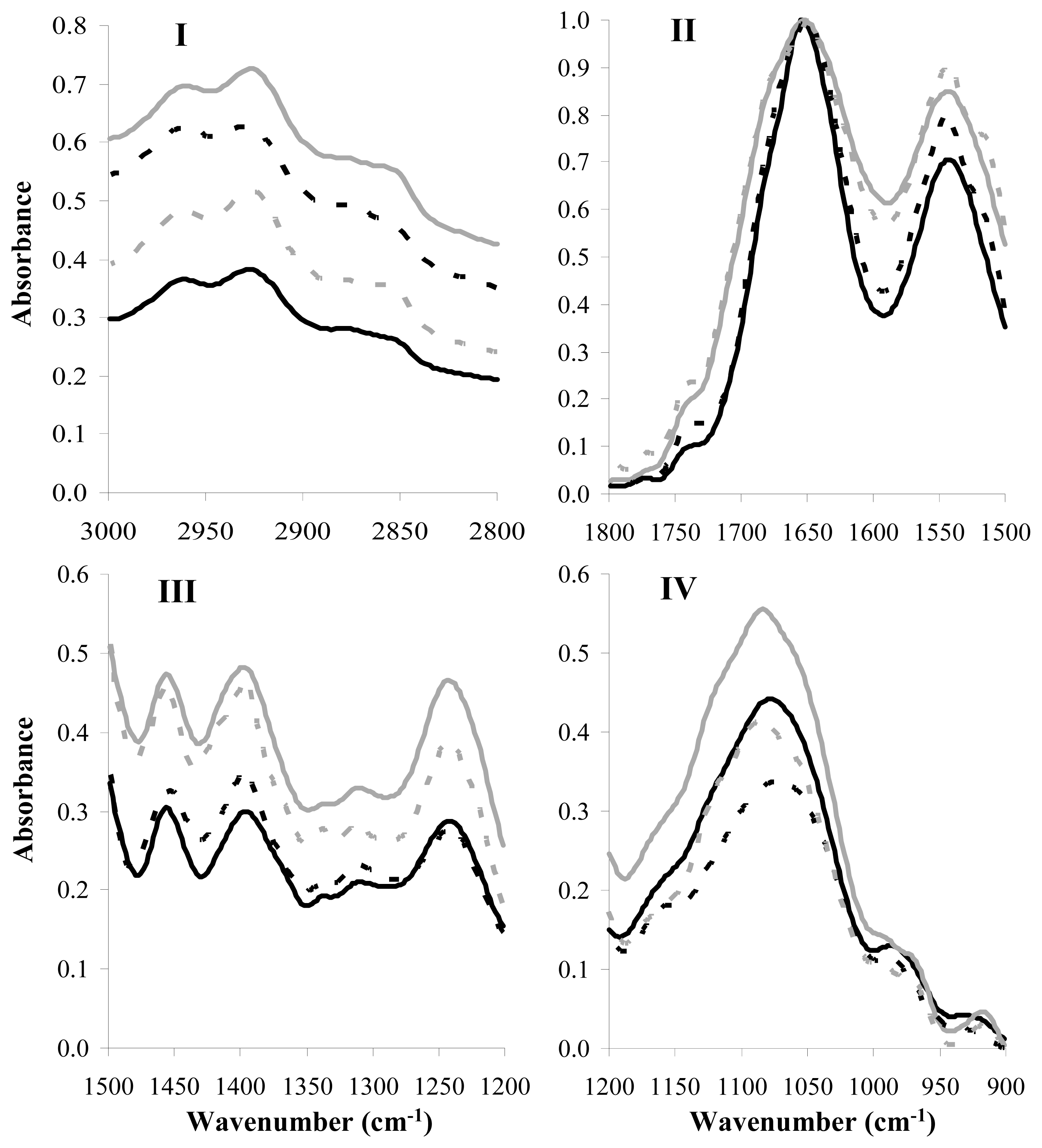
2.3. FT-IR Analysis
3. Experimental Section
3.1. Bacterial Strains and Culture Conditions
3.2. Preparation of Cell-Free Culture Supernatants (CFCSs) and of Pure Macedocin
3.3. Detection of Antimicrobial Activity
3.4. Inhibition Assays
3.5. FT-IR Sample Preparation and Measurements
3.6. FT-IR Data Analysis
4. Conclusions
Supplementary Information
ijms-14-04640-s001.pdfAcknowledgments
References
- Joint FAO/WHO, Report of a Joint FAO/WHO expert consultation on guidelines for the evaluation of probiotics in food; World Health Organization and Food and Agriculture Organization of the United Nations: Ontario, Canada, 2002.
- Bosch, M.; Nart, J.; Audivert, S.; Bonachera, M.A.; Alemany, A.S.; Fuentes, M.C.; Cune, J. Isolation and characterization of probiotic strains for improving oral health. Arch. Oral. Biol 2012, 57, 539–549. [Google Scholar]
- Snel, J.; Marco, M.L.; Kingma, F.; Noordman, W.M.; Rademaker, J.; Kleerebezem, M. Competitive selection of lactic acid bacteria that persist in the human oral cavity. Appl. Environ. Microbiol 2011, 77, 8445–8450. [Google Scholar]
- Kolenbrander, P.E.; Palmer, R.J., Jr; Rickard, A.H.; Jakubovics, N.S.; Chalmers, N.I.; Diaz, P.I. Bacterial interactions and successions during plaque development. Periodontol. 2000 2006, 42, 47–79. [Google Scholar]
- Kang, M.S.; Oh, J.S.; Lee, H.C.; Lim, H.S.; Lee, S.W.; Yang, K.H.; Choi, N.K.; Kim, S.M. Inhibitory effect of Lactobacillus reuteri on periodontopathic and cariogenic bacteria. J. Microbiol 2011, 49, 193–199. [Google Scholar]
- Bonifait, L.; Chandad, F.; Grenier, D. Probiotics for oral health: Myth or reality? J. Can. Dent. Assoc 2009, 75, 585–590. [Google Scholar]
- Stamatova, I.; Meurman, J.H. Probiotics and periodontal disease. Periodontol. 2000 2009, 51, 141–151. [Google Scholar]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev 2000, 13, 547–558. [Google Scholar]
- Nakano, K.; Inaba, H.; Nomura, R.; Nemoto, H.; Takeda, M.; Yoshioka, H.; Matsue, H.; Takahashi, T.; Taniguchi, K.; Amano, A.; et al. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J. Clin. Microbiol 2006, 44, 3313–3317. [Google Scholar]
- Takarada, K.; Kimizuka, R.; Takahashi, N.; Honma, K.; Okuda, K.; Kato, T. A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol. Immunol 2004, 19, 61–64. [Google Scholar]
- Kirkup, B.C., Jr. Bacteriocins as oral and gastrointestinal antibiotics: Theoretical considerations, applied research, and practical applications. Curr. Med. Chem. 2006, 13, 3335–3350. [Google Scholar]
- Wescombe, P.A.; Heng, N.C.; Burton, J.P.; Chilcott, C.N.; Tagg, J.R. Streptococcal bacteriocins and the case for Streptococcus salivarius as model oral probiotics. Future Microbiol 2009, 4, 819–835. [Google Scholar]
- Zoumpopoulou, G.; Foligne, B.; Christodoulou, K.; Grangette, C.; Pot, B.; Tsakalidou, E. Lactobacillus fermentum ACA-DC 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and Salmonella infection in murine models. Int. J. Food Microbiol 2008, 121, 18–26. [Google Scholar]
- Meurman, J.H.; Stamatova, I. Lactic acid bacteria in oral health. In Lactic Acid Bacteria: Microbiological and Functional Aspects; Lahtinen, S., Ouwehand, A.C., Salminen, S., von Wright, A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 403–422. [Google Scholar]
- Lemos, J.A.; Abranches, J.; Burne, R.A. Responses of cariogenic streptococci to environmental stresses. Curr. Issues Mol. Biol 2005, 7, 95–107. [Google Scholar]
- Cao-Hoang, L.; Marechal, P.A.; Le-Thanh, M.; Gervais, P. Synergistic action of rapid chilling and nisin on the inactivation of Escherichia coli. Appl. Microbiol. Biotechnol 2008, 79, 105–109. [Google Scholar]
- Dykes, G.A.; Moorhead, S.M. Combined antimicrobial effect of nisin and a listeriophage against Listeria monocytogenes in broth but not in buffer or on raw beef. Int. J. Food Microbiol 2002, 73, 71–81. [Google Scholar]
- Schobitz, R.; Suazo, V.; Costa, M.; Ciampi, L. Effects of a bacteriocin-like inhibitory substance from Carnobacterium piscicola against human and salmon isolates of Listeria monocytogenes. Int. J. Food Microbiol 2003, 84, 237–244. [Google Scholar]
- Deegan, L.H.; Cotter, P.D.; Hill, C.; Ross, P. Bacteriocins: Biological tools for bio-preservation and shelf-life extension. Int. Dairy Fed 2006, 16, 1058–1071. [Google Scholar]
- Georgalaki, M.D.; van den Berghe, E.; Kritikos, D.; Devreese, B.; van Beeumen, J.; Kalantzopoulos, G.; de Vuyst, L.; Tsakalidou, E. Macedocin, a food-grade lantibiotic produced by Streptococcus macedonicus ACA-DC 198. Appl. Environ. Microbiol 2002, 68, 5891–5903. [Google Scholar]
- Alvarez-Ordonez, A.; Mouwen, D.J.; Lopez, M.; Prieto, M. Fourier transform infrared spectroscopy as a tool to characterize molecular composition and stress response in foodborne pathogenic bacteria. J. Microbiol. Methods 2011, 84, 369–378. [Google Scholar]
- Lu, X.; Al-Qadiri, H.; Lin, M.; Rasco, B. Application of mid-infrared and raman spectroscopy to the study of bacteria. Food Bioprocess Technol 2011, 4, 919–935. [Google Scholar]
- Al-Qadiri, H.M.; Lin, M.; Cavinato, A.G.; Rasco, B.A. Fourier transform infrared spectroscopy, detection and identification of Escherichia coli O157:H7 and Alicyclobacillus strains in apple juice. Int. J. Food Microbiol 2006, 111, 73–80. [Google Scholar]
- Schmitt, J.; Flemming, H.-C. FTIR-spectroscopy in microbial and material analysis. Int. Biodeterior. Biodegrad 1998, 41, 1–11. [Google Scholar]
- Kansiz, M.; Heraud, P.; Wood, B.; Burden, F.; Beardall, J.; McNaughton, D. Fourier transform infrared microspectroscopy and chemometrics as a tool for the discrimination of cyanobacterial strains. Phytochemistry 1999, 52, 407–417. [Google Scholar]
- Zeroual, W.; Choisy, C.; Doglia, S.M.; Bobichon, H.; Angiboust, J.F.; Manfait, M. Monitoring of bacterial growth and structural analysis as probed by FT-IR spectroscopy. Biochim. Biophys. Acta 1994, 1222, 171–178. [Google Scholar]
- Filip, Z.; Hermann, S. An attempt to differentiate Pseudomonas spp. and other soil bacteria by FT-IR spectroscopy. Eur. J. Soil Biol 2001, 37, 137–143. [Google Scholar]
- Papadimitriou, K.; Boutou, E.; Zoumpopoulou, G.; Tarantilis, P.A.; Polissiou, M.; Vorgias, C.E.; Tsakalidou, E. RNA arbitrarily primed PCR and fourier transform infrared spectroscopy reveal plasticity in the acid tolerance response of Streptococcus macedonicus. Appl. Environ. Microbiol 2008, 74, 6068–6076. [Google Scholar]
- Zoumpopoulou, G.; Papadimitriou, K.; Polissiou, M.G.; Tarantilis, P.A.; Tsakalidou, E. Detection of changes in the cellular composition of Salmonella enterica serovar Typhimurium in the presence of antimicrobial compound(s) of Lactobacillus strains using Fourier transform infrared spectroscopy. Int. J. Food Microbiol 2010, 144, 202–207. [Google Scholar]


| Genus/species | Number of strains |
|---|---|
| Bacilli | |
| Lactobacillus acidipiscis | 1 |
| Lactobacillus acidophilus | 3 |
| Lactobacillus brevis | 2 |
| Lactobacillus delbrueckii subsp. bulgaricus | 11 |
| Lactobacillus delbrueckii subsp. delbrueckii | 1 |
| Lactobacillus delbrueckii subsp. lactis | 4 |
| Lactobacillus fermentum | 3 |
| Lactobacillus gasseri | 6 |
| Lactobacillus paracasei subsp. paracasei | 17 |
| Lactobacillus paracasei subsp. tolerans | 1 |
| Lactobacillus plantarum | 52 |
| Lactobacillus rennini | 11 |
| Lactobacillus rhamnosus | 1 |
| Lactobacillus sanfransinscensis | 1 |
| Lactobacillus sp. | 25 |
| Cocci | |
| Enterococcus faecalis | 3 |
| Enterococcus faecium | 2 |
| Lactococcus lactis | 41 |
| Leuconostoc lactis | 2 |
| Leuconostoc mesenteroides subsp. dextranicum | 4 |
| Pediococcus pentosaceus | 7 |
| Streptococcus bovis | 1 |
| Streptococcus macedonicus | 5 |
| Streptococcus thermophilus | 32 |
| Target strain | Inhibition (diameter; mm ± SD) a | |||||||
|---|---|---|---|---|---|---|---|---|
| L. fermentum ACA-DC 179 | L. plantarum ACA-DC 269 | S. macedonicus ACA-DC 198 | ||||||
| MRS CFCS | 10-fold concentrated MRS CFCS | Milk CFCS | 10-fold concentrated milk CFCS | Milk CFCS | 10-fold concentrated milk CFCS | M17 CFCS | 10-fold concentrated M17 CFCS | |
| S. oralis LMG 14532T | 0.7 ± 0.10 | 1.1 ± 0.10 | 0.5 ± 0.06 | 0.9 ± 0.06 | 1.0 ± 0.06 | 1.1 ± 0.10 | – b | – |
| S. sanguinis DSM 20068 | – | – | – | – | 1.0 ± 0.06 | 1.1 ± 0.12 | 0.9 ± 0.06 | 1.2 ± 0.12 |
| S. gordonii LMG 14518T | – | – | – | – | 1.2 ± 0.06 | 1.6 ± 0.10 | 1.0 ± 0.06 | 1.4 ± 0.12 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zoumpopoulou, G.; Pepelassi, E.; Papaioannou, W.; Georgalaki, M.; Maragkoudakis, P.A.; Tarantilis, P.A.; Polissiou, M.; Tsakalidou, E.; Papadimitriou, K. Incidence of Bacteriocins Produced by Food-Related Lactic Acid Bacteria Active towards Oral Pathogens. Int. J. Mol. Sci. 2013, 14, 4640-4654. https://doi.org/10.3390/ijms14034640
Zoumpopoulou G, Pepelassi E, Papaioannou W, Georgalaki M, Maragkoudakis PA, Tarantilis PA, Polissiou M, Tsakalidou E, Papadimitriou K. Incidence of Bacteriocins Produced by Food-Related Lactic Acid Bacteria Active towards Oral Pathogens. International Journal of Molecular Sciences. 2013; 14(3):4640-4654. https://doi.org/10.3390/ijms14034640
Chicago/Turabian StyleZoumpopoulou, Georgia, Eudoxie Pepelassi, William Papaioannou, Marina Georgalaki, Petros A. Maragkoudakis, Petros A. Tarantilis, Moschos Polissiou, Effie Tsakalidou, and Konstantinos Papadimitriou. 2013. "Incidence of Bacteriocins Produced by Food-Related Lactic Acid Bacteria Active towards Oral Pathogens" International Journal of Molecular Sciences 14, no. 3: 4640-4654. https://doi.org/10.3390/ijms14034640







