Gonadotropins Activate Oncogenic Pathways to Enhance Proliferation in Normal Mouse Ovarian Surface Epithelium
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Gonadotropins Enhance Proliferation of Normal OSE
2.1.2. Gonadotropins Regulate Oncogenic Signal Transduction Pathways in Normal Mouse OSE Cultured in 3D
2.1.3. Gonadotropins Enhance Akt Expression in Normal OSE
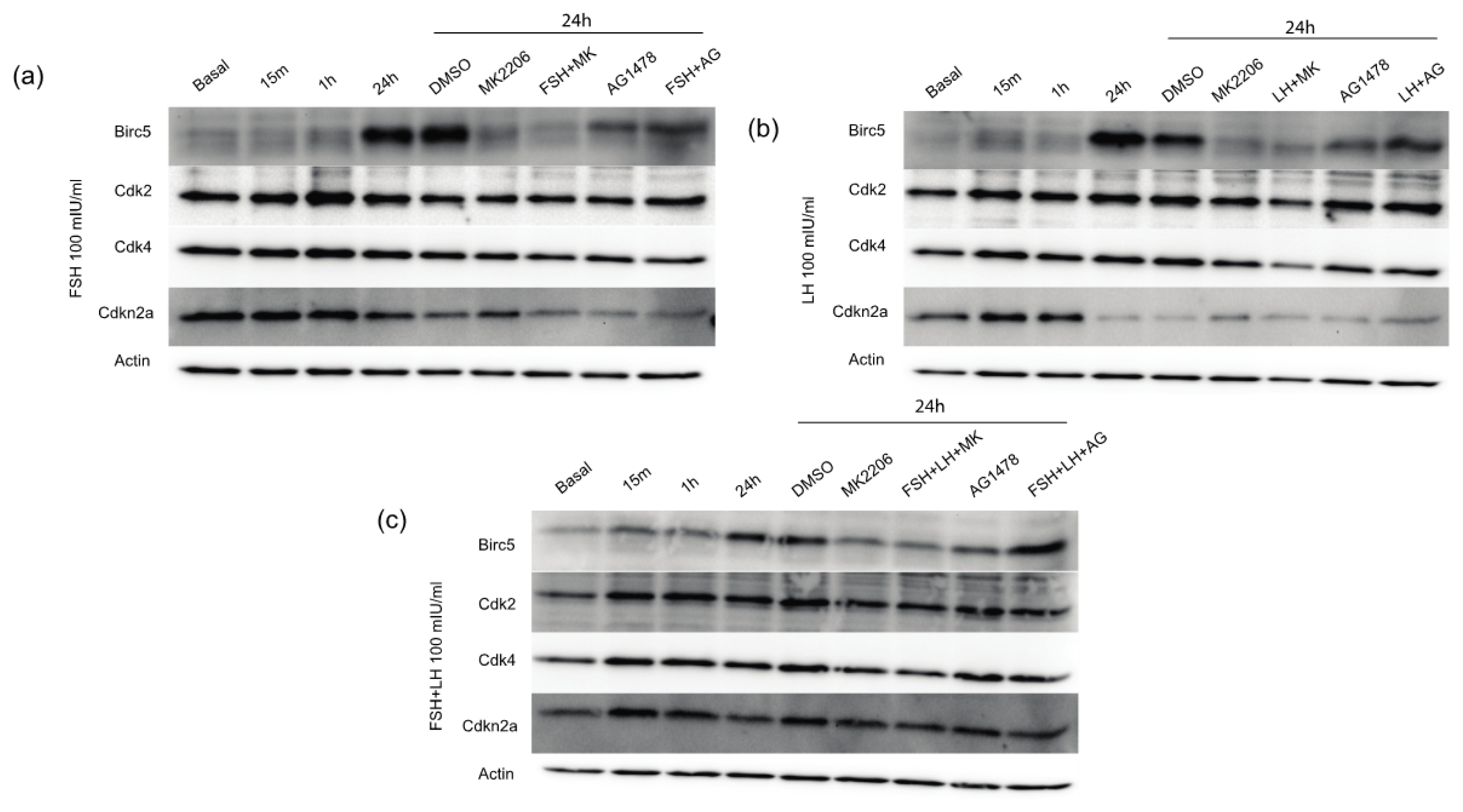
2.1.4. Gonadotropins Increase Expression of Proliferative and Anti-Apoptotic Proteins in Normal OSE
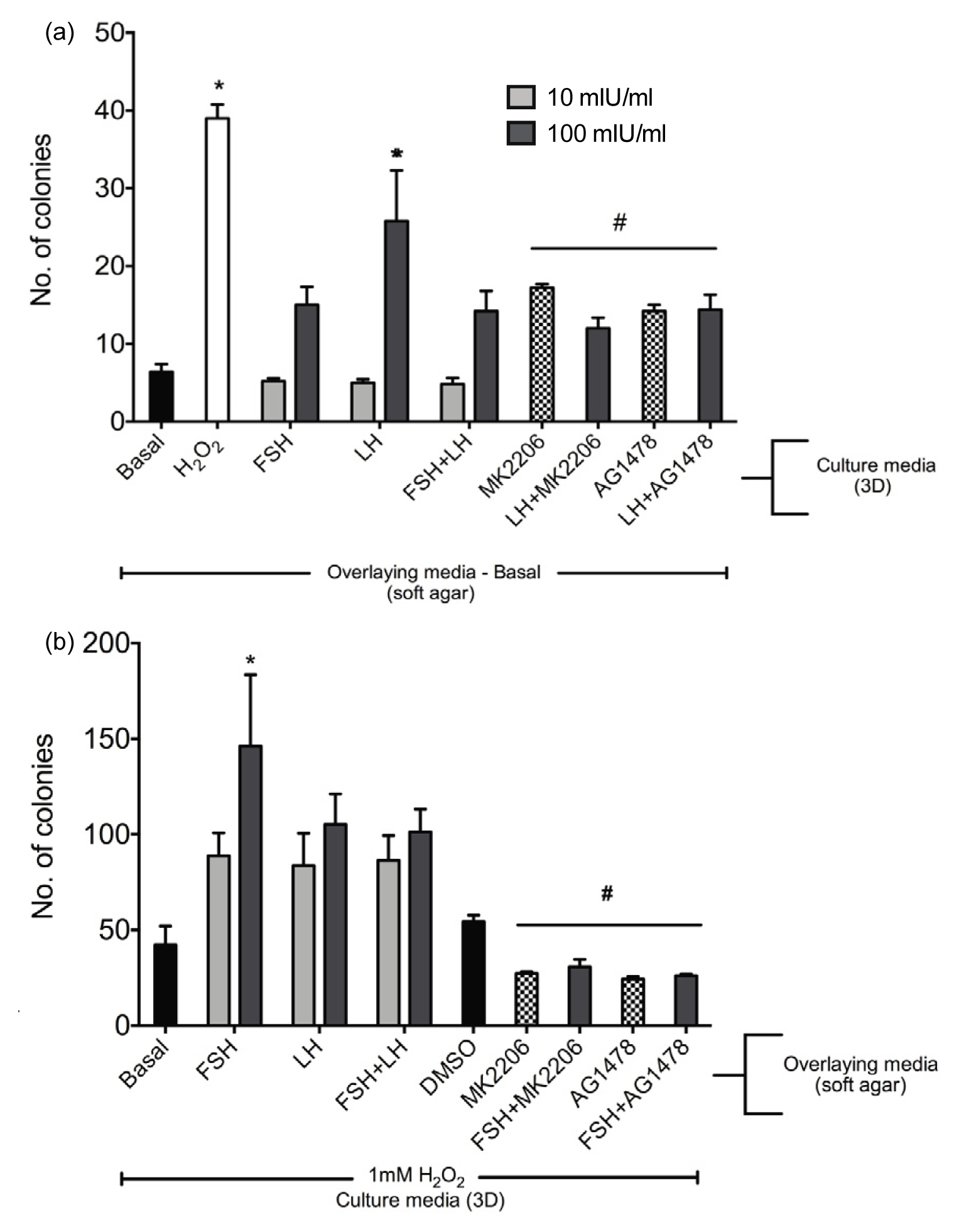
2.1.5. Gonadotropins Enhance Soft Agar Colony Formation
2.2. Discussion
3. Experimental Section
3.1. Animals
3.2. Organ Culture
3.3. Cell Culture
3.4. Immunohistochemistry
3.5. RNA Isolation and RT-PCR
3.6. Cell Viability Assay
3.7. Soft Agar Transformation Assay
3.8. Western Blotting
3.9. Imaging and Counts
3.10. Statistical Analysis
4. Conclusions
Supplementary materials
| Gene | Effect of FSH and/or LH | Ovarian cancer cell line | Reference |
|---|---|---|---|
| Proliferation/Cell cycle control | |||
| A | FSH induces pAkt expression | SKOV3 cells | [15] |
| kt | FSH and LH induce pAkt expression | IOSE80-PC | [26] |
| 1 | |||
| A | |||
| kt | |||
| 2 | |||
| Cdk2 | Overexpression of β-hCG induces elevated expression of Cdk2 and Cdk4 proteins | T29 & T80 cells | [46] |
| Cdk4 | |||
| Cdkn2a | |||
| Chek 2 | |||
| Cyclin D1 | FSH increases expression of Cyclin D1 | SKOV3 cells | [50] |
| EGFR | FSH and LH upregulate EGFR expression | IOSE80-PC and OVCAR3 cells | [26] |
| Mdm2 | |||
| Myc | FSH induced expression of c-Myc | OVCAR3 cells | [51] |
| Trp53 | |||
| Angiogenesis/Metastasis/Adhesion | |||
| Angpt1 | |||
| Mcam | |||
| c-Met | |||
| S100a4 | |||
| Vegfc | FSH and LH stimulate VEGF expression | AO cells | [52] |
| FSH stimulates VEGF expression | SKOV3 cells | [50] | |
| Apoptosis | |||
| Birc5 | LH upregulates survivin expression | SKOV3 and MCV152 cells | [53] |
| FSH upregulates survivin expression | SKOV3 cells | [50] | |
| Casp8 | |||
Acknowledgments
Conflict of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin 2010, 60, 277–300. [Google Scholar]
- Lukanova, A.; Kaaks, R. Endogenous hormones and ovarian cancer: Epidemiology and current hypotheses. Cancer Epidemiol. Biomark. Prev 2005, 14, 98–107. [Google Scholar]
- Karst, A.M.; Levanon, K.; Drapkin, R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc. Natl. Acad. Sci. USA 2011, 108, 7547–7552. [Google Scholar]
- Lee, Y.; Medeiros, F.; Kindelberger, D.; Callahan, M.J.; Muto, M.G.; Crum, C.P. Advances in the recognition of tubal intraepithelial carcinoma: Applications to cancer screening and the pathogenesis of ovarian cancer. Adv. Anat. Pathol 2006, 13, 1–7. [Google Scholar]
- Medeiros, F.; Muto, M.G.; Lee, Y.; Elvin, J.A.; Callahan, M.J.; Feltmate, C.; Garber, J.E.; Cramer, D.W.; Crum, C.P. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am. J. Surg. Pathol 2006, 30, 230–236. [Google Scholar]
- Syed, V.; Ulinski, G.; Mok, S.C.; Yiu, G.K.; Ho, S.M. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res 2001, 61, 6768–6776. [Google Scholar]
- Choi, J.H.; Wong, A.S.; Huang, H.F.; Leung, P.C. Gonadotropins and ovarian cancer. Endocr. Rev 2007, 28, 440. [Google Scholar]
- King, S.M.; Hilliard, T.S.; Wu, L.Y.; Jaffe, R.C.; Fazleabas, A.T.; Burdette, J.E. The impact of ovulation on fallopian tube epithelial cells: Evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr. Relat. Cancer 2011, 18, 627–642. [Google Scholar]
- Auersperg, N.; Woo, M.M.; Gilks, C.B. The origin of ovarian carcinomas: A developmental view. Gynecol. Oncol 2008, 110, 452–454. [Google Scholar]
- Leung, P.C.; Choi, J.H. Endocrine signaling in ovarian surface epithelium and cancer. Hum. Reprod. Update 2007, 13, 143–162. [Google Scholar]
- Bernard, D.J.; Fortin, J.; Wang, Y.; Lamba, P. Mechanisms of fsh synthesis: What we know, what we don’t, and why you should care. Fertil. Steril 2010, 93, 2465–2485. [Google Scholar]
- Dierich, A.; Sairam, M.R.; Monaco, L.; Fimia, G.M.; Gansmuller, A.; LeMeur, M.; Sassone-Corsi, P. Impairing follicle-stimulating hormone (fsh) signaling in vivo: Targeted disruption of the fsh receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl. Acad. Sci. USA 1998, 95, 13612–13617. [Google Scholar]
- Rao, M.C.; Midgley, A.R., Jr; Richards, J.S. Hormonal regulation of ovarian cellular proliferation. Cell 1978, 14, 71–78. [Google Scholar]
- Hunzicker-Dunn, M.; Maizels, E.T. Fsh signaling pathways in immature granulosa cells that regulate target gene expression: Branching out from protein kinase a. Cell. Signal 2006, 18, 1351–1359. [Google Scholar]
- Huang, Y.; Hua, K.; Zhou, X.; Jin, H.; Chen, X.; Lu, X.; Yu, Y.; Zha, X.; Feng, Y. Activation of the pi3k/akt pathway mediates fsh-stimulated vegf expression in ovarian serous cystadenocarcinoma. Cell. Res 2008, 18, 780–791. [Google Scholar]
- Parrott, J.A.; Doraiswamy, V.; Kim, G.; Mosher, R.; Skinner, M.K. Expression and actions of both the follicle stimulating hormone receptor and the luteinizing hormone receptor in normal ovarian surface epithelium and ovarian cancer. Mol. Cell. Endocrinol 2001, 172, 213–222. [Google Scholar]
- Davies, B.R.; Finnigan, D.S.; Smith, S.K.; Ponder, B.A. Administration of gonadotropins stimulates proliferation of normal mouse ovarian surface epithelium. Gynecol. Endocrinol 1999, 13, 75–81. [Google Scholar]
- Burdette, J.E.; Kurley, S.J.; Kilen, S.M.; Mayo, K.E.; Woodruff, T.K. Gonadotropin-Induced superovulation drives ovarian surface epithelia proliferation in cd1 mice. Endocrinology 2006, 147, 2338–2345. [Google Scholar]
- Stewart, S.L.; Querec, T.D.; Gruver, B.N.; O’Hare, B.; Babb, J.S.; Patriotis, C. Gonadotropin and steroid hormones stimulate proliferation of the rat ovarian surface epithelium. J. Cell. Physiol 2004, 198, 119–124. [Google Scholar]
- Wimalasena, J.; Meehan, D.; Cavallo, C. Human epithelial ovarian cancer cell steroid secretion and its control by gonadotropins. Gynecol. Oncol 1991, 41, 56–63. [Google Scholar]
- Tourgeman, D.E.; Lu, J.J.; Boostanfar, R.; Amezcua, C.; Felix, J.C.; Paulson, R.J. Human chorionic gonadotropin suppresses ovarian epithelial neoplastic cell proliferation in vitro. Fertil. Steril 2002, 78, 1096–1099. [Google Scholar]
- Slot, K.A.; de Boer-Brouwer, M.; Houweling, M.; Vaandrager, A.B.; Dorrington, J.H.; Teerds, K.J. Luteinizing hormone inhibits fas-induced apoptosis in ovarian surface epithelial cell lines. J. Endocrinol 2006, 188, 227–239. [Google Scholar]
- Ivarsson, K.; Sundfeldt, K.; Brannstrom, M.; Hellberg, P.; Janson, P.O. Diverse effects of fsh and lh on proliferation of human ovarian surface epithelial cells. Hum. Reprod 2001, 16, 18–23. [Google Scholar]
- Murdoch, W.J. Ovulatory factor in ovarian carcinogenesis. Adv. Exp. Med. Biol 2008, 622, 119–128. [Google Scholar]
- Jackson, K.S.; Inoue, K.; Davis, D.A.; Hilliard, T.S.; Burdette, J.E. Three-Dimensional ovarian organ culture as a tool to study normal ovarian surface epithelial wound repair. Endocrinology 2009, 150, 3921–3926. [Google Scholar]
- Choi, J.H.; Choi, K.C.; Auersperg, N.; Leung, P.C. Gonadotropins upregulate the epidermal growth factor receptor through activation of mitogen-activated protein kinases and phosphatidyl-inositol-3-kinase in human ovarian surface epithelial cells. Endocr. Relat. Cancer 2005, 12, 407–421. [Google Scholar]
- Wong, A.S.; Leung, P.C. Role of endocrine and growth factors on the ovarian surface epithelium. J. Obstet. Gynaecol. Res 2007, 33, 3–16. [Google Scholar]
- Wright, J.W.; Toth-Fejel, S.; Stouffer, R.L.; Rodland, K.D. Proliferation of rhesus ovarian surface epithelial cells in culture: Lack of mitogenic response to steroid or gonadotropic hormones. Endocrinology 2002, 143, 2198–2207. [Google Scholar]
- Gamwell, L.F.; Collins, O.; Vanderhyden, B.C. The mouse ovarian surface epithelium contains a population of ly6a (sca-1) expressing progenitor cells that are regulated by ovulation-associated factors. Biol. Reprod 2012, 87, 80. [Google Scholar]
- Altomare, D.A.; Wang, H.Q.; Skele, K.L.; De Rienzo, A.; Klein-Szanto, A.J.; Godwin, A.K.; Testa, J.R. Akt and mtor phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene 2004, 23, 5853–5857. [Google Scholar]
- Kinross, K.M.; Montgomery, K.G.; Kleinschmidt, M.; Waring, P.; Ivetac, I.; Tikoo, A.; Saad, M.; Hare, L.; Roh, V.; Mantamadiotis, T.; et al. An activating pik3ca mutation coupled with pten loss is sufficient to initiate ovarian tumorigenesis in mice. J. Clin. Invest 2012, 122, 553–557. [Google Scholar]
- Gladhaug, I.P.; Refsnes, M.; Christoffersen, T. Regulation of hepatocyte epidermal growth factor receptors by n-butyrate and dimethyl sulfoxide: Sensitivity to modulation by the tumor promoter tpa. Anticancer Res 1989, 9, 1587–1592. [Google Scholar]
- King, S.M.; Quartuccio, S.M.; Vanderhyden, B.C.; Burdette, J.E. Early transformative changes in normal ovarian surface epithelium induced by oxidative stress require akt upregulation, DNA damage, and epithelial-stromal interaction. Carcinogenesis 2013. [Google Scholar] [CrossRef]
- Osterholzer, H.O.; Johnson, J.H.; Nicosia, S.V. An autoradiographic study of rabbit ovarian surface epithelium before and after ovulation. Biol. Reprod 1985, 33, 729–738. [Google Scholar]
- Bai, W.; Oliveros-Saunders, B.; Wang, Q.; Acevedo-Duncan, M.E.; Nicosia, S.V. Estrogen stimulation of ovarian surface epithelial cell proliferation. In Vitro Cell. Dev. Biol. Anim 2000, 36, 657–666. [Google Scholar]
- Cheng, J.Q.; Godwin, A.K.; Bellacosa, A.; Taguchi, T.; Franke, T.F.; Hamilton, T.C.; Tsichlis, P.N.; Testa, J.R. Akt2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc. Natl. Acad. Sci. USA 1992, 89, 9267–9271. [Google Scholar]
- Sun, M.; Wang, G.; Paciga, J.E.; Feldman, R.I.; Yuan, Z.Q.; Ma, X.L.; Shelley, S.A.; Jove, R.; Tsichlis, P.N.; Nicosia, S.V.; et al. Akt1/pkbalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in nih3t3 cells. Am. J. Pathol 2001, 159, 431–437. [Google Scholar]
- Yuan, Z.Q.; Sun, M.; Feldman, R.I.; Wang, G.; Ma, X.; Jiang, C.; Coppola, D.; Nicosia, S.V.; Cheng, J.Q. Frequent activation of akt2 and induction of apoptosis by inhibition of phosphoinositide-3-oh kinase/akt pathway in human ovarian cancer. Oncogene 2000, 19, 2324–2330. [Google Scholar]
- Bellacosa, A.; de Feo, D.; Godwin, A.K.; Bell, D.W.; Cheng, J.Q.; Altomare, D.A.; Wan, M.; Dubeau, L.; Scambia, G.; Masciullo, V.; et al. Molecular alterations of the akt2 oncogene in ovarian and breast carcinomas. Int. J. Cancer 1995, 64, 280–285. [Google Scholar]
- Cristiano, B.E.; Chan, J.C.; Hannan, K.M.; Lundie, N.A.; Marmy-Conus, N.J.; Campbell, I.G.; Phillips, W.A.; Robbie, M.; Hannan, R.D.; Pearson, R.B. A specific role for akt3 in the genesis of ovarian cancer through modulation of g(2)-m phase transition. Cancer Res 2006, 66, 11718–11725. [Google Scholar]
- Flynn, M.P.; Maizels, E.T.; Karlsson, A.B.; McAvoy, T.; Ahn, J.H.; Nairn, A.C.; Hunzicker-Dunn, M. Luteinizing hormone receptor activation in ovarian granulosa cells promotes protein kinase a-dependent dephosphorylation of microtubule-associated protein 2d. Mol. Endocrinol 2008, 22, 1695–1710. [Google Scholar]
- Sewell, J.M.; Macleod, K.G.; Ritchie, A.; Smyth, J.F.; Langdon, S.P. Targeting the egf receptor in ovarian cancer with the tyrosine kinase inhibitor zd 1839 (“Iressa”). Br. J. Cancer 2002, 86, 456–462. [Google Scholar]
- Bull Phelps, S.L.; Schorge, J.O.; Peyton, M.J.; Shigematsu, H.; Xiang, L.L.; Miller, D.S.; Lea, J.S. Implications of egfr inhibition in ovarian cancer cell proliferation. Gynecol. Oncol 2008, 109, 411–417. [Google Scholar]
- Psyrri, A.; Kassar, M.; Yu, Z.; Bamias, A.; Weinberger, P.M.; Markakis, S.; Kowalski, D.; Camp, R.L.; Rimm, D.L.; Dimopoulos, M.A. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin. Cancer Res 2005, 11, 8637–8643. [Google Scholar]
- Tanaka, Y.; Terai, Y.; Tanabe, A.; Sasaki, H.; Sekijima, T.; Fujiwara, S.; Yamashita, Y.; Kanemura, M.; Ueda, M.; Sugita, M.; et al. Prognostic effect of epidermal growth factor receptor gene mutations and the aberrant phosphorylation of akt and erk in ovarian cancer. Cancer Biol. Ther 2011, 11, 50–57. [Google Scholar]
- Guo, X.; Liu, G.; Schauer, I.G.; Yang, G.; Mercado-Uribe, I.; Yang, F.; Zhang, S.; He, Y.; Liu, J. Overexpression of the beta subunit of human chorionic gonadotropin promotes the transformation of human ovarian epithelial cells and ovarian tumorigenesis. Am. J. Pathol 2011, 179, 1385–1393. [Google Scholar]
- King, S.M.; Quartuccio, S.; Hilliard, T.S.; Inoue, K.; Burdette, J.E. Alginate hydrogels for three-dimensional organ culture of ovaries and oviducts. J. Vis. Exp. 2011. [Google Scholar] [CrossRef]
- King, S.M.; Burdette, J.E. Evaluating the progenitor cells of ovarian cancer: Analysis of current animal models. BMB Rep 2011, 44, 435–445. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst 1990, 82, 1107–1112. [Google Scholar]
- Huang, Y.; Jin, H.; Liu, Y.; Zhou, J.; Ding, J.; Cheng, K.W.; Yu, Y.; Feng, Y. Fsh inhibits ovarian cancer cell apoptosis by up-regulating survivin and down-regulating pdcd6 and dr5. Endocr. Relat. Cancer 2011, 18, 13–26. [Google Scholar]
- Choi, J.H.; Choi, K.C.; Auersperg, N.; Leung, P.C. Overexpression of follicle-stimulating hormone receptor activates oncogenic pathways in preneoplastic ovarian surface epithelial cells. J. Clin. Endocrinol. Metab 2004, 89, 5508–5516. [Google Scholar]
- Wang, J.; Luo, F.; Lu, J.J.; Chen, P.K.; Liu, P.; Zheng, W. Vegf expression and enhanced production by gonadotropins in ovarian epithelial tumors. Int. J. Cancer 2002, 97, 163–167. [Google Scholar]
- Zhang, Z.; Liao, H.; Chen, X.; Zheng, Y.; Liu, Y.; Tao, X.; Gu, C.; Dong, L.; Duan, T.; Yang, Y.; et al. Luteinizing hormone upregulates survivin and inhibits apoptosis in ovarian epithelial tumors. Eur. J. Obstet. Gynecol. Reprod. Biol 2011, 155, 69–74. [Google Scholar]
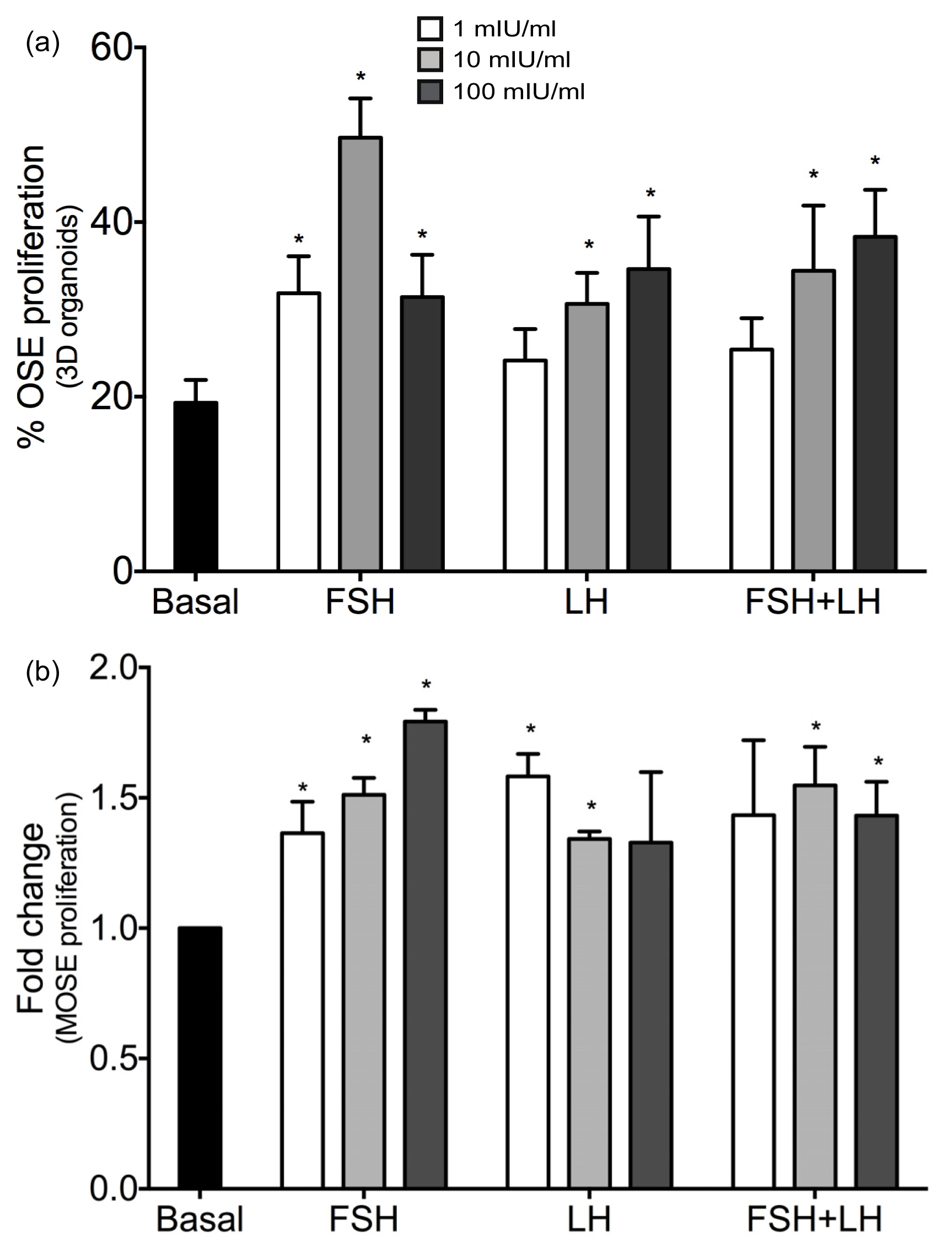
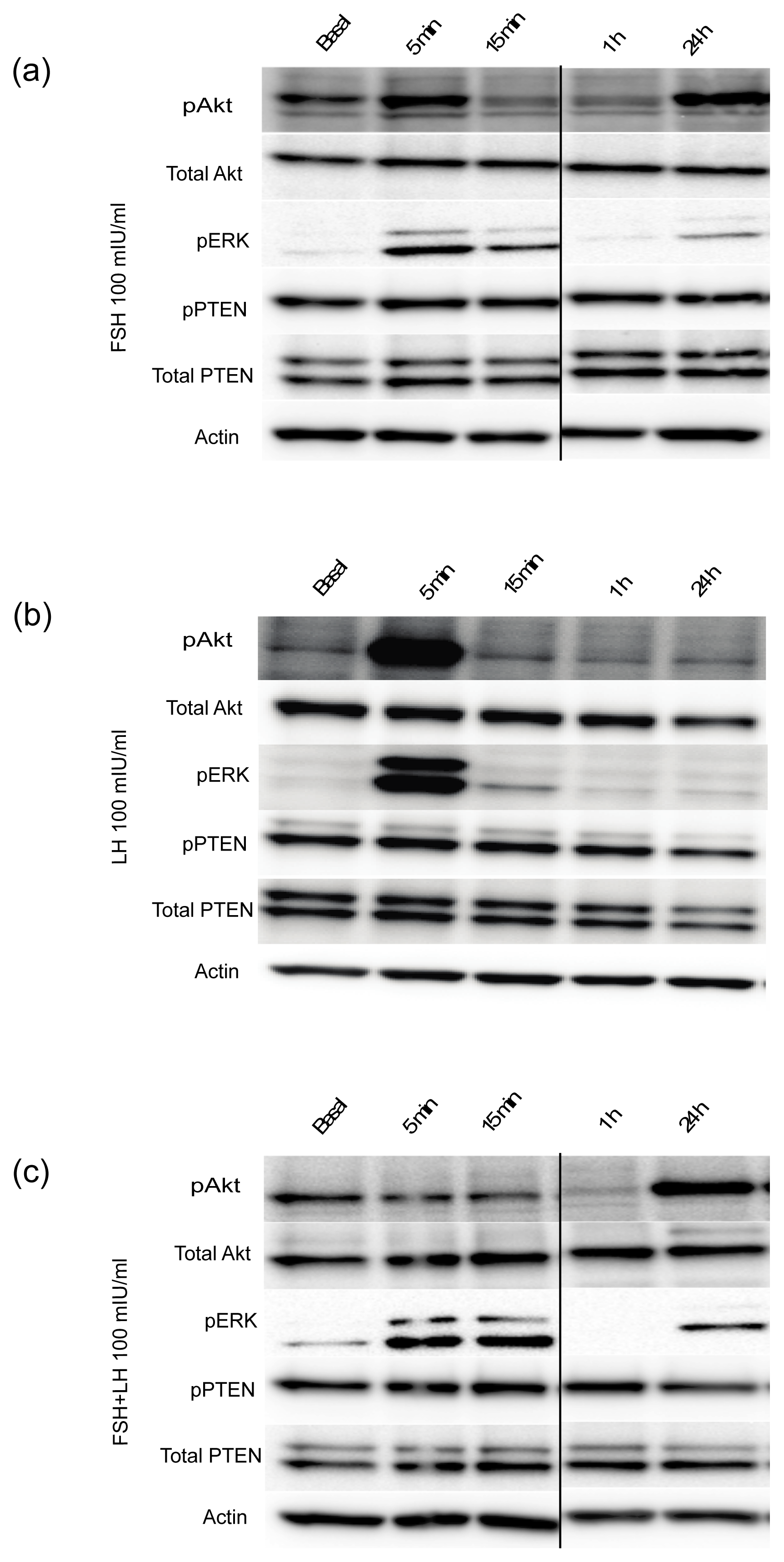
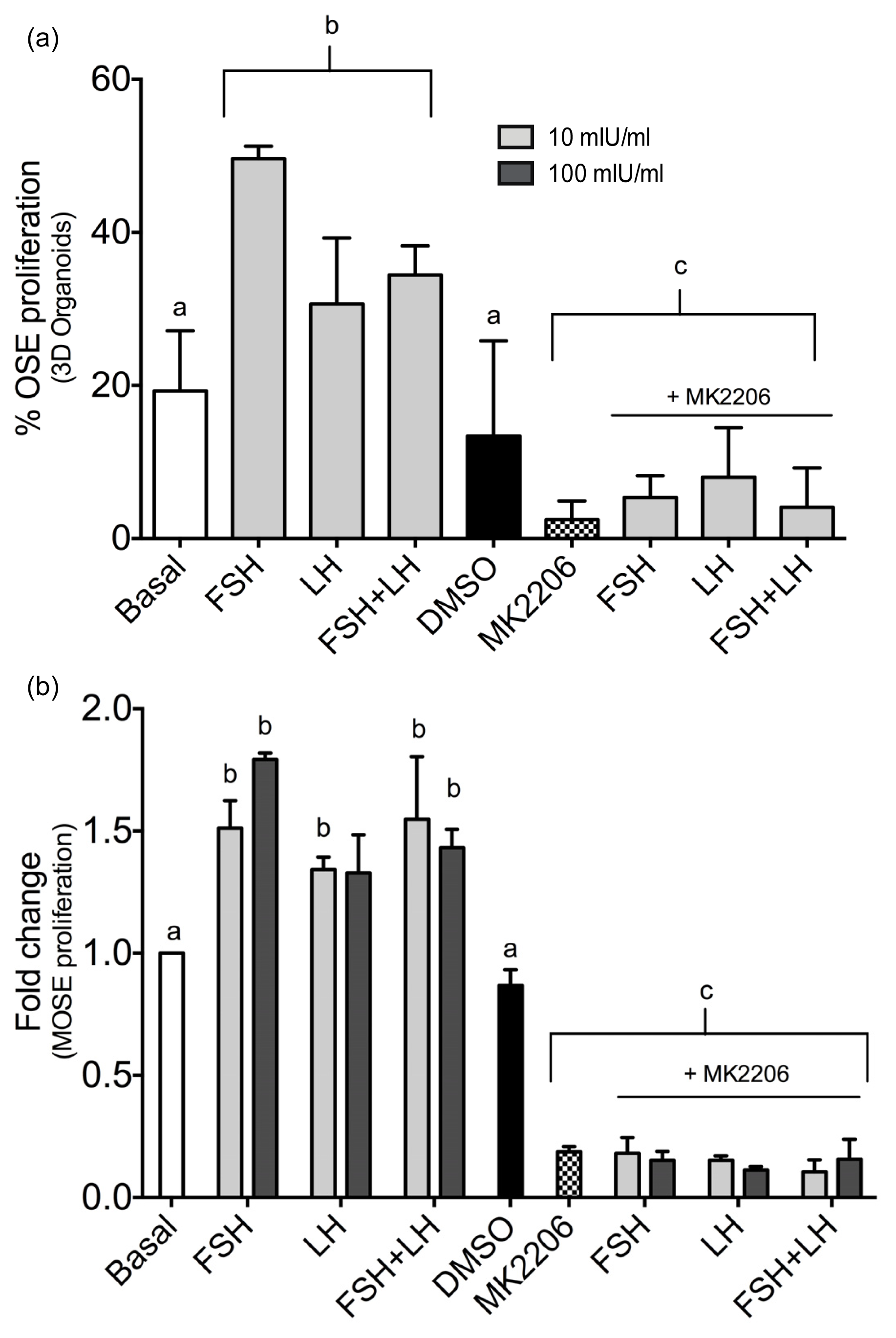
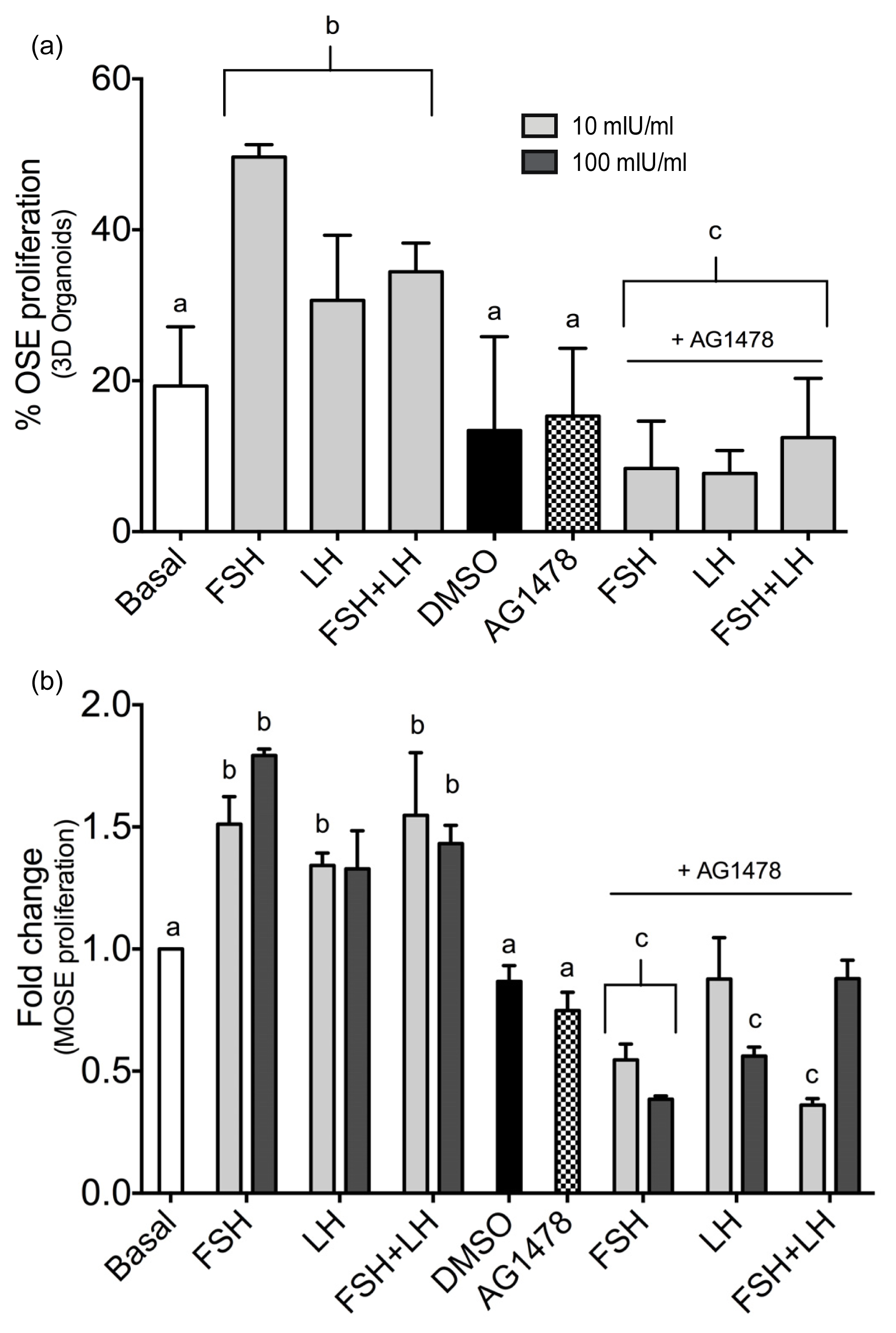
| Proliferation/Cell Cycle Control | |||
|---|---|---|---|
| Gene | FSH | LH | FSH+LH |
| Akt1 | 3.5 | 61 | 55 |
| Akt2 | 58 | 58 | 37 |
| Cdk2 | 3.7 | 1.0 | 2.6 |
| Cdk4 | 22 | 0.9 | 175 |
| Cdkn2a | 38 | 25 | 17 |
| Chek 2 | 1.6 | 0.7 | 1.0 |
| Cyclin D1 | 1.4 | 0.6 | 1.3 |
| EGFR | 5.8 | 1.8 | 5.3 |
| Mdm2 | 3.0 | 1.0 | 2.1 |
| Myc | 1.7 | 0.7 | 1.4 |
| Trp53 | 2.5 | 1.0 | 2.0 |
| Angiogenesis/Metastasis/Adhesion | |||
| Angpt1 | 32 | 16 | 4.9 |
| Mcam | 3.0 | 1.2 | 2.0 |
| c-Met | 2.1 | 0.70 | 2.4 |
| S100a4 | 3.2 | 0.6 | 2.2 |
| Vegfc | 3.5 | 1.1 | 2.4 |
| Apoptosis | |||
| Birc5 | 2.2 | 1.2 | 2.6 |
| Casp8 | 0.92 | 0.1 | 0.45 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hilliard, T.S.; Modi, D.A.; Burdette, J.E. Gonadotropins Activate Oncogenic Pathways to Enhance Proliferation in Normal Mouse Ovarian Surface Epithelium. Int. J. Mol. Sci. 2013, 14, 4762-4782. https://doi.org/10.3390/ijms14034762
Hilliard TS, Modi DA, Burdette JE. Gonadotropins Activate Oncogenic Pathways to Enhance Proliferation in Normal Mouse Ovarian Surface Epithelium. International Journal of Molecular Sciences. 2013; 14(3):4762-4782. https://doi.org/10.3390/ijms14034762
Chicago/Turabian StyleHilliard, Tyvette S., Dimple A. Modi, and Joanna E. Burdette. 2013. "Gonadotropins Activate Oncogenic Pathways to Enhance Proliferation in Normal Mouse Ovarian Surface Epithelium" International Journal of Molecular Sciences 14, no. 3: 4762-4782. https://doi.org/10.3390/ijms14034762




