Preclinical Activity of Simvastatin Induces Cell Cycle Arrest in G1 via Blockade of Cyclin D-Cdk4 Expression in Non-Small Cell Lung Cancer (NSCLC)
Abstract
:1. Introduction
2. Results and Discussion
2.1. SIM Inhibits the Proliferation of NCI-H460 Cells
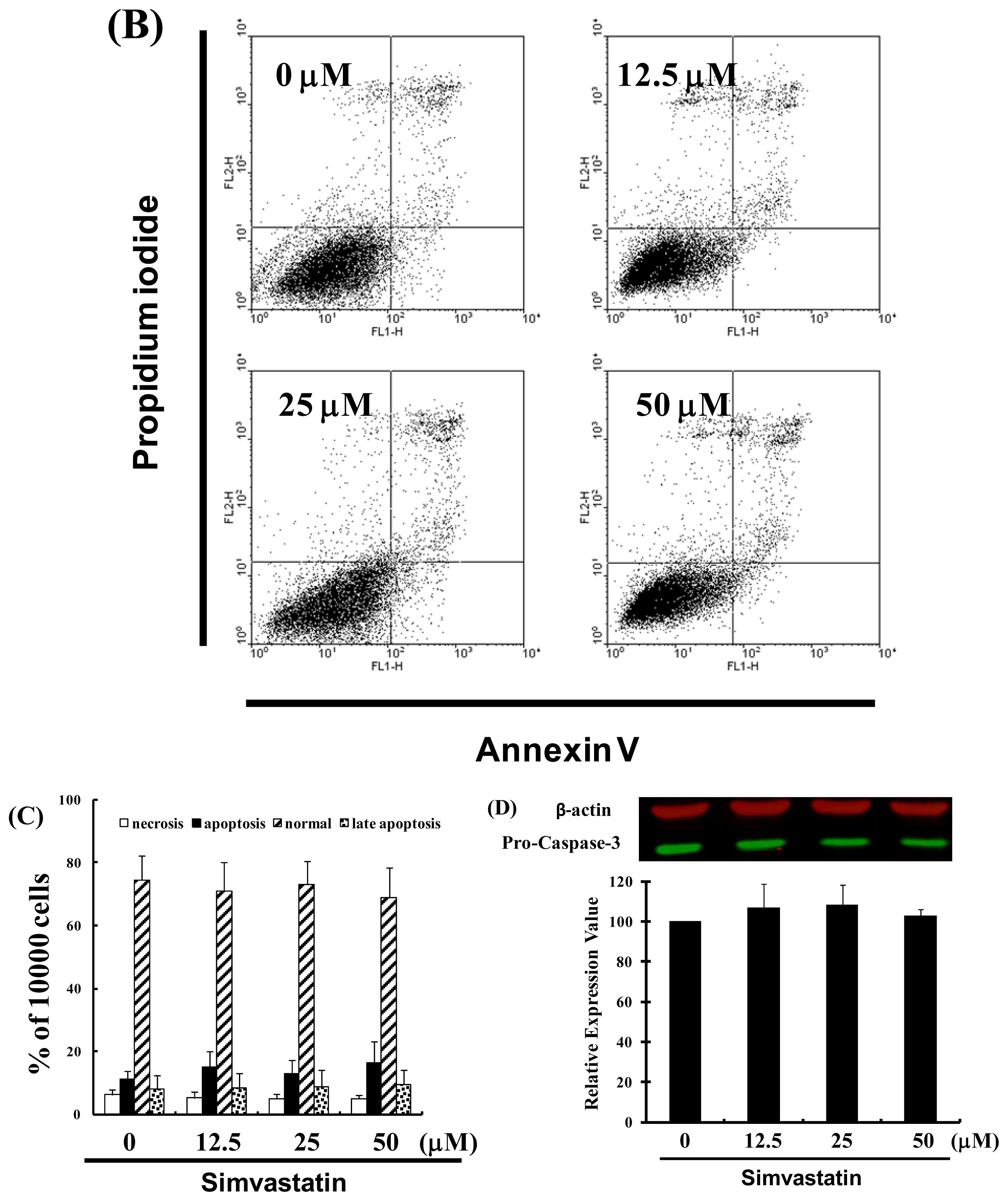
2.2. SIM Impaired the Viability of NCI-H460 Cells without Inducing Apoptosis
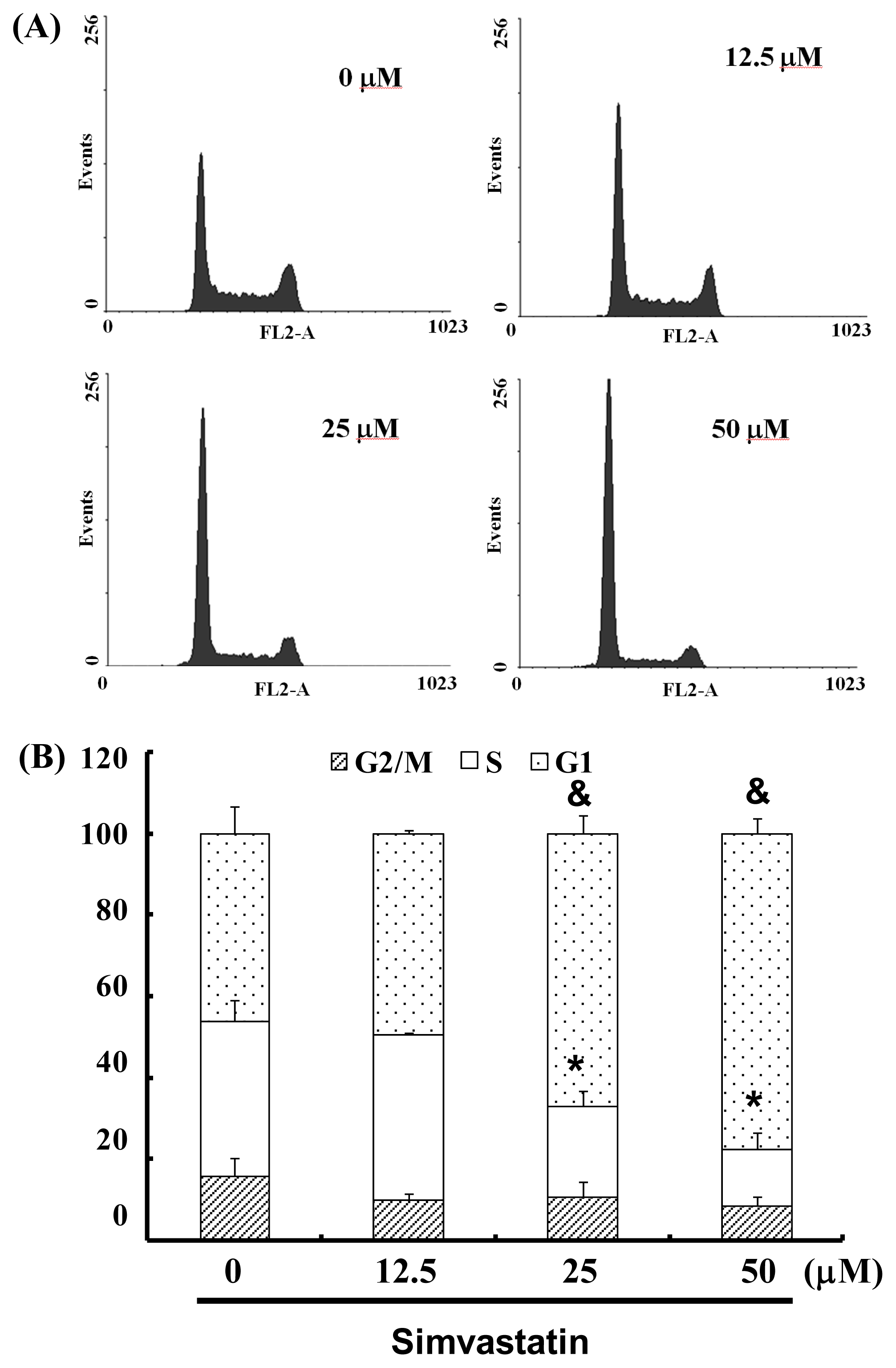
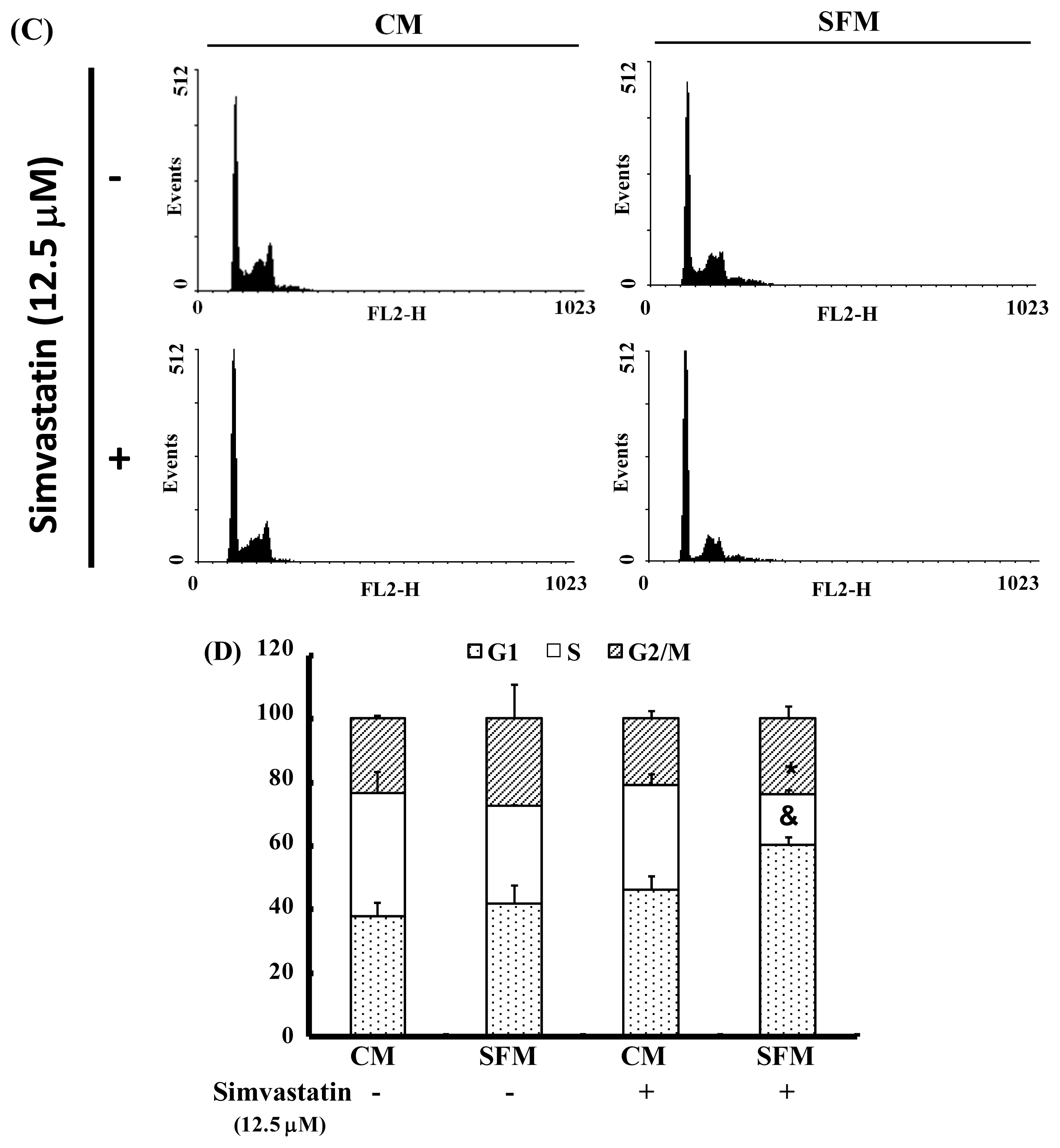
2.3. SIM Treatment Induced Accumulation of G1 Phase in NCI-H460 Cells
2.4. Cell Cycle Arrest Was Induced by SIM in NCI-H460 Cells via Cyclin D and Cdk4 Downregulation and Enhanced CDK Inhibition
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
3.3. Cell Proliferation Assay
3.4. Measurement of Apoptosis
3.5. Cell Cycle Analysis
3.6. Western Blot Assay
3.7. Statistical Analysis
4. Conclusions
References
- Ou, J.P.; Lin, H.Y.; Su, K.Y.; Yu, S.L.; Tseng, I.H.; Chen, C.J.; Hsu, H.C.; Chan, D.C.; Sophia Chen, Y.L. Potential therapeutic role of Z-isochaihulactone in lung cancer through induction of apoptosis via notch signaling. Evid. Based Complement. Alternat. Med 2012, 2012. [Google Scholar] [CrossRef]
- Chien, C.R.; Shih, Y.C. Economic evaluation of bevacizumab in the treatment of non-small cell lung cancer (NSCLC). Clinicoecon. Outcomes Res 2012, 4, 201–208. [Google Scholar]
- Kah, J.; Wüstenberg, A.; Keller, A.D.; Sirma, H.; Montalbano, R.; Ocker, M.; Volz, T.; Dandri, M.; Tiegs, G.; Sass, G. Selective induction of apoptosis by HMG-CoA reductase inhibitors in hepatoma cells and dependence on p53 expression. Oncol. Rep 2012, 28, 1077–1083. [Google Scholar]
- Alexandre, L.; Clark, A.B.; Cheong, E.; Lewis, M.P.; Hart, A.R. Systematic review: Potential preventive effects of statins against oesophageal adenocarcinoma. Aliment. Pharmacol. Ther 2012, 36, 301–311. [Google Scholar]
- Broughton, T.; Sington, J.; Beales, I.L. Statin use is associated with a reduced incidence of colorectal cancer: A colonoscopy-controlled case-control study. BMC Gastroenterol 2012, 12, 36. [Google Scholar]
- Papadopoulos, G.; Delakas, D.; Nakopoulou, L.; Kassimatis, T. Statins and prostate cancer: Molecular and clinical aspects. Eur. J. Cancer 2011, 47, 819–830. [Google Scholar]
- Zhou, P.; Cheng, S.W.; Yang, R.; Wang, B.; Liu, J. Combination chemoprevention: Future direction of colorectal cancer prevention. Eur. J. Cancer Prev 2012, 21, 231–240. [Google Scholar]
- Piechota-Polanczyk, A.; Goraca, A.; Demyanets, S.; Mittlboeck, M.; Domenig, C.; Neumayer, C.; Wojta, J.; Nanobachvili, J.; Huk, I.; Klinger, M. Simvastatin decreases free radicals formation in the human abdominal aortic aneurysm wall via NF-κB. Eur. J. Vasc. Endovasc. Surg 2012, 44, 133–137. [Google Scholar]
- Tu, Y.S.; Kang, X.L.; Zhou, J.G.; Lv, X.F.; Tang, Y.B.; Guan, Y.Y. Involvement of Chk1-Cdc25A-cyclin A/CDK2 pathway in simvastatin induced S-phase cell cycle arrest and apoptosis in multiple myeloma cells. Eur. J. Pharmacol 2011, 670, 356–364. [Google Scholar]
- Higgins, M.J.; Prowell, T.M.; Blackford, A.L.; Byrne, C.; Khouri, N.F.; Slater, S.A.; Jeter, S.C.; Armstrong, D.K.; Davidson, N.E.; Emens, L.A.; et al. A short-term biomarker modulation study of simvastatin in women at increased risk of a new breast cancer. Breast Cancer Res. Treat 2012, 131, 915–924. [Google Scholar]
- Podhorecka, M.; Halicka, D.; Klimek, P.; Kowal, M.; Chocholska, S.; Dmoszynska, A. Simvastatin and purine analogs have a synergic effect on apoptosis of chronic lymphocytic leukemia cells. Ann. Hematol 2010, 89, 1115–1124. [Google Scholar]
- Kochuparambil, S.T.; Al-Husein, B.; Goc, A.; Soliman, S.; Somanath, P.R. Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J. Pharmacol. Exp. Ther 2011, 336, 496–505. [Google Scholar]
- Bababeygy, S.R.; Polevaya, N.V.; Youssef, S.; Sun, A.; Xiong, A.; Prugpichailers, T.; Veeravagu, A.; Hou, L.C.; Steinman, L.; Tse, V. HMG-CoA reductase inhibition causes increased necrosis and apoptosis in an in vivo mouse glioblastoma multiforme model. Anticancer Res 2009, 29, 4901–4908. [Google Scholar]
- Polo, M.P.; Crespo, R.; de Bravo, M.G. Geraniol and simvastatin show a synergistic effect on a human hepatocarcinoma cell line. Cell Biochem. Funct 2011, 29, 452–458. [Google Scholar]
- Ruggenenti, P.; Cattaneo, D.; Rota, S.; Iliev, I.; Parvanova, A.; Diadei, O.; Ene-Iordache, B.; Ferrari, S.; Bossi, A.C.; Trevisan, R.; et al. Effects of combined ezetimibe and simvastatin therapy as compared with simvastatin alone in patients with type 2 diabetes: A prospective randomized double-blind clinical trial. Diabetes Care 2010, 33, 1954–1956. [Google Scholar]
- Schaafsma, D.; McNeill, K.D.; Mutawe, M.M.; Ghavami, S.; Unruh, H.; Jacques, E.; Laviolette, M.; Chakir, J.; Halayko, A.J. Simvastatin inhibits TGFβ1-induced fibronectin in human airway fibroblasts. Respir. Res 2011, 12, 113. [Google Scholar]
- Karadeniz Cakmak, G.; Irkorucu, O.; Ucan, B.H.; Emre, A.U.; Bahadir, B.; Demirtas, C.; Tascilar, O.; Karakaya, K.; Acikgoz, S.; et al. Simvastatin improves wound strength after intestinal anastomosis in the rat. J. Gastrointest. Surg 2009, 13, 1707–1716. [Google Scholar]
- Lei, J.; Gu, X.; Ye, Z.; Shi, J.; Zheng, X. Antiaging effects of simvastatin on vascular endothelial cells. Clin. Appl. Thromb. Hemost 2012. [Google Scholar] [CrossRef]
- Murtaza, G. Solubility enhancement of simvastatin: A review. Acta Pol. Pharm 2012, 69, 581–590. [Google Scholar]
- Chen, M.J.; Tang, W.Y.; Hsu, C.W.; Tsai, Y.T.; Wu, J.F.; Lin, C.W.; Cheng, Y.M.; Hsu, Y.C. Apoptosis induction in primary human colorectal cancer cell lines and retarded tumor growth in SCID mice by sulforaphane. Evid. Based Complement. Alternat. Med 2012, 2012, 415231. [Google Scholar]
- Kim, Y.R.; Byun, H.S.; Jeon, J.; Choi, B.L.; Park, K.A.; Won, M.; Zhang, T.; Shin, S.; Lee, H.; Oh, J. Apoptosis signal-regulating kinase1 is inducible by protein kinase Cδ and contributes to phorbol ester-mediated G1 phase arrest through persistent JNK activation. Cell Biochem. Biophys 2011, 61, 199–207. [Google Scholar]


© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liang, Y.-W.; Chang, C.-C.; Hung, C.-M.; Chen, T.-Y.; Huang, T.-Y.; Hsu, Y.-C. Preclinical Activity of Simvastatin Induces Cell Cycle Arrest in G1 via Blockade of Cyclin D-Cdk4 Expression in Non-Small Cell Lung Cancer (NSCLC). Int. J. Mol. Sci. 2013, 14, 5806-5816. https://doi.org/10.3390/ijms14035806
Liang Y-W, Chang C-C, Hung C-M, Chen T-Y, Huang T-Y, Hsu Y-C. Preclinical Activity of Simvastatin Induces Cell Cycle Arrest in G1 via Blockade of Cyclin D-Cdk4 Expression in Non-Small Cell Lung Cancer (NSCLC). International Journal of Molecular Sciences. 2013; 14(3):5806-5816. https://doi.org/10.3390/ijms14035806
Chicago/Turabian StyleLiang, Yu-Wei, Chi-Chang Chang, Chao-Ming Hung, Tzu-Yu Chen, Tzuu-Yuan Huang, and Yi-Chiang Hsu. 2013. "Preclinical Activity of Simvastatin Induces Cell Cycle Arrest in G1 via Blockade of Cyclin D-Cdk4 Expression in Non-Small Cell Lung Cancer (NSCLC)" International Journal of Molecular Sciences 14, no. 3: 5806-5816. https://doi.org/10.3390/ijms14035806



