LC-MS/MS Determination of Isoprostanes in Plasma Samples Collected from Mice Exposed to Doxorubicin or Tert-Butyl Hydroperoxide
Abstract
:1. Introduction
2. Results and Discussion
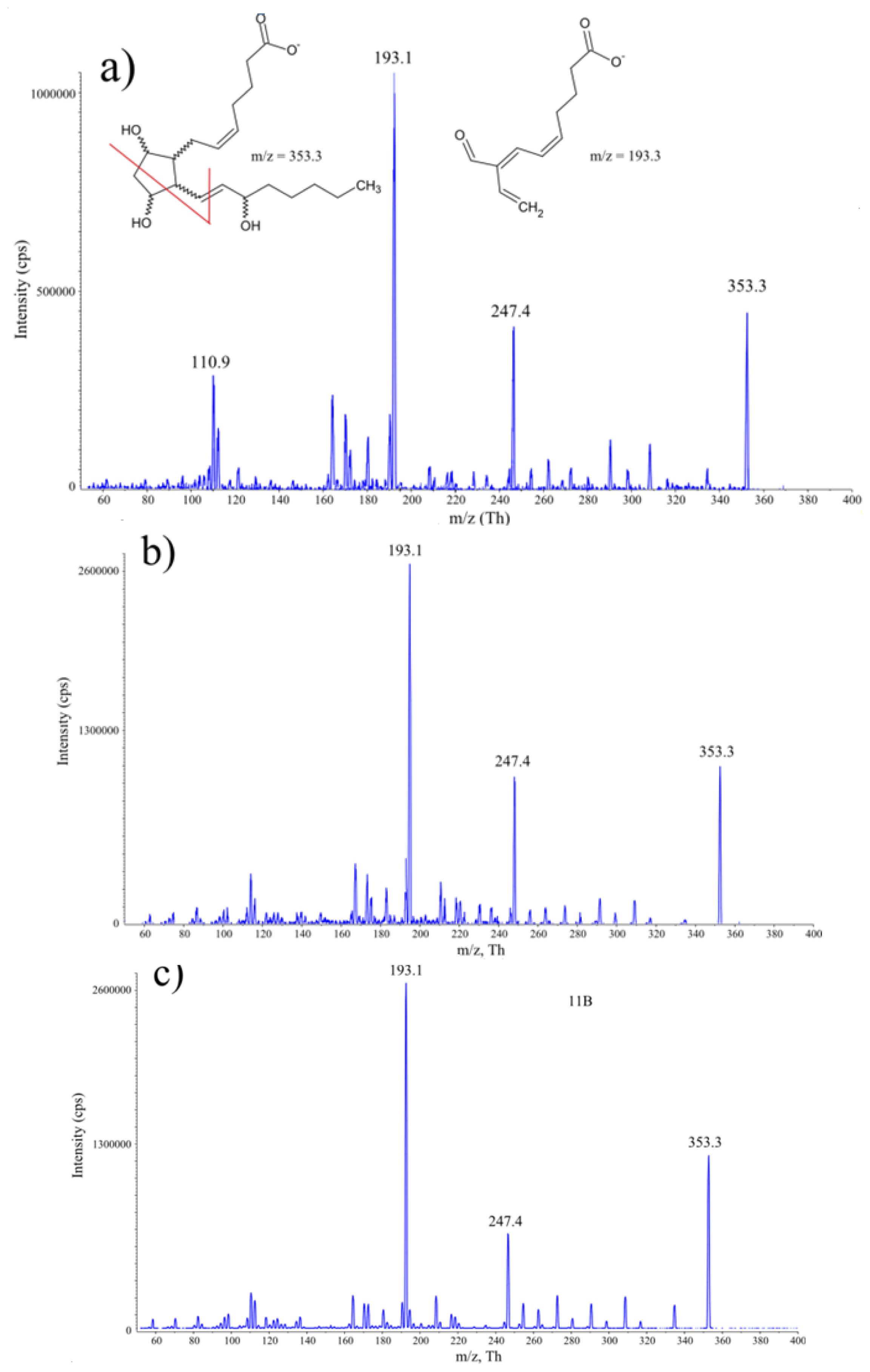
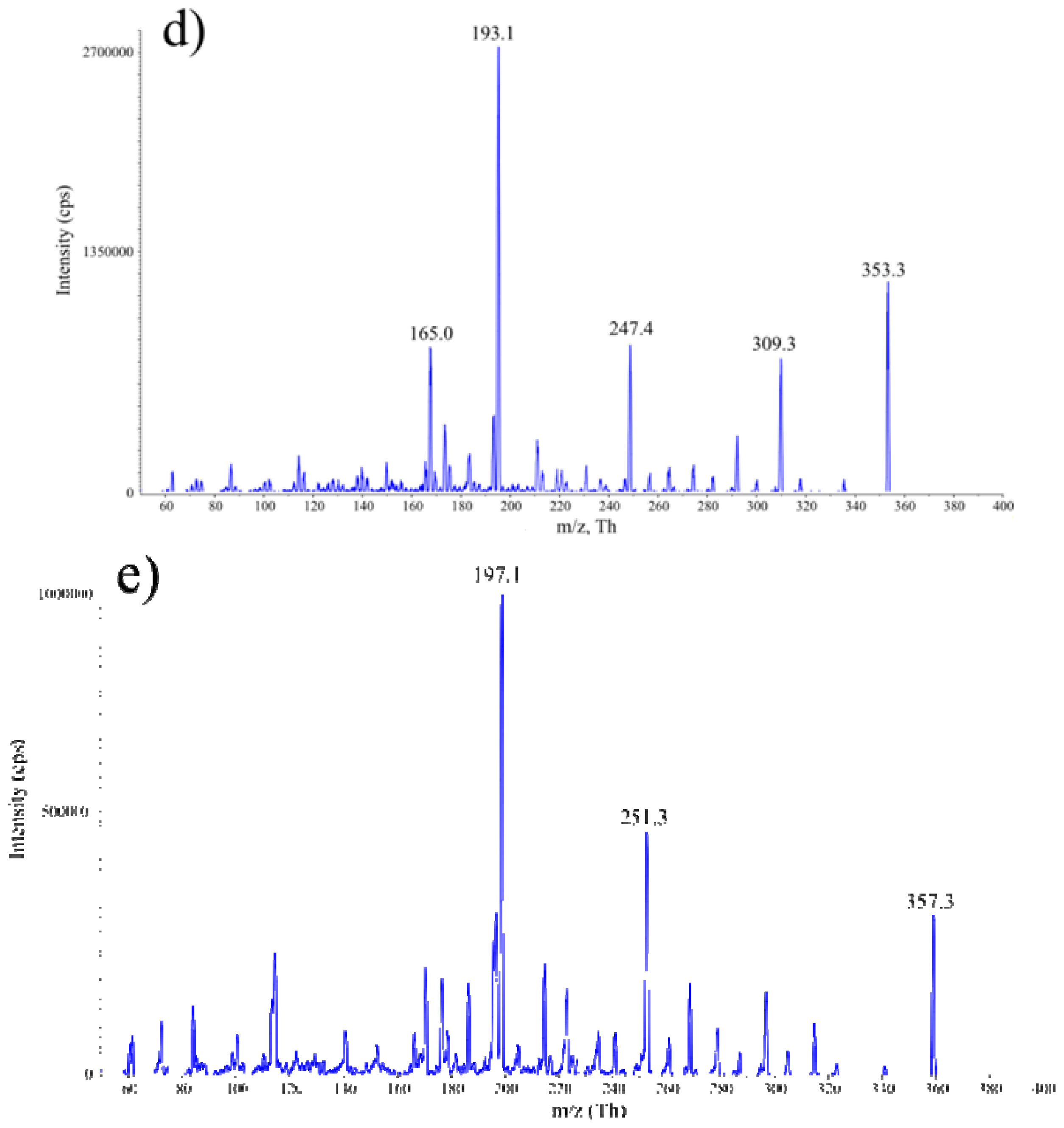
2.1. Development of HPLC-MS/MS Conditions
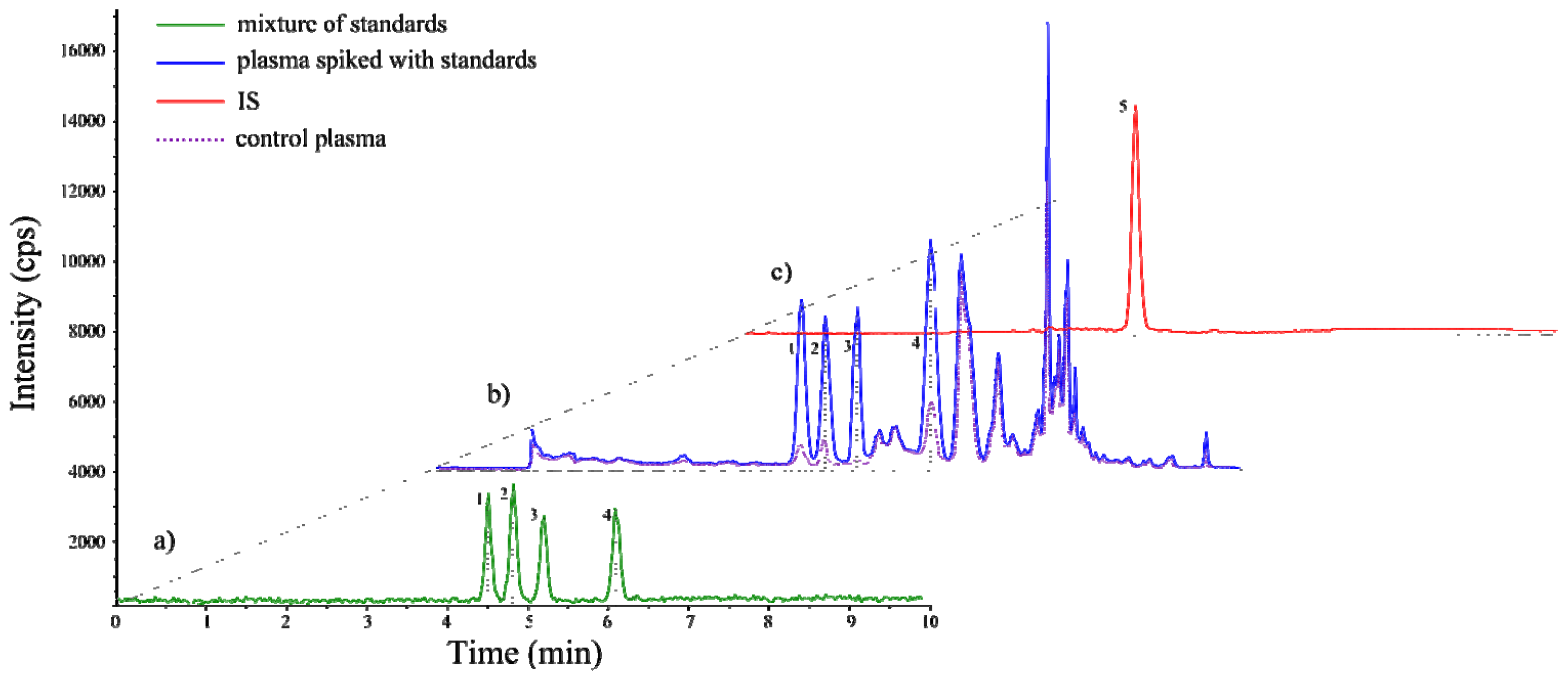
2.2. Method Validation
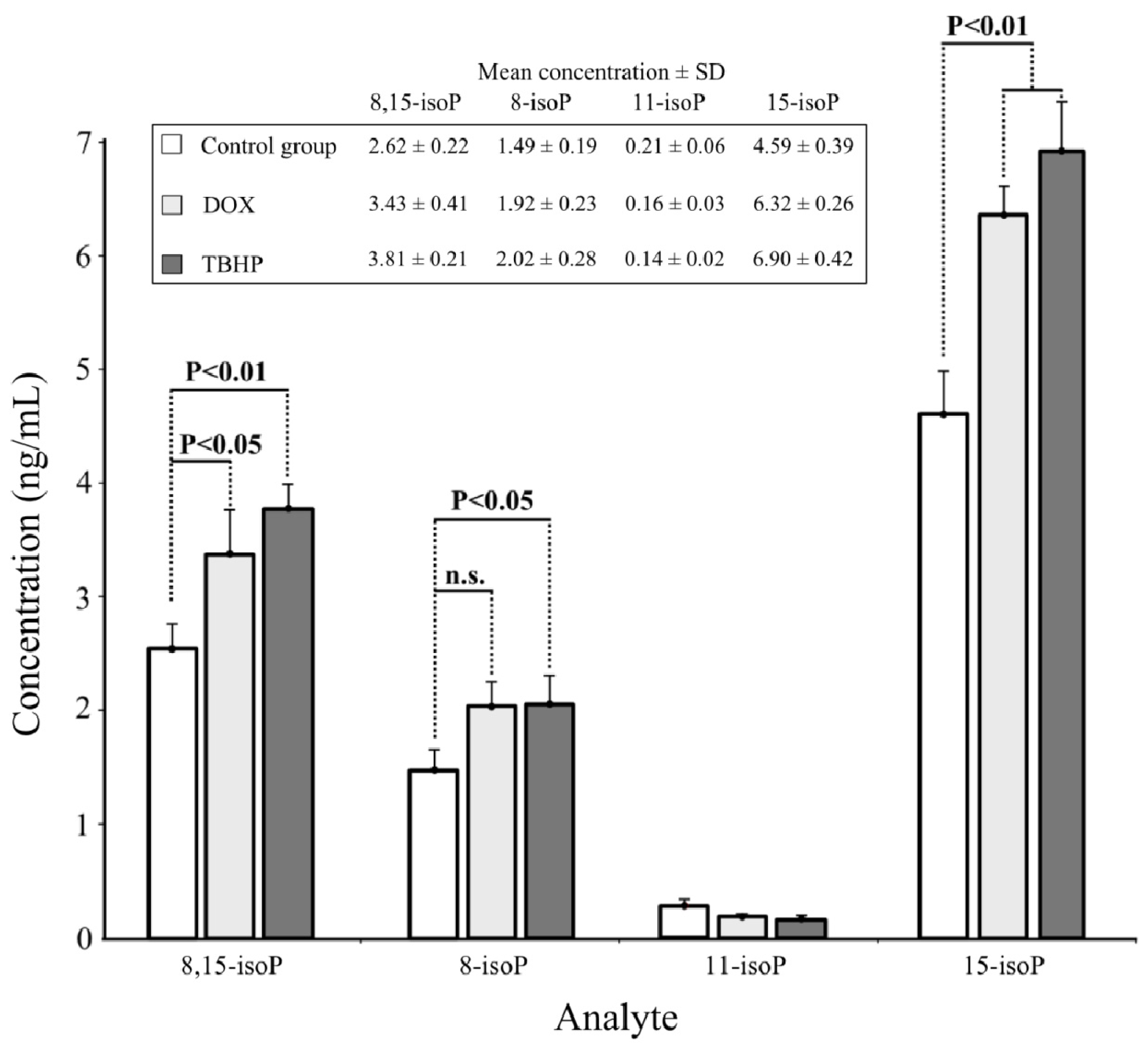
2.3. Real Sample Analysis
3. Materials and Methods
3.1. Reagents and Materials
3.2. Exposure of Mice to Oxidative Stress Inducers
3.3. Collection of Plasma Samples
3.4. Sample Preparation for Chromatographic Analyses
3.5. HPLC-MS/MS Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Mihelich, E.D. Structure and stereochemistry of novel endoperoxides isolated from the sensitized photooxidation of methyl linoleate. Implications for prostaglandin biosynthesis. J. Am. Chem. Soc 1980, 102, 7141–7143. [Google Scholar]
- Roberts, L.J.; Morrow, J.D. Measurements of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med 2000, 28, 505–513. [Google Scholar]
- Basu, S. F2-isoprostanes in human health and diseases: From molecular mechanisms to clinical implications. Antioxid. Redox Signal 2008, 10, 1405–1434. [Google Scholar]
- Pratico, D. The neurobiology of isoprostanes and Alzheimer’s disease. Biochim. Biophys. Acta 2010, 1801, 930–933. [Google Scholar]
- Geco, A.; Minghetti, L.; Levi, G. Isoprostanes, novel markers of oxidative injury help understanding the pathogenesis of neurodegenerative diseases. Neurochem. Res 2000, 25, 1357–1364. [Google Scholar]
- Seet, R.C.S.; Lee, C.J.; Lim, E.C.H.; Tan, J.J.H.; Quek, A.M.L.; Chong, W.; Looi, W.; Huang, S.; Wang, H.; Chan, Y.; et al. Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic. Biol. Med 2010, 48, 560–566. [Google Scholar]
- Meagher, E.A.; Barry, O.P.; Burke, A.; Lucey, M.R.; Lawson, J.A.; Rokach, J.U.; FitzGerald, G.A. Alcohol induced generation of lipid peroxidation products in humans. J. Clin. Invest 1998, 104, 805–813. [Google Scholar]
- Konishi, M.; Iwasa, M.; Araki, J.; Kobayashi, Y.; Katsuki, A.; Sumida, Y.; Nakagawa, N.; Kojima, Y.; Watanabe, S.; Adachi, Y.; et al. Increased lipid peroxidation in patients with non-alcoholic fatty liver disease and chronic hepatitis C as measured by the plasma level of 8-isoprostane. J. Gastroen. Hepatol 2006, 21, 1821–1825. [Google Scholar]
- Hasan, R.A.; O’Brien, E.; Mancuso, P. Lipoxin A4 and 8-isoprostane in the exhaled breath condensate of children hospitalized for status asthmaticus. Pediatr. Crit. Care Med 2012, 13, 141–145. [Google Scholar]
- Inonu, H.; Doruk, S.; Sahin, S.; Erkorkmaz, U.; Celik, D.; Celikel, S.; Seyfikli, Z. Oxidative stress levels in exhaled breath condensate associated with COPD and smoking. Respir. Care 2012, 57, 413–419. [Google Scholar]
- Il'yasova, D.; Spasojevic, I.; Base, K.; Zhang, H.; Wang, F.; Young, S.P.; Millington, D.S.; D’Agostino, R.B., Jr; Wagenknecht, L.E. Urinary F2-isoprostanes as a biomarker of reduced risk of type 2 diabetes. Diabetes Care 2012, 35, 173–174. [Google Scholar]
- Morrow, J.D. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler. Thromb. Vasc. Biol 2005, 25, 279–286. [Google Scholar]
- Schwedhelm, E.; Bartling, A.; Lenzen, H.; Tsikas, D.; Mass, R.; Brummer, J.; Gutzki, M.; Berger, J.; Frolich, J.C.; Boger, R.H. Urinary 8-isoprostaglandin-F2 as a risk marker in patients with coronary heart disease. Circulation 2004, 109, 843–848. [Google Scholar]
- Gago-Dominguez, M.; Castelao, J.E.; Pike, M.C.; Sevanian, A.; Haile, R.W. Role of lipid peroxidation in the epidemiology and prevention of breast cancer. Cancer Epidemiol. Biomark. Prev 2005, 14, 2829–2839. [Google Scholar]
- Nourooz-Zadeh, J.; Halliwell, B.; Anggard, E.E. Evidence for the formation of F3-isoprostanes during peroxidation of eicosapentaenoic acid. Biochem. Biophys. Res. Commun 1997, 236, 467–472. [Google Scholar]
- Roberts, L.J., II; Fessel, J.P.; Davies, S.S. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Brain Pathol. 2005, 15, 143–148. [Google Scholar]
- Winnik, W.; Kitchin, K.T. Measurement of oxidative stress parameters using liquid chromatography–tandem mass spectroscopy (LC-MS/MS). Toxicol. Appl. Pharmacol 2008, 233, 100–106. [Google Scholar]
- Janicka, M.; Kot-Wasik, A.; Kot, J.; Namiesnik, J. Isoprostanes-biomarkers of lipid peroxidation: their utility in evaluating oxidative stress and analysis. Int. J. Mol. Sci 2010, 11, 4631–4659. [Google Scholar]
- Lee, C.J.; Huang, S.H.; Jenner, A.M.; Halliwell, B. Measurement of F2-isoprostanes, hydroxyeicosatetraenoic products, and oxysterols from a single plasma sample. Free Radic. Biol. Med 2008, 44, 1314–1322. [Google Scholar]
- O’Sullivan, S.; Mueller, M.J.; Dahlén, S.E.; Kumlin, M. Analyses of prostaglandin D2 metabolites in urine: Comparison between enzyme immunoassay and negative ion chemical ionisation gas chromatography-mass spectrometry. Prostaglandins Other Lipid Mediat 1999, 57, 149–165. [Google Scholar]
- Liu, W.; Morrow, J.D.; Yin, H. Quantification of F2-isoprostanes as a reliable index of oxidative stress in vivo using gas chromatography–mass spectrometry (GC-MS) method. Free Radic. Biol. Med 2009, 47, 1101–1107. [Google Scholar]
- Samitas, K.; Chorianopoulos, D.; Vittorakis, S.; Zervas, E.; Economidou, E.; Papatheodorou, G.; Loukides, S.; Gaga, M. Exhaled cysteinyl-leukotrienes and 8-isoprostane in patients with asthma and their relation to clinical severity. Respir. Med 2009, 103, 750–756. [Google Scholar]
- Liu, X.; Whitefield, P.D.; Ma, Y. Quantification of F2-isoprostane isomers in cultured human lung epithelial cells after silica oxide and metal oxide nanoparticle treatment by liquid chromatography/tandem mass spectrometry. Talanta 2010, 81, 1599–1606. [Google Scholar]
- Tylor, A.W.; Bruno, S.R.; Frei, B.; Traber, M.G. Benefits of prolonged gradient separation for high-performance liquid chromatography–tandem mass spectrometry quantitation of plasma total 15-series F2-isoprostanes. Anal. Biochem 2006, 350, 41–51. [Google Scholar]
- Haschke, M.; Zhang, Y.L.; Kahle, C.; Klawitter, J.; Korecka, M.; Shaw, L.M.; Christians, U. HPLC-Atmospheric pressure chemical ionization MS/MS for quantification of 15-F2t-isoprostane in human urine and plasma. Clin. Chem 2007, 53, 489–497. [Google Scholar]
- Kennedy, C.H.; Church, D.F.; Winston, G.W.; Pryor, W.A. Tert-butyl-hydroperoxide induced radical production in rat liver mitochondia. Free Radic. Biol. Med 1992, 12, 381–387. [Google Scholar]
- Paur, E.; Youngman, R.J.; Lengfelder, E.; Elstner, E.F. Mechanism of adriamycin-dependent oxygen activation catalysed by NADPH-cytochrome c-(ferrodoxin)-oxidoreductase. Z. Naturforsch 1984, 39c, 261–267. [Google Scholar]
- Ohashi, N.; Yoshikawa, M. Rapid and sensitive quantification of 8-isoprostaglandin F2α in human plasma and urine by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. B 2000, 746, 17–24. [Google Scholar]
- Janicka, M.; Kubica, P.; Kot-Wasik, A.; Kot, J.; Namienik, J. Sensitive determination of isoprostanes in exhaled breath condensate samples with use of liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2012, 893–894, 144–149. [Google Scholar]
- Klawitter, J.; Haschke, M.; Shokati, T.; Klawitter, J.; Christians, U. Quantification of 15-F2t-isoprostane in human plasma and urine: results from enzyme-linked immunoassay and liquid chromatography/tandem mass spectrometry cannot be compared. Rapid Commun. Mass Spectrom 2011, 25, 463–468. [Google Scholar]
- Sicilia, T.; Mally, A.; Schauer, U.; Pähler, A.; Völkel, W. LC-MS/MS methods for the detection of isoprostanes (iPF2alpha-III and 8,12-iso-iPF2alpha-VI) as biomarkers of CCl4-induced oxidative damage to hepatic tissue. J. Chromatogr. B 2008, 861, 48–55. [Google Scholar]
| Analyte | Curve equation | R2 | LOD (pg/mL) | LOQ (pg/mL) | |
|---|---|---|---|---|---|
| Plasma sample | Water sample | ||||
| 8,15-isoP | y = 0.000084x + 0.0019 | y = 0.000084x + 0.0006 | 0.999 | 15 | 50 |
| 8-isoP | y = 0.000094x + 0.015 | y = 0.000094x + 0.011 | 0.999 | 12 | 40 |
| 11-isoP | y = 0.000075x + 0.00053 | y = 0.000075x + 0.00041 | 0.999 | 17 | 51 |
| 15-isoP | y = 0.000070x + 0.0013 | y = 0.000070x + 0.0008 | 0.999 | 15 | 50 |
| Analyte | Recovery (%) †,‡ | ||
|---|---|---|---|
| 50 pg/mL | 100 pg/mL | 250 pg/mL | |
| 8,15-isoP | 98.8 ± 5.4 | 99.5 ± 3.4 | 99.8 ± 1.2 |
| 8-isoP | 100.6 ± 5.1 | 99.5 ± 3.2 | 100.7 ± 1.9 |
| 11-isoP | 99.4 ± 7.8 | 97.9 ± 2.3 | 99.5 ± 2.7 |
| 15-isoP | 99.2 ± 8.6 | 100.9 ± 2.5 | 99.4 ± 1.8 |
| Analyte | Recovery (%) †,‡ | |||
|---|---|---|---|---|
| Intra-day | Inter-day | |||
| 8,15-isoP | 98.9 ± 3.8 | 98.2 ± 2.9 | 100.6 ± 2.0 | 99.2 ± 1.2 |
| 8-isoP | 99.9 ± 4.3 | 99.6 ± 3.0 | 100.1 ± 3.2 | 99.9 ± 0.2 |
| 11-isoP | 97.2 ± 7.1 | 97.9 ± 1.9 | 100.2 ± 3.7 | 98.5 ± 1.6 |
| 15-isoP | 109.0 ± 1.5 | 108.0 ± 3.9 | 113.3 ± 6.1 | 110.1 ± 2.6 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Janicka, M.; Kot-Wasik, A.; Paradziej-Łukowicz, J.; Sularz-Peszyńska, G.; Bartoszek, A.; Namieśnik, J. LC-MS/MS Determination of Isoprostanes in Plasma Samples Collected from Mice Exposed to Doxorubicin or Tert-Butyl Hydroperoxide. Int. J. Mol. Sci. 2013, 14, 6157-6169. https://doi.org/10.3390/ijms14036157
Janicka M, Kot-Wasik A, Paradziej-Łukowicz J, Sularz-Peszyńska G, Bartoszek A, Namieśnik J. LC-MS/MS Determination of Isoprostanes in Plasma Samples Collected from Mice Exposed to Doxorubicin or Tert-Butyl Hydroperoxide. International Journal of Molecular Sciences. 2013; 14(3):6157-6169. https://doi.org/10.3390/ijms14036157
Chicago/Turabian StyleJanicka, Monika, Agata Kot-Wasik, Jolanta Paradziej-Łukowicz, Grażyna Sularz-Peszyńska, Agnieszka Bartoszek, and Jacek Namieśnik. 2013. "LC-MS/MS Determination of Isoprostanes in Plasma Samples Collected from Mice Exposed to Doxorubicin or Tert-Butyl Hydroperoxide" International Journal of Molecular Sciences 14, no. 3: 6157-6169. https://doi.org/10.3390/ijms14036157




