Peripheral Reproductive Organ Health and Melatonin: Ready for Prime Time
Abstract
:1. Introduction
2. Melatonin: Improving Peripheral Reproductive Health
3. Melatonin: Female Reproductive Health
3.1. Ovary
3.2. Placenta
3.3. Amnion and Amniotic Fluid
3.4. Parturition
4. Melatonin: Male Reproductive Health
4.1. Sperm in Situ
4.2. Male Accessory Sex Organs
4.3. Protection of Ejaculated Animal Sperm
4.4. Protection of Ejaculated Human Sperm
5. Epilogue
Conflict of Interest
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, Y.; Mori, W. Isolation of melatonin, the pineal factor that lightens melanocytes. J. Am. Chem. Soc 1958, 80, 2587. [Google Scholar]
- Launay, J.M.; Lemaitre, B.J.; Husson, H.P.; Dreux, C.; Hartmann, L.; da Prada, M. Melatonin synthesis by rabbit platelets. Life Sci 1982, 31, 1487–1494. [Google Scholar]
- Reiter, R.J.; Richardson, B.A.; Matthews, S.A.; Lane, S.J.; Ferguson, B.N. Rhythms of immunoreactive melatonin in the retina and Harderian glands of rats: Persistence after pinealectomy. Life Sci 1983, 32, 1299–1236. [Google Scholar]
- Huether, G.; Poeggeler, B.; Reimer, A.; George, A. Effects of tryptophan administration on circulating melatonin levels in chicks and rats: Evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci 1992, 51, 946–953. [Google Scholar]
- Abe, M.; Itoh, M.T.; Miyata, M.; Shimizu, K.; Sumi, Y. Circadian rhythm of serotonin N-acetyltransferace activity in rat lens. Exp. Eye Res 2000, 70, 805–808. [Google Scholar]
- Reed, B.L. The control of circadian pigment changes in the pencil fish: A proposed role for melatonin. Life Sci 1968, 7, 961–973. [Google Scholar]
- Panke, E.S.; Rollag, M.D.; Reiter, R.J. Pineal melatonin concentrations in the Syrian hamster. Endocrinology 1979, 104, 194–197. [Google Scholar]
- Birkeland, A.J. Plasma melatonin levels and nocturnal transitions between sleep and wakefulness. Neuroendocrinology 1982, 34, 126–131. [Google Scholar]
- Lewy, A.J.; Sack, R.L.; Miller, L.S.; Hoban, T.M.; Singer, C.M.; Samples, J.R.; Krauss, G.L. The use of plasma melatonin levels and light in the assessment and treatment of chronobiologic sleep and mood disorders. J. Neural Transm 1986, 21, 311–322. [Google Scholar]
- Reiter, R.J. Pineal control of a seasonal reproductive rhythm in male golden hamsters exposed to natural daylight and temperature. Endocrinology 1973, 92, 423–430. [Google Scholar]
- Reiter, R.J.; Vaughan, M.K.; Blask, D.E.; Johnson, L.Y. Melatonin: Its inhibition of pineal antigonadotrophic activity in male hamsters. Science 1974, 185, 1169–1171. [Google Scholar]
- Lincoln, G.A.; Short, R.V. Seasonal breeding: Nature’s contraceptive. Rec. Progr. Horm. Res 1980, 36, 1–52. [Google Scholar]
- Reiter, R.J. The melatonin rhythm: Both a clock and a calendar. Expenientia 1993, 49, 654–664. [Google Scholar]
- Molina-Carballo, A.; Munoz-Hoyos, A.; Reiter, R.J.; Sanchez-Forte, M.; Moreno-Madrid, F.; Rufo-Campos, M.; Molina-Font, J.A.; Acuna-Castroviejo, D. Utility of high doses of melatonin as adjunctive anticonvulsant therapy in a child with severe myoclonic epilepsy: Two years’ experience. J. Pineal Res 1997, 23, 97–106. [Google Scholar]
- Swarnakar, S.; Paul, S.; Singh, L.P.; Reiter, R.J. Matrix metalloproteinases in health and disease: Regulation by melatonin. J. Pineal Res 2011, 50, 8–20. [Google Scholar]
- Tosini, G.; Baba, K.; Hwang, C.K.; Iuvane, D.M. Melatonin, an underappreciated player in retinal physiology and pathophysiology. Exp. Eye Res 2012, 103, 82–89. [Google Scholar]
- Escames, G.; Ozturk, G.; Bano-Otalora, B.; Pozo, M.J.; Madrid, J.A.; Reiter, R.J.; Serrano, E.; Concepcion, M.; Acuna-Castroviejo, D. Exercise and melatonin in humans: Reciprocal benefits. J. Pineal Res 2012, 52, 1–11. [Google Scholar]
- Cardinali, D.P.; Srinivasan, V.; Brzezinski, A.; Brown, G.M. Melatonin and its analogs in insomnia and depression. J. Pineal Res 2012, 52, 365–375. [Google Scholar]
- Axelrod, J.; Wurtman, R.J.; Snyder, S.H. Control of hydroxyindole-O-methyltransferase activity in the rat pineal gland by environment lighting. J. Biol. Chem 1965, 240, 949–954. [Google Scholar]
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: Implications for an ancient antioxidant system. Cell. Mol. Biol. Res 1996, 41, 391–395. [Google Scholar]
- Tilden, A.R.; Becker, M.A.; Amma, L.L.; Arciniega, J.; McGaw, A.K. Melatonin production in an aerobic photosynthetic bacterium: An evolutionarily early association with darkness. J. Pineal Res 1997, 22, 102–106. [Google Scholar]
- Poeggeler, B.; Hardeland, R. Detection and quantification of melatonin in a dinoflagellate, Gonyaulax polyedra: Solutions to the problem of methoxyindole destruction in non-vertebrate material. J. Pineal Res 1994, 17, 1–10. [Google Scholar]
- Hardeland, R.; Balzer, I.; Poeggeler, B.; Fuhrberg, B.; Uria, H.; Behrmann, G.; Wolf, R.; Meyer, T.J.; Reiter, R.J. On the primary functions of melatonin in evolution: Mediation of photoperiodic signals in a unicell, photooxidation, and scavenging of free radicals. J. Pineal Res 1995, 18, 104–111. [Google Scholar]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiawa, H.W.; Schlvat, W. Melatonin in edible plants identified by radioimmunoassay and high performance liquid chromatography-mass spectrometry. J. Pineal Res 1995, 18, 28–31. [Google Scholar]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamato, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int 1995, 35, 627–634. [Google Scholar]
- Kolar, J.; Machackova, I. Melatonin in higher plants: Occurrence and possible functions. J. Pineal Res 2005, 39, 333–341. [Google Scholar]
- Stankov, B.; Reiter, R.J. Melatonin receptors: Current status, facts and hypotheses. Life Sci 1990, 46, 971–982. [Google Scholar]
- Reppert, S.M.; Henshaw, D.; Schwartz, W.J.; Weaver, D.R. The circadian-gated timing of birth in rats: Disruption by maternal SCN lesions or by removal of the fetal brain. Brain Rec 1987, 403, 398–402. [Google Scholar]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar]
- Acuna-Castroviejo, D.; Reiter, R.J.; Menendez-Pelaez, A.; Pablos, M.I.; Burgos, A. Characterization of high-affinity melatonin binding sites in purified cell nuclei. J. Pineal Res 1994, 16, 100–112. [Google Scholar]
- Steinhilber, D.; Brungs, M.; Werz, O.; Weisenberg, I.; Danielsson, C.; Kahlen, J.P.; Nayeri, S.; Schrader, M.; Carlberg, C. The nuclear receptor for melatonin represses 5-lipoxygenase gene expression in human B lymphocytes. J. Biol. Chem 1995, 270, 7037–7040. [Google Scholar]
- Lardone, P.J.; Guerrero, J.M.; Fernandez-Santos, J.M.; Rubio, A.; Martin-Lacave, F.; Carrillo-Vico, A. Melatonin synthesized in T-lymphocytes as a ligand of the retinoic acid-related orphan receptor. J. Pineal Res 2011, 51, 454–462. [Google Scholar]
- Benitez-King, G.; Huerto-Delgadillo, L.; Anton-Tay, F. Binding of H3-melatonin to calmodulin. Life Sci 1993, 53, 201–207. [Google Scholar]
- Pozo, D.; Reiter, R.J.; Calvo, J.R.; Guerrero, J.M. Inhibition of cerebellar nitric oxide synthase and cyclic GMP production by melatonin via complex formation with calmodulin. J. Cell Biochem 1997, 65, 430–442. [Google Scholar]
- Boutin, J.A. Melatonin binding site MT3 is QR2: State of the art. J. Soc. Biol 2007, 201, 97–103. [Google Scholar]
- Wang, X. The antiapoptotic activity in neurodegenerative diseases. CNS Neurosci. Ther 2009, 15, 345–357. [Google Scholar]
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J 1993, 1, 57–60. [Google Scholar]
- Hardeland, R.; Reiter, R.J.; Poeggeler, B.; Tan, D.X. The significance of the metabolism of the neurohormone melatonin: Antioxidative protection and formation of bioactive substances. Neurosci. Biobehav. Rev 1993, 17, 347–357. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Oi, W.; Kim, S.J.; El-Sokkary, G.H. Ischemia/reperfusion-induced arrhythmias in the isolated rat heart: Prevention by melatonin. J. Pineal Res 1999, 25, 184–191. [Google Scholar]
- Reiter, R.J.; Paradies, S.D.; Manchester, L.C.; Tan, D.X. Reducing oxidative/nitrosative stress: A newly-discovered gene for melatonin. Crit. Rev. Biochem. Mol. Biol 2009, 44, 175–200. [Google Scholar]
- Sewerynek, E.; Abe, M.; Reiter, R.J.; Barlow-Walden, L.R.; Chen, L.; McCabe, T.J.; Roman, L.J.; Diaz-Lopez, B. Melatonin administration prevents lipopolysaccharide-induced oxidative damage in phenobarbital-treated animals. J. Cell. Biochem 1995, 58, 436–444. [Google Scholar]
- Melchiorri, D.; Reiter, R.J.; Sewerynek, E.; Hara, M.; Chen, L.; Nistico, G. Paraquat toxicity and oxidative damage: Reduction by melatonin. Biochem. Pharmacol 1996, 51, 1095–1099. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Koppisepi, S. Medical implications of melatonin: Receptor-mediated and receptor-independent actions. Adv. Med. Soc 2007, 52, 11–28. [Google Scholar]
- Hardeland, R.; Pandi-Perumal, S.R. Melatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab 2005, 2, 22. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept 2000, 9, 137–159. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res 2007, 42, 28–42. [Google Scholar]
- Hardeland, R.; Tan, D.X.; Reiter, R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res 2009, 47, 109–126. [Google Scholar]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res 2013, in press. [Google Scholar]
- Gomez, F.J.; Raba, J.; Cerutti, S.; Silva, M.F. Monitoring melatonin and its isomes in Vitis vinifera cv Malbed by UHPLC-MS/MS from grape to bottle. J. Pineal Res 2012, 52, 349–355. [Google Scholar]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Rosales-Corral, S.; Coto-Montes, A.; Boga, J.A.; Reiter, R.J. Emergence of naturally occurring melatonin isomers and their proposed nomenclature. J. Pineal Res 2012, 53, 113–121. [Google Scholar]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Irit, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J. Pineal Res 2013, in press. [Google Scholar]
- Iriti, M.; Varoni, E.M.; Vitalini, S. Melatonin in traditional Mediterranean diets. J. Pineal Res 2010, 49, 101–105. [Google Scholar]
- Luchetti, F.; Canonico, B.; Betti, M.; Arcangeletti, M.; Pilotti, F.; Piroddi, M.; Canesi, L.; Papa, S.; Galli, F. Melatonin signaling and cell protection function. FASEB J 2010, 24, 3603–3624. [Google Scholar]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants and perspectives in nutritional and agricultural science. J. Exp. Biol 2012, 63, 577–597. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Paredes, S.D.; Mayo, J.C.; Sainz, R.M. Melatonin and reproduction revisited. Biol. Reprod 2009, 81, 445–456. [Google Scholar]
- Mauriz, J.L.; Callado, D.S.; Veneroso, C.; Reiter, R.J.; Gonzalez-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res 2013, 54, 1–14. [Google Scholar]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Avanzas, R. The role of melatonin in acute myocardial infarction. Front. Biosci 2012, 17, 2433–2441. [Google Scholar]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr. J 2013, in press. [Google Scholar]
- Maldonado, M.D.; Murrillo-Cabezas, F.; Terron, M.P.; Flores, L.F.; Tan, D.X.; Manchester, L.C.; Reiter, R.J. The potential of melatonin in reducing morbidity-mortality after craniocerebral trauma. J. Pineal Res 2006, 42, 1–11. [Google Scholar]
- Gitto, E.; Pellegrino, S.; Gitto, P.; Barberi, I.; Reiter, R.J. Oxidative stress of the newborn in the pre- and postnatal period and the clinical utility of melatonin. J. Pineal Res 2008, 46, 128–139. [Google Scholar]
- Hoffman, R.A.; Reiter, R.J. Pineal gland: Influence on gonads of male hamsters. Science 1995, 148, 1609–1611. [Google Scholar]
- Reiter, R.J. The pineal and its hormones in the control of reproduction in mammals. Endocr. Rev 1980, 1, 109–131. [Google Scholar]
- Brainard, G.C.; Petterbarg, L.J.; Richardson, B.A.; Reiter, R.J. Pineal melatonin in Syrian hamsters: Circadian and seasonal rhythms in animals maintained under laboratory and nocturnal conditions. Neuroendocrinology 1982, 35, 342–348. [Google Scholar]
- Reiter, R.J. Evidence for refractoriness of the pituitary-gonadal axis to the pineal gland in golden hamsters and its possible implications in annual reproductive rhythms. Anal. Rec 1972, 173, 365–371. [Google Scholar]
- Carter, D.S.; Goldman, B.D. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): Duration is the critical parameter. Endocrinology 1983, 113, 1261–1266. [Google Scholar]
- Karsch, F.J.; Bittman, E.L.; Foster, D.L.; Goodman, R.L.; Legan, S.J.; Robinson, J.E. Neuroendocrine basis of seasonal reproduction. Rec. Progr. Horm. Res 1984, 41, 185–232. [Google Scholar]
- Lincoln, G.A. Decoding the nightly melatonin signal through circadian clockwork. Mol. Cell. Endocrinol 2006, 252, 69–73. [Google Scholar]
- Barrett, P.; Bolboren, M. Molecular pathways involved in the seasonal body weight and reproductive responses governed by melatonin. J. Pineal Res 2012, 52, 376–388. [Google Scholar]
- Vanecek, J. Cellular mechanisms of melatonin action. Physiol. Rev 1998, 78, 687–721. [Google Scholar]
- Ishii, H.; Tanaka, N.; Kobayashi, M.; Kato, M.; Sakuma, Y. Gene structures, biochemical characterization and distribution of rat melatonin receptors. J. Physiol. Sci 2009, 59, 37–47. [Google Scholar]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol 2011, 93, 350–384. [Google Scholar]
- Ekmekcioglu, C. Melatonin receptors in humans: Biological role and clinical relevance. Biomed. Pharmacother 2006, 60, 97–108. [Google Scholar]
- Niles, L.P.; Wang, J.; Shen, L.; Lobb, D.K.; Younglai, E.V. Melatonin receptor mRNA expression in human granulosa cells. Mol. Cell. Endocrinol 1999, 156, 107–110. [Google Scholar]
- Soares, J.M., Jr; Masana, M.I.; Ersahin, D.; Dubocovich, M.L. Functional melatonin receptors in rat ovaries at various stages of the estrous cycle. J. Pharmacol. Exp. Ther. 2003, 306, 694–702. [Google Scholar]
- Brzezinski, A.; Seibel, M.M.; Lynch, H.J.; Deng, M.; Wurtman, R.J. Melatonin in human preovulatory follicular fluid. J. Clin. Endocrinol. Metab 1987, 64, 865–867. [Google Scholar]
- Ronnberg, L.; Kauppila, A.; Leppaluoto, J.; Martikainen, H.; Vakkuri, O. Circadian and seasonal variation in human preovulatory fluid melatonin concentration. J. Clin. Endocrinol. Metab 1990, 71, 492–496. [Google Scholar]
- Nakamura, Y.; Tamura, H.; Takayama, H.; Kato, H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil. Steril 2003, 80, 1012–1016. [Google Scholar]
- Espey, L.L. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol. Reprod 1994, 50, 233–238. [Google Scholar]
- Shi, J.M.; Tian, X.Z.; Zhan, G.B.; Wang, L.; Gao, C.; Zhu, S.E.; Zeng, S.M.; Tian, J.H.; Liu, G.S. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J. Pineal Res 2009, 47, 318–323. [Google Scholar]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological implication. Fertil. Steril 2009, 92, 328–343. [Google Scholar]
- Salhab, M.; Dhorne-Pollets, S.; Auclair, S.; Guyader-Joly, C.; Brisard, D.; Dalbies-Tran, R.; Dupont, J.; Ponsart, G.; Mermillod, P.; Uzbekova, S. In vitro maturation of oocytes alters gene expression and signaling pathways in bovine cumulus cells. Mol. Reprod. Dev 2013, 80, 166–182. [Google Scholar]
- Johnston, J.D.; Bashforth, R.; Diack, A.; Andersson, H.; Lincoln, G.A.; Hazelrigg, D.G. Rhythmic melatonin secretion does not correlate with the expression of arylalkylamine N-acetyltransferase, inducible cyclic AMP early repressor period 1 or cytochrome 1 mRNA in the sheep pineal. Neuroscience 2004, 124, 789–795. [Google Scholar]
- Liu, T.; Borjigin, J. N-acetyltransferase is not the rate limiting enzyme of melatonin synthesis at night. J. Pineal Res 2005, 39, 91–96. [Google Scholar]
- Brannstrom, M.; Enskog, A. Leukocyte networks and ovulation. J. Reprod. Immunol 2002, 57, 47–60. [Google Scholar]
- Tamanini, C.; de Ambrogi, M. Angiogenesis in developing follicle and corpus luteum. Reprod. Domest. Anim 2004, 39, 206–216. [Google Scholar]
- Richards, J.S. Ovulation: New factors that prepare the oocyte for fertilization. Mol. Cell. Endocrinol 2005, 234, 75–79. [Google Scholar]
- Brannstrom, M.; Norman, R.J. Involvement of leucocytes and cytokines in the ovulatory process and corpus luteum function. Hum. Reprod 1993, 8, 1762–1775. [Google Scholar]
- Behrman, H.R.; Kodaman, P.H.; Preston, S.L.; Gao, S. Oxidative stress and the ovary. J. Soc. Gynecol. Investig 2001, 8, S40–S42. [Google Scholar]
- Sugino, N. Reactive oxygen species in ovarian physiology. Reprod. Med. Biol 2005, 4, 31–44. [Google Scholar]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res 2011, 51, 1–16. [Google Scholar]
- Allegra, M.; Reiter, R.J.; Tan, D.X.; Gentile, C.; Tesoriere, L.; Livrea, M.A. The chemistry of melatonin’s interaction with reactive species. J. Pineal Res 2003, 34, 1–10. [Google Scholar]
- Antolin, I.; Rodriguez, C.; Sainz, R.M.; Mayo, J.C.; Uria, H.; Kotler, M.L.; Rodriguez-Colunga, M.J.; Toliva, D.; Menendez-Pelaez, A. Neurohormone melatonin prevents cell damage: Effect on gene expression for antioxidant enzymes. FASEB J 1996, 10, 882–890. [Google Scholar]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res 2004, 36, 1–9. [Google Scholar]
- Tomas-Zapico, C.; Coto-Montes, A. A proposed mechanism to explain the stimulating effect of melatonin on antioxidative enzymes. J. Pineal Res 2005, 39, 99–104. [Google Scholar]
- Tamura, H.; Nakamura, Y.; Terron, M.P.; Flores, L.J.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and pregnancy in the human. Reprod. Toxicol 2008, 25, 291–303. [Google Scholar]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res 2008, 44, 280–287. [Google Scholar]
- El-Raey, M.; Geshi, M.; Somfai, T.; Kaneda, M.; Hirako, M.; Abdel-Ghaffar, A.E.; Sosa, G.A.; Abau El-Roos, M.E.A.; Nagai, T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev 2011, 78, 250–262. [Google Scholar]
- Qian, Y.; Shi, W.Q.; Ding, J.T.; Sha, J.H.; Fan, B.Q. Predictive value of the area of expanded cumulus mass on development of porcine oocytes matured and fertilized in vitro. J. Reprod. Dev 2003, 49, 167–174. [Google Scholar]
- Gutinsky, C.; Dalvit, G.C.; Pintos, L.N.; Thompson, J.G.; Beconi, M.T.; Cetica, P.D. Influence of hyaluronic acid synthesis and cumulus mucification on bovine oocyte in vitro maturation, fertilisation and embryo development. Reprod. Fertil. Dev 2007, 19, 488–497. [Google Scholar]
- Benitez-King, G.; Soto-Vega, E.; Dominguez-Rodriguez, G. Melatonin modulates microfilament phenotypes in epithelial cells: Implications for adhesion and inhibition of cancer cell migration. Histol. Histopathol 2009, 24, 789–799, ,. [Google Scholar]
- Acuna-Castroviejo, D.; Escames, G.; Rodriguez, M.; Lopez, L.C. Melatonin role in the mitochondrial function. Front. Biosci 2007, 12, 947–963. [Google Scholar]
- Rocha, R.M.P.; Lima, L.F.; Alves, A.M.C.V.; Celestino, J.J.H.; Matos, M.H.T.; Lima-Verde, I.B.; Bernuci, M.P.; Lopes, C.A.P.; Bao, S.N.; Campello, C.C.; et al. Interaction between melatonin and follicle-stimulating hormone promotes in vitro development of caprine preantral follicles. Dom. Anim. Endocrinol 2013, 44, 1–9. [Google Scholar]
- Dhalpuria, S.; Vyas, S.; Purohit, G.N.; Patkak, K.M. Songraphic monitoring of early follicle growth induced by melatonin implants in camels and the subsequent fertility. J. Ultrasound 2012, 15, 135–141. [Google Scholar]
- Wilson, P.R.; Walker, I.H.; Bond, D.B.; Middleberg, A.; Staples, L.D. Field evaluation of melatonin implants to advance the breeding season in 1-year-old formed red deer hinds. N. Z. Vet. J 1991, 39, 23–28. [Google Scholar]
- Chemineau, P.; Berthelot, X.; Daneau, A.; Maurice, F.; Viguie, C.; Malpaux, B. Can melatonin be used in out-of-season reproduction in domestic mammals? Contracept. Fertil. Sex 1993, 21, 733–738, [in French]. [Google Scholar]
- De Nicolo, G.; Morris, S.T.; Kenyon, P.R.; Morel, P.C.; Parkinson, T.J. Melatonin-improved reproductive performance in sheep bred out of season. Anim. Reprod. Sci 2008, 109, 124–133. [Google Scholar]
- Scott, P.R.; Sargison, N.D.; Macrae, A.L.; Gaugh, M.R. Melatonin treatment prior to the normal breeding season increases fetal number in United Kingdom sheep flocks. Vet. J 2009, 182, 198–202. [Google Scholar]
- Sirotkin, A.V.; Schaeffer, H.J. Direct regulation of mammalian reproductive organs by serotonin and melatonin. J. Endocrinol 1997, 154, 1–5. [Google Scholar]
- Murayama, T.; Kawashima, M.; Takahashi, T.; Yasuoka, T.; Kuwayama, T.; Tanaka, K. Direct action of melatonin on hen ovarian granulosa cells to lower responsiveness to luteinizing hormone. Proc. Soc. Exp. Biol 1997, 215, 386–392. [Google Scholar]
- Tamura, H.; Nakamura, Y.; Takiguchi, S.; Kashida, S.; Yamagata, Y.; Sugino, N.; Kato, H. Melatonin directly suppresses steroid production by preovulatory follicles in the cyclic hamster. J. Pineal Res 1998, 35, 135–141. [Google Scholar]
- Woo, M.M.M.; Tai, C.J.; Kang, S.K.; Nathwani, P.M.; Pang, S.F.; Leung, P.C.K. Direct action of melatonin in human granulosa-luteal cells. J. Clin. Endocrinol. Metab 2001, 86, 4789–4797. [Google Scholar]
- Fiske, V.M.; Parker, K.L.; Ulmer, R.A.; Ow, C.H.; Aziz, N. Effect of melatonin alone or in combination with human chorionic gonadotropin or ovine luteinizing hormone on the in vitro secretion of estrogens or progesterone by granulosa cells of rats. Endocrinology 1984, 114, 407–410. [Google Scholar]
- Baratta, M.; Tamanini, C. Effect of melatonin on the in vitro secretion of progesterone and estradiol-17β by ovine granulosa cells. Acta Endocrinol 1992, 127, 366–370. [Google Scholar]
- Tanavde, V.S.; Maitra, A. In vitro modulation of steroidogenesis and gene expression by melatonin: A study with porcine antral follicles. Endocrinol. Res 2003, 29, 399–410. [Google Scholar]
- Webley, G.E.; Luck, M.R. Melatonin directly stimulates the secretion of progesterone by human bovine granulosa cells luteinized in vitro. J. Reprod. Fertil 1986, 78, 711–717. [Google Scholar]
- Adriaens, I.; Jacquet, P.; Cortvrindt, R.; Janssen, K.; Smitz, J. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology 2006, 228, 333–343. [Google Scholar]
- Hemadi, M.M.; Abolhassani, F.; Akbari, M.; Sobhani, A.; Pasbakhsh, P.; Ahrlund-Richter, L.; Modaresi, M.H.; Salehnia, M. Melatonin promotes the cumulus-oocyte complexes quality of vitrified-thawed murine ovaries with increases mean number of follicles survival and ovary size following heterotopic transplantation. Eur. J. Pharmacol 2009, 618, 84–90. [Google Scholar]
- Mazoochi, T.; Salehnia, M.; Valojerdi, M.R.; Mowla, S.J. Morphologic, ultrastructural, and biochemical identification of apoptosis in vitrified-warmed mouse ovarian tissue. Fertil. Steril 2008, 90, 1480–1486. [Google Scholar]
- Venegas, C.; Garcia, J.A.; Escames, G.; Ortiz, F.; Lopez, A.; Doerrier, G.; Garcia-Corzo, L.; Lopez, L.C.; Reiter, R.J.; Acuna-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res 2012, 52, 217–227. [Google Scholar]
- Vaughan, G.M.; Pelham, R.W.; Pang, S.F.; Laughlin, L.L.; Wilson, K.M.; Sandock, K.L.; Vaughan, M.K.; Koslow, S.H.; Reiter, R.J. Nocturnal elevation of plasma melatonin and urinary 5-hydroxyindoleacetic acid in young men: Attempts at modification by brief changes in environmental lighting and sleep and by autonomic drugs. J. Clin. Endocrinol. Metab 1976, 42, 752–764. [Google Scholar]
- Sun, X.; Liu, T.; Deng, J.; Borjigin, J. Long term invivo microdialysis. J. Pineal Res 2003, 35, 118–124. [Google Scholar]
- Lewy, A.J. Effects of light on human melatonin production and the human circadian system. Prog. Neuropsychopharmacol. Biol. Psychiatry 1983, 1, 551–556. [Google Scholar]
- Yu, H.S.; Pang, S.F.; Tang, P.L. Increase in the level of retinal melatonin and its persistence of its diurnal rhythm after pinealectomy. J. Endocrinol 1981, 91, 477–481. [Google Scholar]
- Huether, G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 1993, 49, 665–670. [Google Scholar]
- Bubenik, G.A. Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Biol. Signals Recept 2001, 10, 350–366. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid and an antioxidant vitamin. J. Pineal Res 2003, 34, 74–79. [Google Scholar]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Membrane melatonin receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol 2012, 351, 152–166. [Google Scholar]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol 2006, 38, 313–316. [Google Scholar]
- Iwazaki, S.; Nakazawa, K.; Sakai, J.; Kometani, K.; Mitsutoshi, I.; Yoshimura, Y.; Maruyama, T. Melatonin as a local regulator of human placental function. J. Pineal Res 2005, 39, 261–265. [Google Scholar]
- Stehle, J.H.; Sadde, A.; Rawashdeh, O.; Ackermann, K.; Jilg, A.; Sebesteng, T.; Maronde, E. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J. Pineal Res 2011, 51, 17–43. [Google Scholar]
- Maronde, E.; Saade, A.; Ackermann, K.; Gougran-Botros, H.; Pagan, C.; Box, R.; Bourgeron, T.; Dehghani, F.; Stehte, J.H. Dynamics of enzymatic protein complexes offer a novel principle for the regulation of melatonin synthesis in the human pineal gland. J. Pineal Res 2011, 51, 145–155. [Google Scholar]
- Lanoix, D.; Beghdadi, H.; Lafond, J.; Vaillancourt, C. Human placental trophoblasts synthesize melatonin and express its receptors. J. Pineal Res 2008, 45, 50–60. [Google Scholar]
- Sainz, R.M.; Mayo, J.C.; Rodriguez, C.; Tan, D.X.; Lopez-Burillo, S.; Reiter, R.J. Melatonin and cell death: Differential actions in normal and cancer cells. Cell. Mol. Life Sci 2003, 60, 1407–1426. [Google Scholar]
- Lanoix, D.; Lacasse, A.A.; Reiter, R.J.; Vaillancourt, C. Melatonin, the smart killer: The human trophoblast as a model. Mol. Cell. Endocrinol 2012, 348, 1–11. [Google Scholar]
- Vaillancourt, C.; Lanoix, D.; LeBellego, F.; Daoud, G.; Lafond, J. Involvement of MAPK signaling in human villous trophoblast differentiation. Mini Rev. Med. Chem 2009, 9, 962–973. [Google Scholar]
- Castellucci, M.; Kaufmann, P.; Bischof, F. Extracellular matrix influences hormone and protein production by human chorionic villi. Cell Tiss. Res 1990, 262, 135–142. [Google Scholar]
- Fisher, S.J.; Cui, T.Y.; Zhang, L.; Hartman, L.; Grahl, K.; Zhang, G.Y.; Tarpey, J.; Damsky, C.H. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J. Cell. Biol 1989, 109, 891–902. [Google Scholar]
- Saundararajan, R.; Rao, A.J. Trophoblast “pseudo-tumorigenesis”: Significance and contributory factors. Reprod. Biol. Endocrinol 2004, 2, 15. [Google Scholar]
- Mounier, C.; Barbeau, B.; Vaillancourt, C. Endocrinology and cell signaling in human villous trophoblast. Meth. Mol. Biol 2009, 550, 89–102. [Google Scholar]
- Fulia, F.; Gitto, E.; Cuzzocrea, S.; Reiter, R.J.; Dugo, L.; Gitto, P.; Barberi, S.; Cordaro, S.; Barberi, I. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: Reduction by melatonin. J. Pineal Res 2001, 31, 343–349. [Google Scholar]
- Richter, H.G.; Hansell, J.A.; Rout, S.; Giussani, D.A. Melatonin improves placental efficiency and birth weight and increases the placental expression of antioxidant enzymes in undernourished pregnancy. J. Pineal Res 2009, 46, 357–364. [Google Scholar]
- Chen, Y.C.; Tain, Y.L.; Sheen, J.M.; Huang, L.T. Melatonin in neonates and children. J. Formosa Med. Assoc 2012, 111, 57–66. [Google Scholar]
- Mitchell, M.D.; Sayers, L.; Keirse, M.J.; Anderson, A.B.; Turnbull, A.C. Melatonin in amniotic fluid during human parturition. Br. J. Obstet. Gynecol 1978, 85, 684–686. [Google Scholar]
- Kivela, A.; Kauppila, A.; Leppaluoto, J.; Vakkuri, O. Serum and amniotic fluid melatonin during human labor. J. Clin. Endocrinol. Metab 1989, 69, 1065–1069. [Google Scholar]
- Ciesla, W. Low ACTH and high melatonin concentrations in amniotic fluid as hormonal markers of high risk of fetal abnormalities. Preliminary studies. Perinat. Diagn 1998, 18, 980–983. [Google Scholar]
- Kaneko, Y.; Hayaski, T.; Yu, S.; Tajiri, N.; Bae, E.C.; Solomita, M.A.; Cheda, S.H.; Weinbren, N.L.; Parolini, O.; Borlongan, C.V. Human amniotic epithelial cells express melatonin receptor MT1, but not melatonin receptor MT2: A new perspective on neuroprotection. J. Pineal Res 2011, 50, 272–280. [Google Scholar]
- Xu, D.X.; Wang, H.; Ning, H.; Zhao, L.; Chen, Y.H. Maternally administered melatonin differentially regulates lipopolysaccharide-induced proinflammatory and anti-inflammatory cytokines in maternal serum, amniotic fluid, fetal liver, and fetal brain. J. Pineal Res 2007, 43, 74–79. [Google Scholar]
- Glattre, E.; Bjerkedal, T. The 24-hour rhythmicity of birth: A population study. Acta Obstet. Gynecol. Scand 1983, 62, 31–36. [Google Scholar]
- Cagnacci, A.; Soldani, R.; Melis, G.B.; Volpe, A. Diurnal rhythms of labor and delivery in women: Modulation by parity and seasons. Am. J. Obstet. Gynecol 1998, 178, 140–145. [Google Scholar]
- Ducsay, C.A.; Yellon, S.M. Photoperiod regulation of uterine activity and melatonin rhythms in pregnant rhesus macaque. Biol. Reprod 1991, 44, 967–974. [Google Scholar]
- Dickmeis, T. Glucocorticoids and the circadian clock. J. Endocrinol 2009, 200, 3–22. [Google Scholar]
- Von Gall, C.; Garabette, M.L.; Kell, C.A.; Frenzel, S. Rhythmic gene expression in pituitary cells depends on temporally defined heterologous sensitization by the neurohormone melatonin. Nat. Neurosci 2002, 5, 234–238. [Google Scholar]
- Reppert, S.M.; Weaver, D.R.; Ebisawa, T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994, 13, 1175–1186. [Google Scholar]
- Takayama, H.; Nakamura, Y.; Tamura, H.; Yamagata, Y.; Harada, A.; Nakata, M.; Surgino, N.; Kato, H. Pineal gland (melatonin) affects the parturition time, but not luteal function and fetal growth, in pregnant rats. Endocr. J 2003, 50, 37–43. [Google Scholar]
- Nathanielsz, P.W.; Guiseani, D.A.; Mecenas, C.A.; Wu, W.; Winter, J.A.; Garcia-Villar, R.; Baguma-Nibasheka, M.; Honnebier, M.B.; McDonald, T.J. Regulation of the switch from myometrial contractures to contractions in late pregnancy: Studies in pregnant sheep and monkey. Reprod. Fertil. Rev 1995, 7, 595–602. [Google Scholar]
- Leake, R.D.; Weitzman, R.E.; Glatz, T.H.; Fisher, D.A. Plasma oxytocin concentrations in men, nonpregnant women and pregnant women before and during spontaneous labor. J. Clin. Endocrinol. Metab 1981, 53, 730–733. [Google Scholar]
- Thornton, S.; Davison, J.M.; Baylis, P.H. Plasma oxytocin during the first and second stages of spontaneous human labor. Acta Endocrinol 1992, 126, 425–429. [Google Scholar]
- Chard, T. Fetal and maternal oxytocin in human parturition. Am. J. Perinatol 1989, 6, 145–152. [Google Scholar]
- Petraglia, F.; Imperatore, A.; Challis, J.R.G. Neuroendocrine mechanisms in pregnancy and parturition. Endocr. Rev 2010, 31, 783–816. [Google Scholar]
- Schlabritz-Loutsevitch, N.; Hellner, N.; Middendorf, R.; Muller, D.; Olcese, J. The human myometrium as a target for melatonin. J. Clin. Endocrinol. Metab. 2003, 88, 908–913. [Google Scholar]
- Sharkey, J.T.; Puttaramu, R.; Word, R.A.; Olcese, J. Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J. Clin. Endocrinol. Metab 2009, 94, 421–427. [Google Scholar]
- Sharkey, J.; Cable, C.; Olcese, J. Melatonin sensitizes human myometrial cells to oxytocin in a PKCα/ERK-dependent manner. J. Clin. Endocrinol. Metab 2010, 95, 2902–2908. [Google Scholar]
- Olcese, J.; Lozier, S.; Paradise, B.S. Melatonin and the circadian timing of human parturition. Reprod. Sci 2013, 20, 168–174. [Google Scholar]
- Nakamara, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yamagata, Y.; Kurube, A.; Sergino, N.; Kato, H. Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res 2001, 30, 29–33. [Google Scholar]
- Kivela, A. Serum melatonin during human pregnancy. Acta. Endocrinol 1991, 124, 233–237. [Google Scholar]
- Gravett, M.G.; Rubens, C.E.; Nunes, T.M. Global report on preterm birth and stillbirth (2 of 7): Discovery science. BMC Pregnancy Childbirth 2010, 10. [Google Scholar] [CrossRef]
- Kwon, S.Y.; McIntyre, P.B.; Flecker, A.S.; Campbell, L.M. Mercury biomagnification in the food web of a neotropical stream. Sci. Total Environ 2012, 417–418, 92–97. [Google Scholar]
- Kirk, J.L.; Lehnherr, I.; Andersson, M.; Braune, B.M.; Chan, L.; Dastoor, A.P.; Durnford, D.; Gleason, A.L.; Loseto, L.L.; Steffen, A.; et al. Mercury in Arctic marine ecosystems: Sources, pathways and exposure. Environ. Res 2012, 119, 64–87. [Google Scholar]
- Koedrith, P.; Seo, Y.R. Advances in carcinogenic metal toxicity and potential molecular markers. Int. J. Mol. Sci 2011, 12, 9576–9595. [Google Scholar]
- Pal, P.B.; Pal, S.; Das, J.; Sil, P.C. Modulation of mercury-induced mitochondrial-dependent apoptosis by glycine in hepatocytes. Amino Acids 2012, 42, 1669–1683. [Google Scholar]
- Rao, M.V.; Gangadharan, B. Antioxidant potential of melatonin against mercury induced intoxication in spermatozoa in vitro. Toxicol In Vitro 2008, 22, 935–942. [Google Scholar]
- Sarabia, L.; Maurer, I.; Bustos-Obregon, E. Melatonin prevents damage elicited by organophosphorous pesticide diazinon on mouse sperm DNA. Ecotoxicol. Environ. Saf 2009, 72, 663–668. [Google Scholar]
- Peiris-John, R.J.; Wickremasinghe, R. Impact of low-level exposure to organophosphates on human reproduction and survival. Trans. R. Soc. Trop. Med. Hyg 2008, 102, 239–245. [Google Scholar]
- Jurewicz, J.; Hanke, W.; Radwan, M.; Bonde, J.P. Environmental factors and semen quality. Int. J. Occup. Med Environ Health 2009, 22, 305–329. [Google Scholar]
- Vargas, A.; Bustos-Obregon, E.; Hartley, R. Effects of hypoxia on epididymal sperm parameters and protective role of ibuprofen and melatonin. Biol. Res 2011, 44, 161–167. [Google Scholar]
- Tamme, L.A.; Still, D.L.; Acromite, M.T. Hypoxia and flight performance of military instructor pilots in a flight simulator. Aviat. Space Environ. Med 2010, 81, 654–659. [Google Scholar]
- Li, L.; Xu, J.N.; Wong, Y.H.; Wong, J.T.; Pang, S.F.; Shiu, S.Y. Molecular and cellular analyses of melatonin receptor-mediated cAMP signaling in rat corpus epididymis. J. Pineal Res 1998, 25, 219–228. [Google Scholar]
- Shiu, S.Y.; Li, L.; Siu, S.W.; Xi, S.C.; Fong, S.W.; Pang, S.F. Biological basis and possible physiological implications of melatonin receptor-mediated signaling in the rat epididymis. Biol. Signals Recept 2000, 9, 172–187. [Google Scholar]
- Li, L.; Wong, J.T.; Pang, S.F.; Shiu, S.Y. Melatonin-induced stimulation of rat corpus epididymal epithelial cell proliferation. Life Sci 1999, 65, 1067–1076. [Google Scholar]
- Sonmez, M.; Yüce, A.; Türk, G. The protective effects of melatonin and vitamin E on antioxidant enzyme activities and epididymal sperm characteristics of homocysteine treated male rats. Reprod. Toxicol 2007, 23, 226–231. [Google Scholar]
- Kurcer, Z.; Hekimoglu, A.; Aral, F.; Baba, F.; Sahna, E. Effect of melatonin on epididymal sperm quality after testicular ischemia/reperfusion in rats. Fertil. Steril 2010, 93, 1545–1549. [Google Scholar]
- Reiter, R.J. Exogenous and endogenous control of the annual reproductive cycle in the male golden hamster: Participation of the pineal gland. J. Exp. Zool 1975, 191, 111–120. [Google Scholar]
- Chaves, E.M.; Aguilera-Merlo, C.; Cruceno, A.; Fogal, T.; Piezzi, R.; Scardapane, L.; Dominquez, S. Seasonal morphological variations and age-related changes of the seminal vesicle of viscacha (Lagostomus maximus maximus): An ultrastructural and immunohistochemical study. Anat. Rec 2012, 295, 886–895. [Google Scholar]
- Jung-Hynes, B.; Huang, W.; Reiter, R.J.; Ahmad, N. Melatonin synchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J. Pineal Res 2010, 49, 60–68. [Google Scholar]
- Shiu, S.Y.; Leung, W.Y.; Tam, C.W.; Liu, V.W.; Yao, K.M. Melatonin MT(1) receptor-induced translational up-regulation of p27(Kip 1) in prostate cancer antiproliferation is mediated via inhibition of constitutively active nuclear factor kappa B (NF-κB): Potential implications on prostate cancer chemoprevention and therapy. J. Pineal Res 2012, in press. [Google Scholar]
- Karasek, M.; Pawlikowski, M. Pineal gland, melatonin and cancer. Neuroendocrinol. Lett 1999, 20, 139–144. [Google Scholar]
- Shiu, S.Y. Toward rational and evidence-based use of melatonin in prostate cancer prevention and treatment. J. Pineal Res 2007, 43, 1–9. [Google Scholar]
- Casao, A.; Gallego, M.; Abecia, J.A.; Forcada, F.; Perez-Pe, R.; Muino-Blanco, T.; Cebrian-Perez, J.A. Identification and immunolocalisation of melatonin MT(1) and MT(2) receptors in Rasa aragonesa ram spermatozoa. Reprod. Fertil. Dev 2012, 24, 953–961. [Google Scholar]
- Kaya, A.; Aksoy, M.; Baspinar, N.; Yildiz, C.; Ataman, M.B. Effect of melatonin implantation to sperm donor rams on post-thaw viability and acrosomal integrity of sperm cells in the breeding and non-breeding season. Reprod. Domest. Anim 2001, 36, 211–215. [Google Scholar]
- Casao, A.; Vega, S.; Palacin, I.; Perez-Pe, R.; Levina, A.; Quintin, F.J.; Sevilla, E.; Abecia, J.A.; Cebrian-Perez, J.A.; Forcada, F.; et al. Effects of melatonin implants during non-breeding season on sperm motility and reproductive parameters in Rasa aragonesa rams. Reprod. Domest. Anim 2010, 45, 425–432. [Google Scholar]
- Webster, J.R.; Suttie, J.M.; Veenoliet, B.A.; Manley, T.R.; Littlejohn, R.P. Effect of melatonin implants in secretion of luteinizing hormone in intact and castrated rams. J. Reprod. Fertil 1991, 92, 21–31. [Google Scholar]
- Lincoln, G.A.; Clarke, I.J. Refractoriness to a static melatonin signal develops in the pituitary gland for the control of prolactin secretion in the ram. Biol. Reprod 1997, 57, 460–467. [Google Scholar]
- Rosa, H.J.; Juniper, D.T.; Bryant, M.J. Effects of recent sexual experience and melatonin treatment of rams on plasma testosterone concentrations, sexual behavior and ability to induce ovulation in seasonally anestrous ewes. J. Reprod. Fertil 2000, 120, 169–176. [Google Scholar]
- Casao, A.; Luna, C.; Serrano, E. Quantification of Melatonin on Oxidized Proteins and Lipids in Ram Semen in the Breeding and Non-breeding Season. In XIII Jornadas sobre Produccion Animal; AIDA: Zaragoza, Spain, 2009; pp. 723–725. [Google Scholar]
- Casao, A.; Mendoza, N.; Perez-Pe, R.; Grasa, P.; Abecia, J.A.; Forcada, F.; Cebrian-Perez, J.A.; Muino-Blanco, T. Melatonin prevents capacitation and apoptotic-like changes of ram spermatozoa and increases fertility rate. J. Pineal Res 2010, 48, 39–46. [Google Scholar]
- Balao da Silva, C.M.; Macias-Garcia, B.; Miro-Moran, A.; Gonzalez-Fernandez, L.; Morillo-Rodriguez, A.; Ortega-Fervisola, C.; Gallardo-Balanos, J.M.; Stillwell, G.; Topea, J.A.; Pena, F.J. Melatonin reduces lipid peroxidation and apoptotic-like changes in stallion spermatozoa. J. Pineal Res 2011, 51, 172–179. [Google Scholar]
- Bendahmane, M.; Lynch, C.; Tulsiani, D.R.P. Calmodulin signals capacitation and triggers the agonist-induced acrosome reaction in mouse spermatozoa. Arch. Biochem. Biophys 2001, 390, 1–8. [Google Scholar]
- Tukiani, D.R.F.; Zeng, H.T.; Ibou-Haila, A. Biology of sperm capacitation: Evidence for multiple signaling pathways. Reprod. Fertil 2007, 63, 357–372. [Google Scholar]
- Succu, S.; Berlinguer, F.; Pasciu, V.; Satta, V.; Leoni, G.G.; Naltana, S. Melatonin protects ram spermatozoa from cryopreservation injuries in a dose-dependent manner. J. Pineal Res 2011, 50, 310–318. [Google Scholar]
- Ortega Ferrusola, C.; Gonzalez-Fernandez, L.; Macias Garcia, B.; Salazar-Sandoval, C.; Morillo-Rodriguez, A.; Rodriguez Martinez, H.; Tapia, J.A.; Pena, F.J. Effect of cryopreservation on nitric oxide production in stallion spermatozoa. Biol. Reprod 2009, 81, 1106–1111. [Google Scholar]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M. Storage of boar semen. Anim. Reprod. Sci 2000, 62, 143–172. [Google Scholar]
- Martin-Hildago, D.; Baron, F.J.; Bragado, M.J.; Carmona, P.; Robina, A.; Garcia-Marin, L.J.; Gil, M.C. The effect of melatonin on the quality of extended boar semen after long-term storage at 17 °C. Theriogenology 2011, 75, 1550–1560. [Google Scholar]
- Garcia, J.J.; Reiter, R.J.; Guerrero, J.M.; Escames, G.; Yu, B.; Oh, C.S.; Munoz-Hayos, A. Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Lett 1997, 408, 297–300. [Google Scholar]
- Martin, M.; Macias, M.; Escames, G.; Reiter, R.J.; Agapito, M.T.; Ortiz, G.G.; Acuna-Castroviejo, D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J. Pineal Res 2000, 28, 242–248. [Google Scholar]
- Martin, M.; Macias, M.; Leon, J.P.; Escames, G.; Khaldy, H.; Acuna-Castroviejo, D. Melatonin increases the activity of oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int. J. Biochem. Cell Biol 2002, 34, 348–357. [Google Scholar]
- Leon, J.; Acuna-Castroviejo, D.; Sainz, R.M.; Mayo, J.C.; Tan, D.X.; Reiter, R.J. Melatonin and mitochondrial function. Life Sci 2004, 75, 765–790. [Google Scholar]
- Jou, M.J.; Peng, T.I.; Yu, P.Z.; Jou, S.B.; Reiter, R.J.; Chen, J.Y.; Wu, H.Y.; Chen, C.C.; Hsu, L.F. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J. Pineal Res 2007, 43, 389–403. [Google Scholar]
- Benitez-King, G.; Anton-Tay, F. Calmodulin mediates melatonin cytoskeletal effects. Experientia 1993, 49, 35–41. [Google Scholar]
- Johnson, L.A. Sexing mammalian sperm for production of offspring: The state-of-the-art. Anim. Reprod. Sci 2000, 60–61, 93–107. [Google Scholar]
- Agawal, A.; Probakaran, A.S. Mechanism, measurements, and prevention of oxidative stress in male reproductive physiology. Indian J. Exp. Biol 2005, 43, 963–974. [Google Scholar]
- Li, X.X.; Yang, X.G.; Lu, Y.Q.; Lu, S.S.; Zhang, M.; Yao, H.L.; Meng, L.J.; Lu, K.H. Protective effects of melatonin against oxidative stress in flow cytometry-sorted buffalo sperm. Reprod. Dom. Anim 2012, 47, 299–307. [Google Scholar]
- Fujinoki, M. Melatonin-enhanced hyperactivation of hamster sperm. Reproduction 2008, 136, 533–541. [Google Scholar]
- Fujinoki, M.; Suzuki, T.; Takayama, T.; Shibahara, H.; Ohtake, H. Profiling of proteins phosphorylated or dephosphorylated during hyperactivation via activation on hamster spermatozoa. Reprod. Med. Biol 2006, 5, 123–135. [Google Scholar]
- Pitout, N.J.; VanVuuren, R.J.J.; van Aswegen, C.H.; Theron, J.J. Melatonin and sperm motility. S. Afr. Med. J 1991, 79, 683. [Google Scholar]
- Du Toit, D.; Bornman, M.S.; van Aswegen, C.H.; du Plessis, D.J. Sialic acid concentration and sperm motility. Arch. Androl 1994, 32, 21–23. [Google Scholar]
- Browning, C.; Beresford, J.; Fraser, N.; Giles, H. Pharmacological characterization of human recombinant melatonin mt1 and MT2 receptors. Br. J. Pharmacol 2000, 129, 877–886. [Google Scholar]
- Bornman, M.S.; Oosthuizen, J.M.C.; Barnard, H.C.; Schulenburg, G.W.; Boomker, D.; Reif, S. Melatonin and sperm motility. Andrologia 1989, 21, 483–485. [Google Scholar]
- Luboshitzky, R.; Shen-Orr, Z.; Nave, R.; Lavi, S.; Lavie, P. Melatonin administration alters semen quality in healthy men. J. Androl 2002, 23, 572–578. [Google Scholar]
- Lerchl, A. Melatonin administration alters semen quality in normal men. J. Androl 2004, 25, 185–187. [Google Scholar]
- Acuna-Castroviejo, D.; Lopez, L.C.; Escames, G.; Lopez, A.; Garcia, J.A.; Reiter, R.J. Melatonin-mitochondria interplay in health and disease. Curr. Top. Med. Chem 2011, 11, 221–240. [Google Scholar]
- Shang, X.; Huang, Y.; Ye, Z.; Yu, X.; Gu, W. Protection of melatonin against damage of sperm mitochondrial function induced by reactive oxygen species. Zhonghaw Nan Ke Xue 2004, 10, 604–607, [In Chinese]. [Google Scholar]
- Du Plessis, S.S.; Hagenaar, K.; Lampiao, F. The in vitro effects of melatonin on human sperm function and its scavenging activities on NO and ROS. Andrologia 2010, 42, 112–116. [Google Scholar]
- Espino, J.; Bejarano, I.; Ortiz, A.; Lozano, G.M.; Garcia, J.F.; Pariente, J.A.; Rodriguez, A.B. Melatonin as a potential tool against oxidative damage and apoptosis in ejaculated human spermatozoa. Fertil. Steril 2010, 94, 1915–1917. [Google Scholar]
- Espino, J.; Ortiz, A.; Bejarano, I.; Lozano, G.M.; Monllor, F.; Garcia, J.F.; Rodriguez, A.B.; Pariente, J.A. Melatonin protects human spermatozoa from apoptosis via melatonin receptor- and extracellular signal-regulated kinase-mediated pathways. Fertil. Steril 2011, 95, 2290–2296. [Google Scholar]
- Ortiz, A.; Espino, J.; Bejarano, I.; Lozano, G.M.; Monllor, F.; Garcia, J.F.; Pariente, J.A.; Rodriguez, A.B. High endogenous melatonin concentrations enhance sperm quality and short-term in vitro exposure improves aspects of sperm motility. J. Pineal Res 2011, 50, 132–139. [Google Scholar]
- Oba, S.; Nakamura, K.; Sahashi, Y.; Hattori, A.; Nagata, C. Consumption of vegetables alters urinary 6-sulfatoxymelatonin concentrations. J. Pineal Res 2008, 45, 17–23. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evaluation in eukaryotes. J. Pineal Res 2013, 54, 127–138. [Google Scholar]
- Reiter, R.J.; Richardson, B.A.; Johnson, L.J.; Ferguson, B.N.; Dink, D.T. Pineal melatonin rhythm: Reduction in aging Syrian hamsters. Science 1980, 210, 1372–1373. [Google Scholar]
- Reiter, R.J.; Craft, C.M.; Johnson, J.E., Jr; King, T.S.; Richardson, B.A.; Vaughan, G.M.; Vaughan, M.K. Age-associated reduction in nocturnal pineal melatonin levels in female rats. Endocrinology 1981, 109, 1295–1297. [Google Scholar]
- Iguchi, H.; Kato, K.I.; Ibayashi, H. Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J. Clin. Endocrinol. Metab 1982, 55, 27–29. [Google Scholar]
- Sack, R.L.; Lewy, A.J.; Erb, D.L.; Vollmer, W.M.; Singer, C.M. Human melatonin production decreases with age. J. Pineal Res 1989, 3, 379–388. [Google Scholar]
- Benot, S.; Goberna, R.; Reiter, R.J.; Garcia-Maurino, S.; Osuna, C.; Guerrero, J.M. Physiological levels of melatonin contribute to the antioxidant capacity of human serum. J. Pineal Res 1999, 27, 59–64. [Google Scholar]
- Sanchez-Hildalgo, M.; de la Lastra, C.A.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Gomez-Corvera, A.; Caballero, B.; Guerrero, J.M. Age-related changes in melatonin synthesis in extrapineal tissues. Exp. Gerontol 2009, 44, 328–334. [Google Scholar]
- Witt-Enderby, P.A.; Radio, N.M.; Doctor, D.S.; Davis, V.L. Therapeutic treatments potentially mediated by melatonin receptors: Potential clinical uses of melatonin in the prevention of osteoporosis, cancer and as an adjuvant therapy. J. Pineal Res 2006, 41, 297–305. [Google Scholar]
- Sanchez-Barcelo, E.; Mediavilla, M.D.; Tan, D.X.; Reiter, R.J. Clinical uses of melatonin: Evaluation of clinical trials. Curr. Med. Chem 2010, 17, 2070–2095. [Google Scholar]
- Sanchez-Barcelo, E.; Mediavilla, M.D.; Tan, D.X.; Reiter, R.J. Scientific basis for the potential use of melatonin in bone diseases: Osteoporosis and adolescent idiopathic scoliosis. J. Osteoporos 2010. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Vigo, D.E.; Olivar, N.; Vidal, M.F.; Furio, A.M.; Brusco, L.I. Therapeutic application of melatonin in mild cognitive impairment. Am. J. Neurodegener. Dis 2012, 1, 280–291. [Google Scholar]
- Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Cato-Montes, A.; Boga, J.A.; Manchester, L.C.; Fuentes-Broto, L.; Korkmaz, A.; Ma, S.; Tan, D.X.; Reiter, R.J. Alzheimer’s disease: Pathological mechanisms and the beneficial role of melatonin. J. Pineal Res 2012, 52, 167–202. [Google Scholar]
- Reiter, R.J. Melatonin: Lowering the high price of free radicals. News Physiol. Sci 2000, 15, 246–250. [Google Scholar]
- Reyes, J.G.; Farias, J.G.; Henriquez-Olavavieta, S.; Madrid, E.; Parraga, M.; Zepeda, A.B.; Moreno, R.D. The hypoxic testicle; physiology and pathophysiology. Oxid. Med. Cell. Long 2013, in press. [Google Scholar]
- Aversa, S.; Pellegrino, S.; Barberi, I.; Reiter, R.J.; Gitto, E. Potential utility of melatonin as an antioxidant during pregnancy and during the perinatal period. J. Matern. Fetal Neonat. Med 2012, 25, 207–221. [Google Scholar]
- Lavranos, G.; Balla, M.; Tzortzopoulou, A.; Syriou, V.; Angelopoulou, R. Investigating ROS sources in male infertility: A common end for numerous pathways. Reprod. Toxidol 2012, 34, 298–307. [Google Scholar]
- Reiter, R.J. Normal patterns of melatonin levels in the pineal gland and body fluids of humans and experimental animals. J. Neural. Transm 1986, 21, 35–54. [Google Scholar]
- Schenker, S.; Yang, Y.; Perez, A.; Acuff, R.V.; Papas, A.M.; Henderson, G.; Lee, M.P. Antioxidant transport by the human placenta. Clin. Nutr 1998, 17, 159–167. [Google Scholar]
- Okatani, Y.; Okamoto, K.; Hayaski, K.; Wakatsuki, A.; Tamura, S.; Sugara, Y. Maternal-fetal transfer of melatonin in pregnant woman near term. J. Pineal Res 1998, 26, 129–134. [Google Scholar]
- Wakatsuki, A.; Okatani, Y.; Shinokara, K.; Ikenoue, N.; Fukaya, T. Melatonin protects against isthemia/reperfusion-induced oxidative damage to mitochondria in fetal rat brain. J. Pineal Res 2001, 31, 167–172. [Google Scholar]
- Miller, S.L.; Yan, E.B.; Castillo-Melendez, M.; Jenkin, G.; Walker, D.W. Melatonin provides neuroprotection in the late-gestation fetal sheep brain in response to umbilical cord occlusion. Dev. Neurosci 2005, 27, 200–210. [Google Scholar]
- Jahnke, G.; Marr, M.; Myers, C.; Wilson, R.; Travlos, S.; Price, C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci 1999, 50, 271–279. [Google Scholar]
- Alvarez, B.E.; Rodriguez, E.D.; Alvarez, C.F.; Martinez, P.N.; Lopez, B.D. Ovarian aging: Melatonin regulation of the cytometric and endocrine evaluative pattern. Curr. Aging Sci 2012, in press. [Google Scholar]
- Bubenik, G.A.; Blask, D.E.; Brown, G.M.; Maestroni, G.; Pang, S.F.; Reiter, R.J.; Viswanathan, M.; Zisapel, N. Prospects on the clinical utilization of melatonin. Biol. Signals Recept 1998, 7, 195–219. [Google Scholar]
- Gitto, E.; Reiter, R.J.; Korbownick, M.; Tan, D.X.; Gitto, D.; Barberi, S.; Barberi, I. Causes of oxidative stress in the pre- and perinatal period. Neonatology 2002, 81, 146–157. [Google Scholar]
- Reiter, R.J. Melatonin: Clinical relevance. Best Pract. Res. Clin. Endocrinol. Metab 2003, 17, 273–285. [Google Scholar]
- Blask, D.E. Melatonin, sleep disturbance and cancer risk. Sleep Med. Rev 2009, 13, 257–264. [Google Scholar]
- Korkmaz, A.; Reiter, R.J.; Topal, T.; Manchester, L.C.; Oter, S.; Tan, D.X. Melatonin, an established antioxidant worthy of clinical use. Mol. Med 2009, 15, 43–50. [Google Scholar]
- Wang, X.; Sirianni, A.; Pei, Z.; Cormier, L.; Smith, K.; Jiang, J.; Zhan, S.; Wang, H.; Zhao, R.; Yano, H.; et al. The melatonin MT1 receptor axis modulates mutant Huntingtin-mediated toxicity. J. Neurosci 2011, 31, 14496–14507. [Google Scholar]
- Gitto, E.; Aversa, S.; Reiter, R.J.; Barberi, I.; Pelligrino, S. Update on the use of melatonin in pediatrics. J. Pineal Res 2011, 50, 21–28. [Google Scholar]
- Proietti, S.; Cucina, A.; Reiter, R.J.; Bizzarsi, M. Molecular mechanisms of melatonin’s inhibitory actions on breast cancer. Mol. Cell. Life Sci 2013, in press. [Google Scholar]

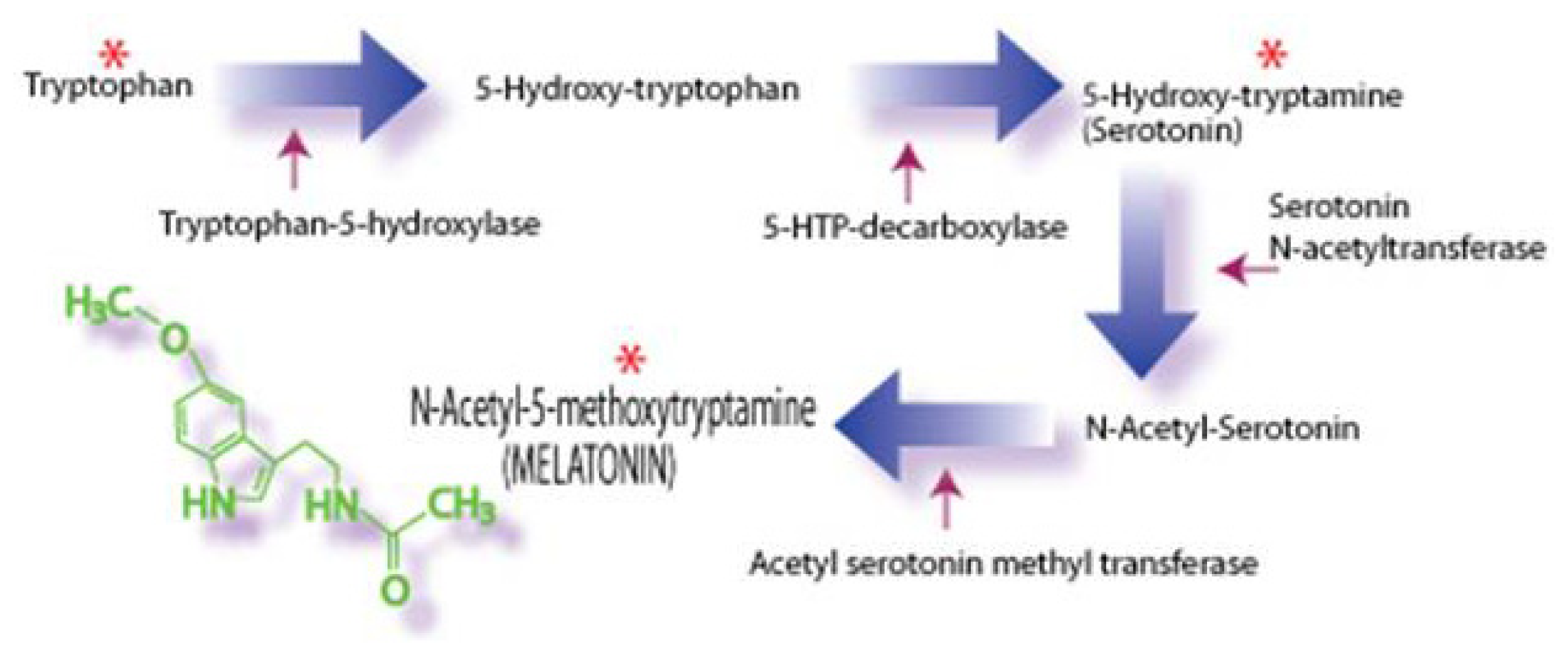
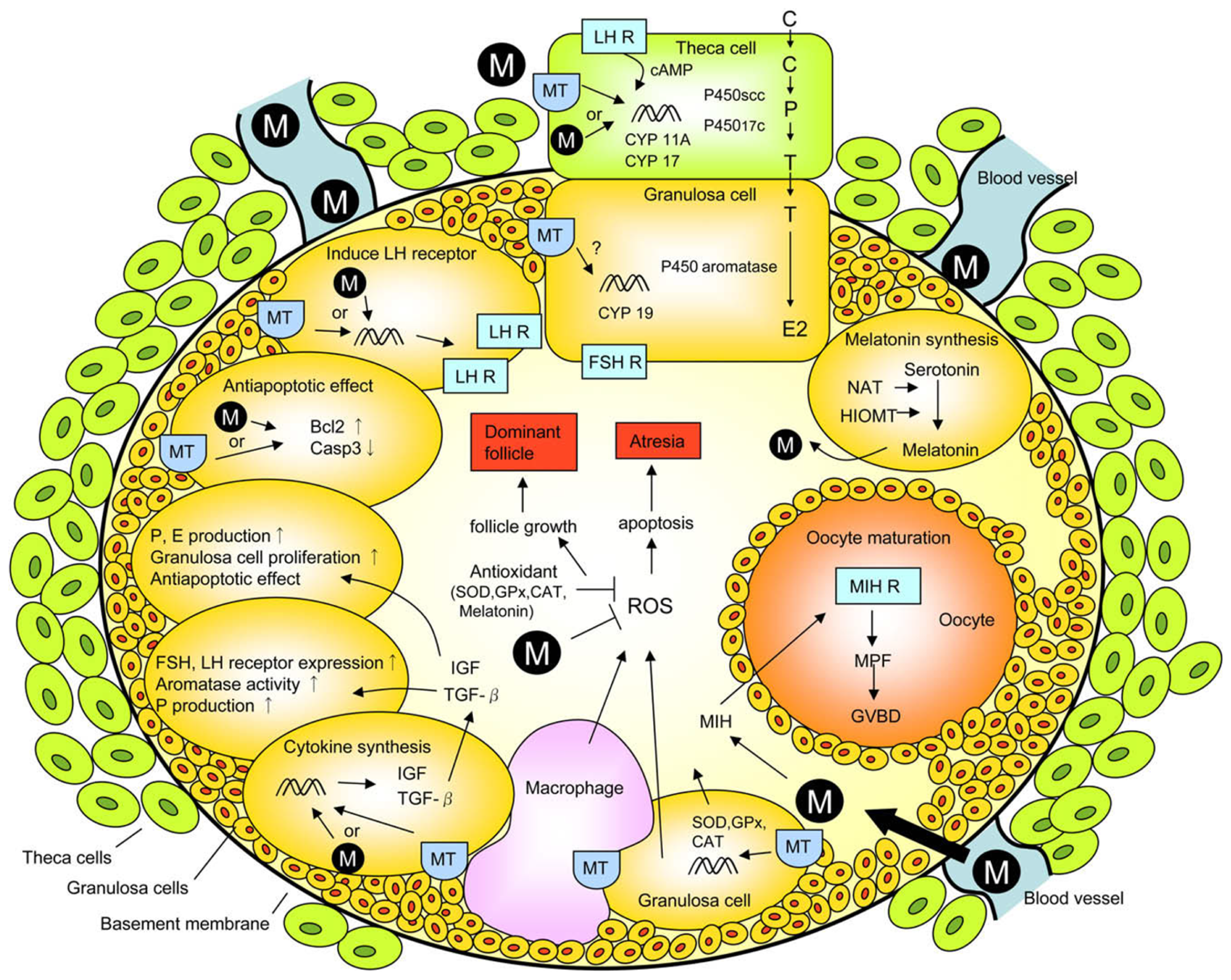
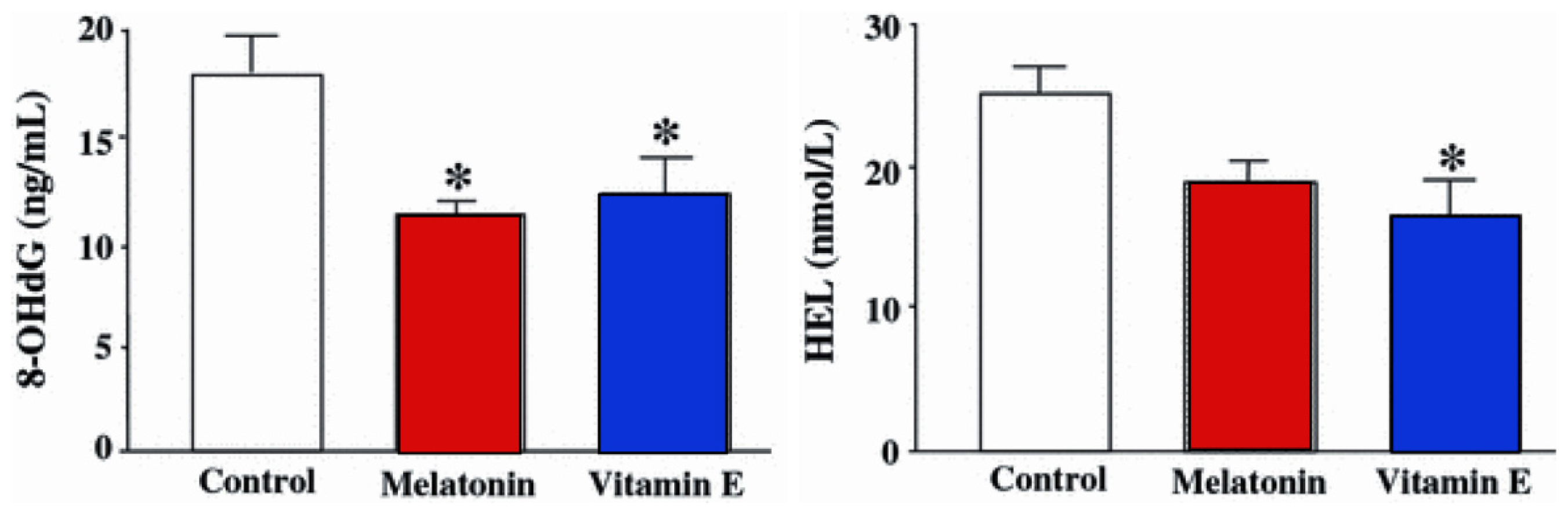

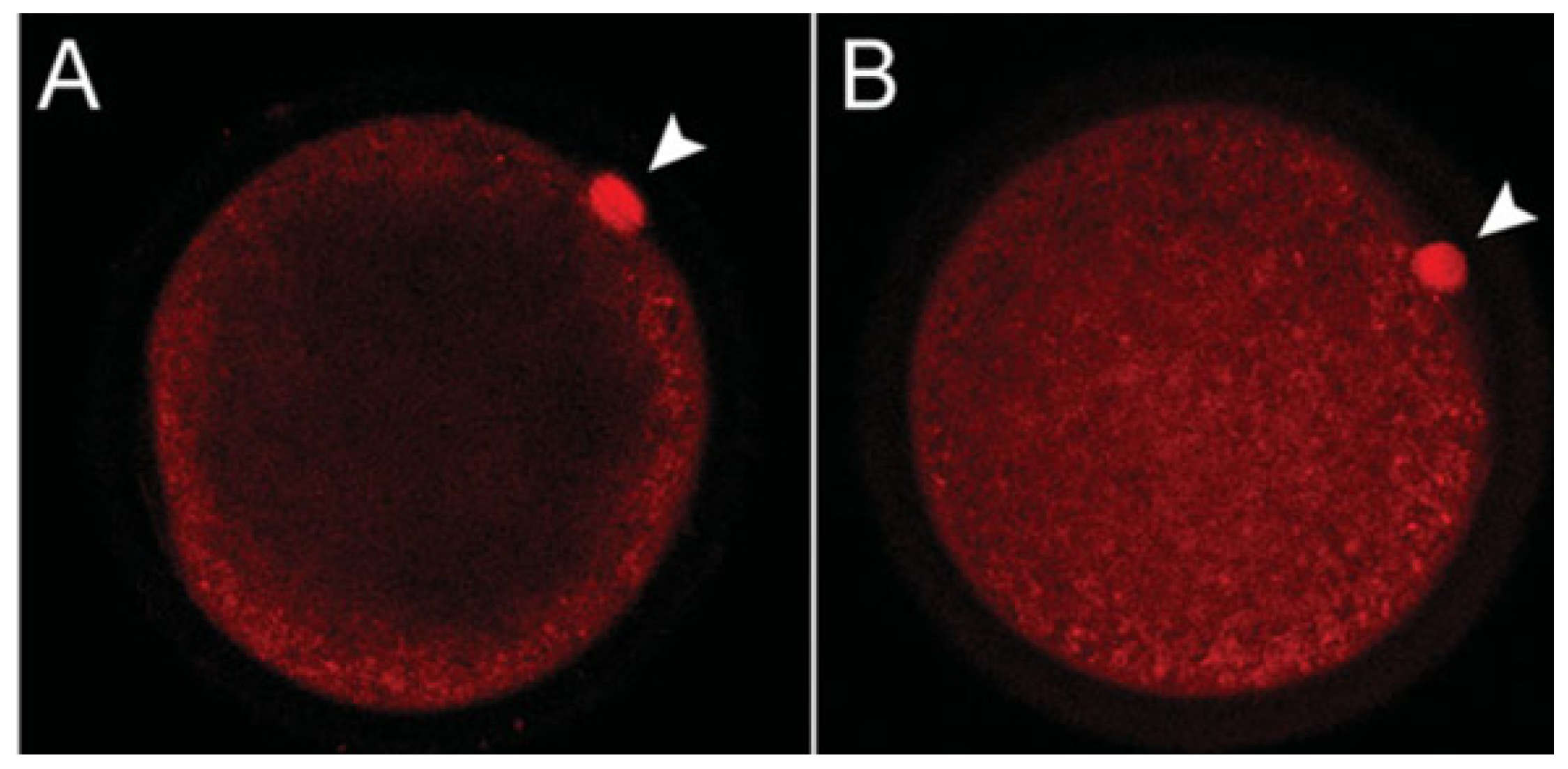


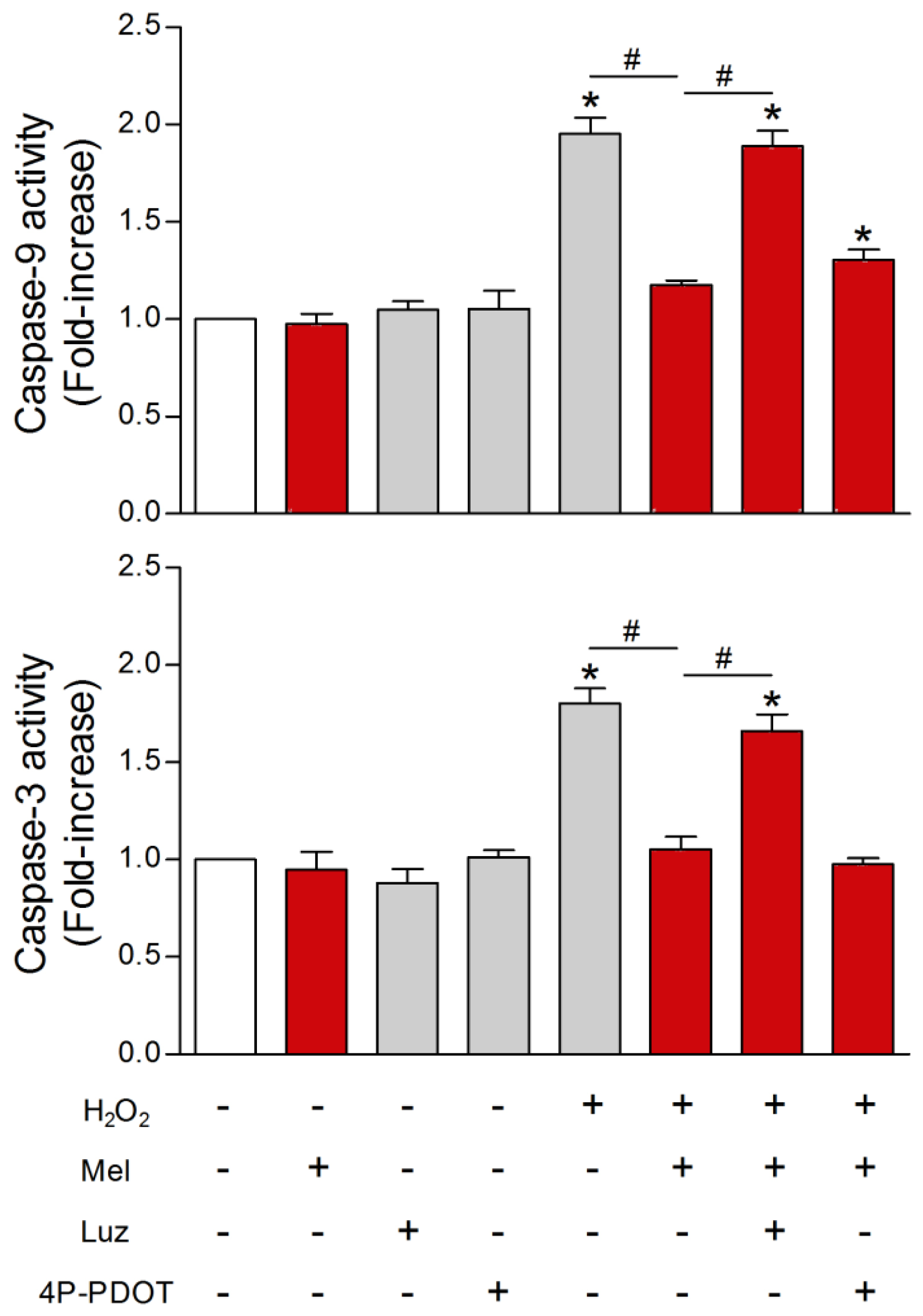
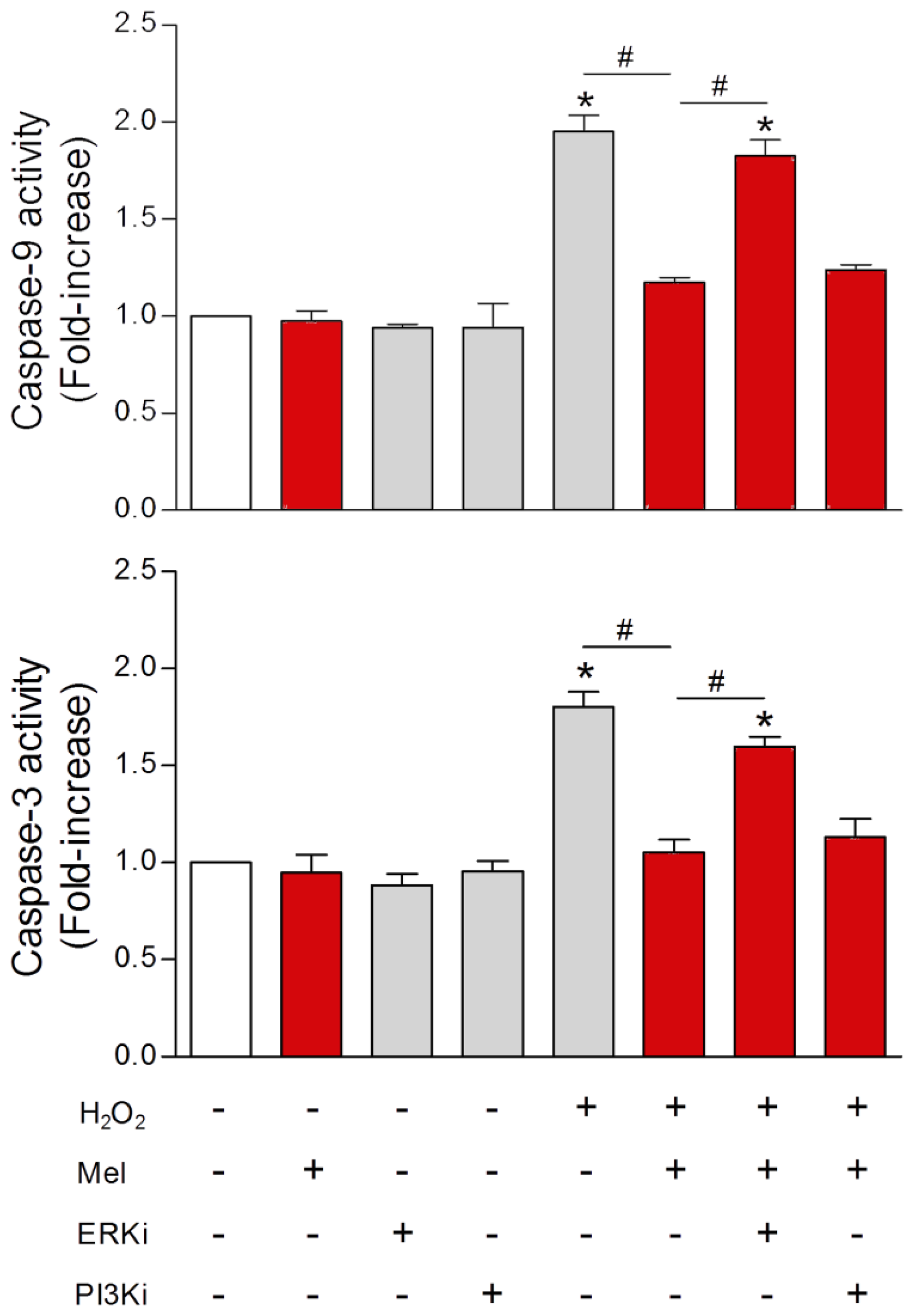

| Melatonin Supplementation (56 patients) | No Melatonin Supplementation (59 patients) | |
|---|---|---|
| Fertilization rate in a previous IVF-ET cycle | 20.2% ± 19.0% | 20.9% ± 16.5% |
| Fertilization rate | 50.0% ± 38.0% | 22.8% ± 19.0% |
| Pregnancy rate | 11/56 (19.6%) | 6/59 (10.2%) |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reiter, R.J.; Rosales-Corral, S.A.; Manchester, L.C.; Tan, D.-X. Peripheral Reproductive Organ Health and Melatonin: Ready for Prime Time. Int. J. Mol. Sci. 2013, 14, 7231-7272. https://doi.org/10.3390/ijms14047231
Reiter RJ, Rosales-Corral SA, Manchester LC, Tan D-X. Peripheral Reproductive Organ Health and Melatonin: Ready for Prime Time. International Journal of Molecular Sciences. 2013; 14(4):7231-7272. https://doi.org/10.3390/ijms14047231
Chicago/Turabian StyleReiter, Russel J., Sergio A. Rosales-Corral, Lucien C. Manchester, and Dun-Xian Tan. 2013. "Peripheral Reproductive Organ Health and Melatonin: Ready for Prime Time" International Journal of Molecular Sciences 14, no. 4: 7231-7272. https://doi.org/10.3390/ijms14047231





