Size-Controlled and Optical Properties of Monodispersed Silver Nanoparticles Synthesized by the Radiolytic Reduction Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formation of Ag Nanoparticles in Colloidal PVA
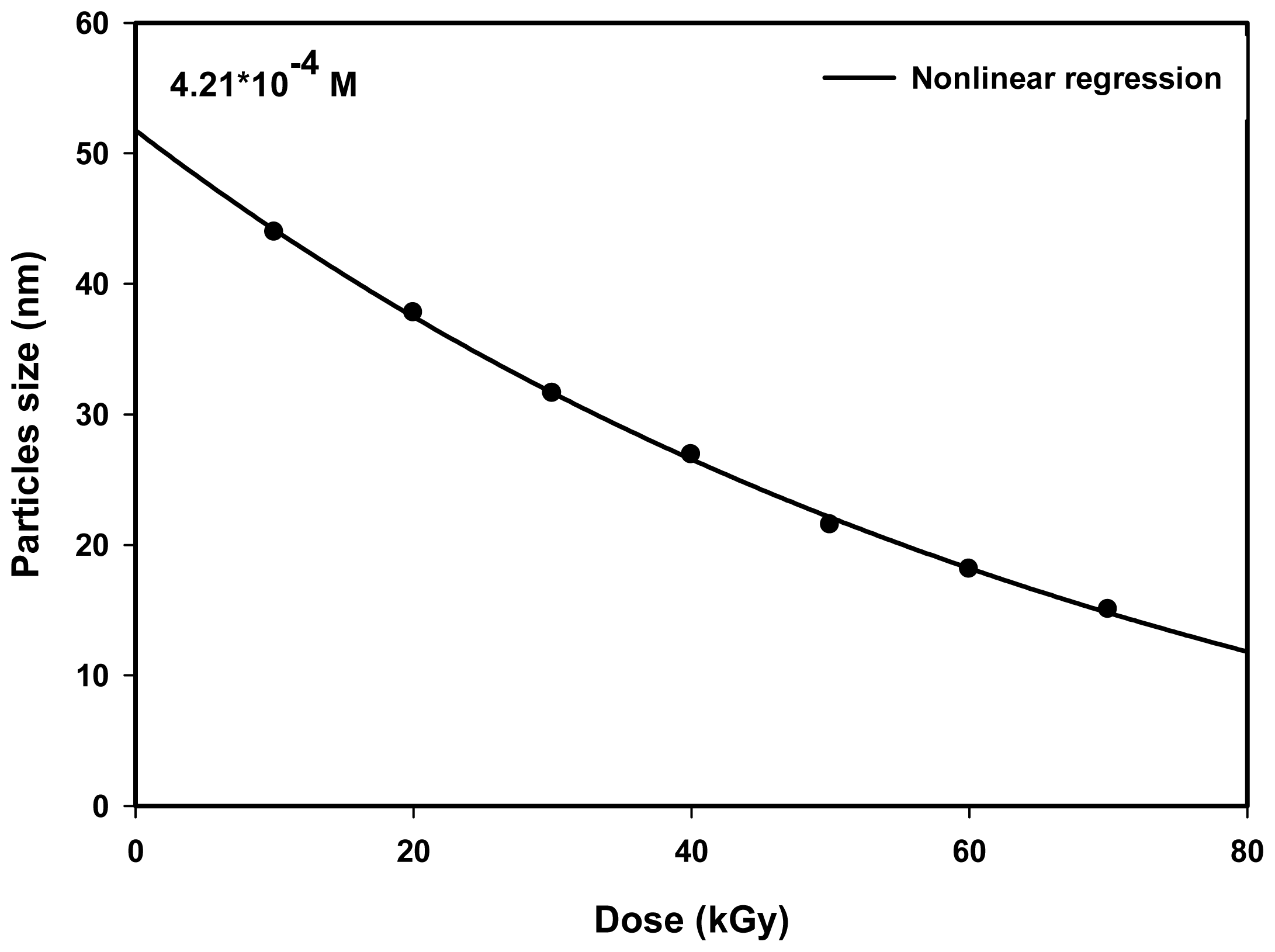
2.2. Effect of Dose
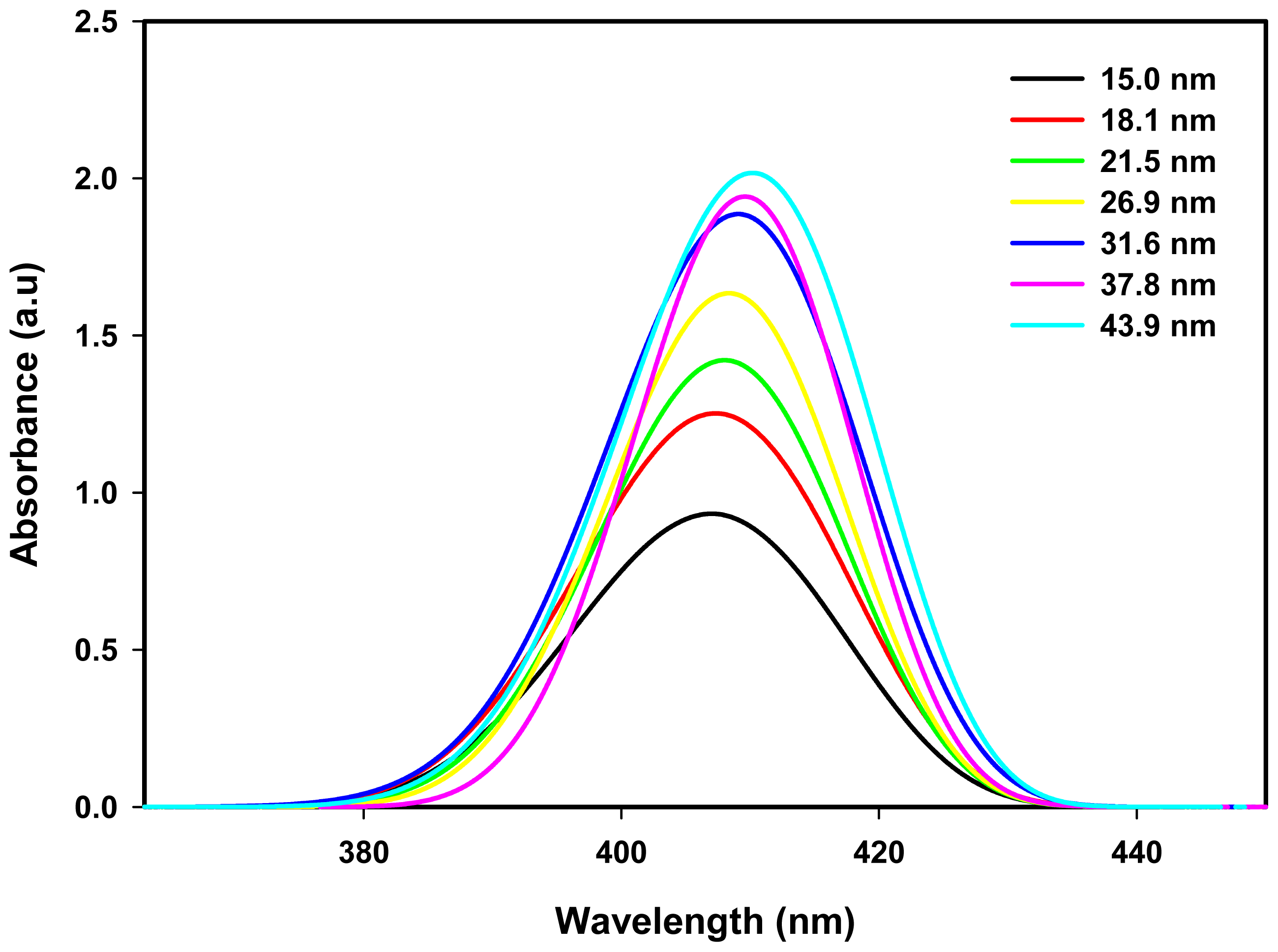
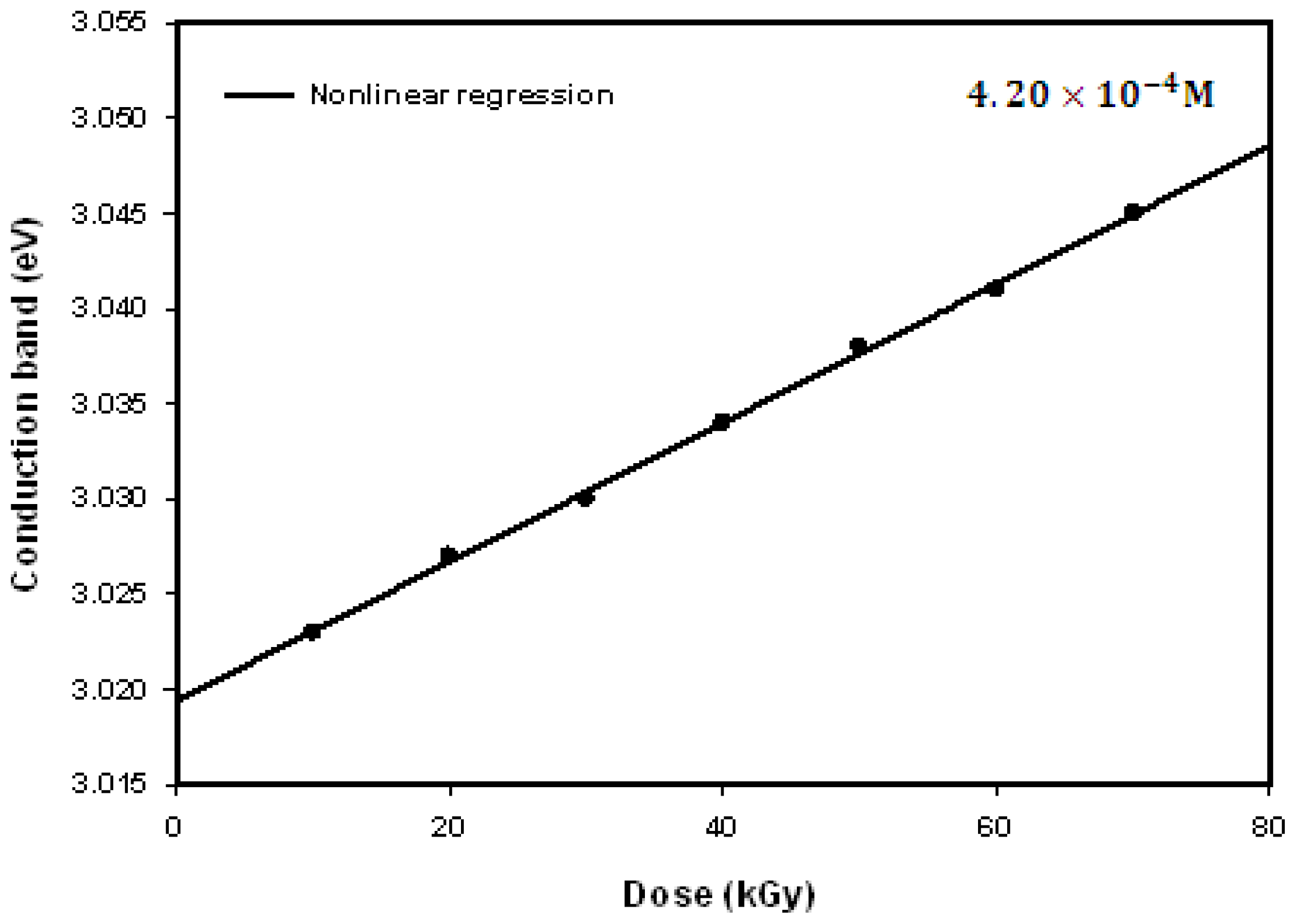
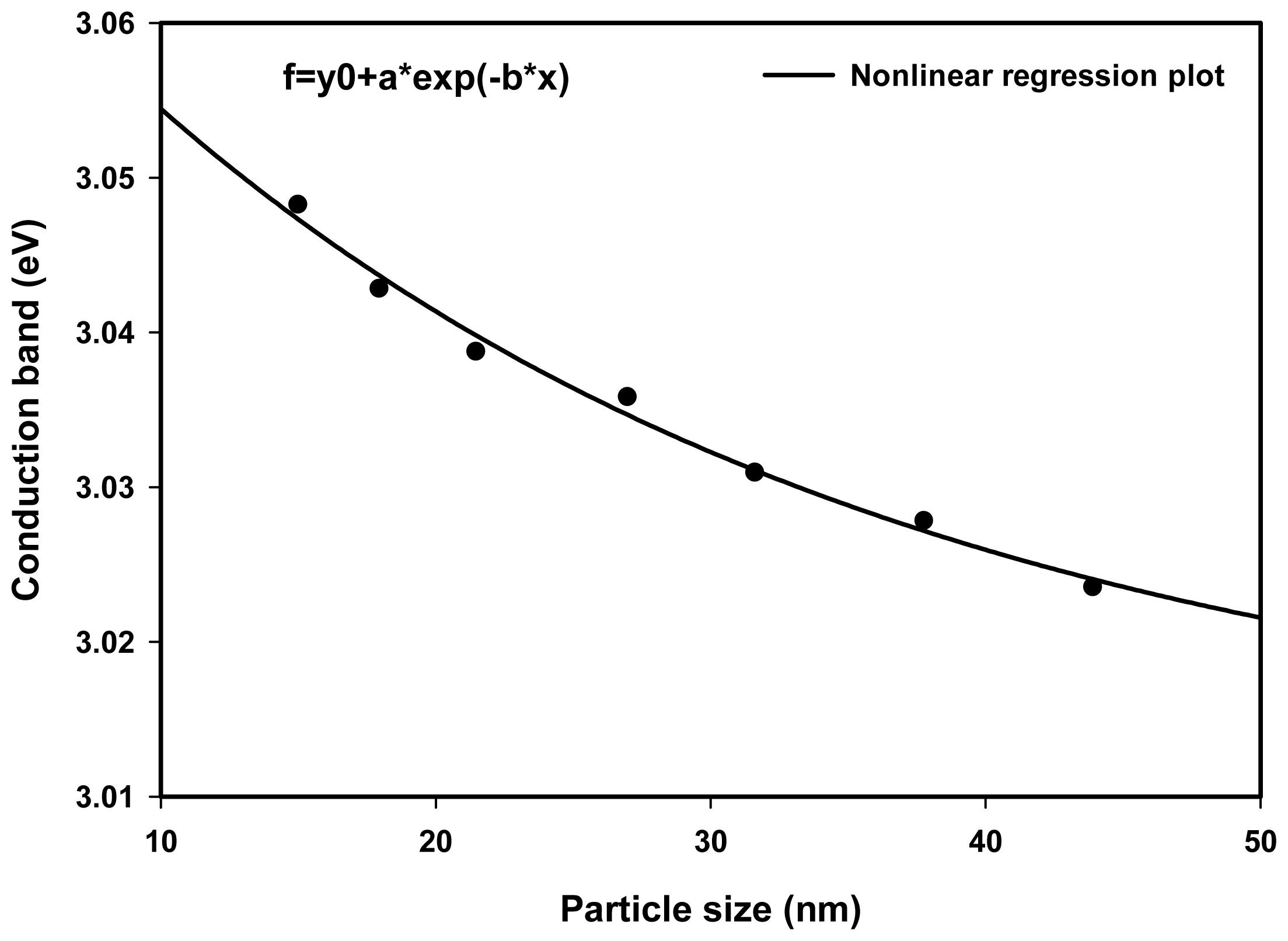
2.3. Optical Properties
3. Experimental Section
4. Conclusions
Acknowledgments
References
- Hornyak, G.L.; Tibbals, H.F.; Dutta, J.; Moor, J.J. Introduction to Nanoscience and Nanotechnology; CRC Press: New York, NY, USA, 2009. [Google Scholar]
- Belloni, J.; Mostafavi, M. Radiation Induced Metal Clusters. Nucleation Mechanisms and Chemistry. In Metal Clusters in Chemistry; Braunstein, P., Oro, L.A., Raithby, P.R., Eds.; Wiley: New York, NY, USA, 1999; pp. 1213–1247. [Google Scholar]
- Zhong, C.J.; Luo, J.; Fang, B.; Wanjala, B.N.; Njoki, P.N. Nanostructured catalysts in fuel cells. Nanotechnology 2010, 21, 1–20. [Google Scholar]
- Dadosh, T. Synthesis of uniform silver nanoparticles with a controllable size. Mater. Lett 2009, 63, 2236–2238. [Google Scholar]
- Chaney, S.B.; Shanmukh, S.; Dluhy, R.A.; Zhao, Y.P. Aligned Silver Nanorod Arrays Produce High Sensitivity Surface-Enhanced Raman Spectroscopy Substrates. Appl. Phys. Lett 2005, 87, 031908. [Google Scholar]
- Riveros, G.; Green, S.; Cortes, A.; Gomez, H.; Marotti, R.E.; Dalchiele, E.A. Silver nanowire arrays electrochemically grown into nanoporous anodic alumina templates. Nanotechnology 2006, 17, 561–570. [Google Scholar]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. J. Chem. Int. Ed 2005, 44, 7852–7872. [Google Scholar]
- Paulraj, P.; Janaki, N.; Sandhya, S.; Pandian, K. Single pot synthesis of polyaniline protected silver nanoparticles by interfacial polymerization and study its application on electrochemical oxidation of hydrazine. Colloids Surf. A Physicochem. Eng. Aspects 2011, 377, 28–34. [Google Scholar]
- Korotcenkov, G.; Han, S.D.; Stetter, J.R. Review of Electrochemical Hydrogen Sensors. Chem. Rev 2009, 109, 1402–1433. [Google Scholar]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, M.S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different Antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med 2007, 3, 168–171. [Google Scholar]
- Pal, S.; Kyung, Y.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol 2007, 73, 1712–1720. [Google Scholar]
- Rastogi, P.K.; Ganesan, V.; Krishnamoorthi, S. Microwave assisted polymer stabilized synthesis of silver nanoparticles and its application in the degradation of environmental pollutants. Mat. Sci. Eng. B 2012, 177, 456–461. [Google Scholar]
- Schrand, A.M.; Braydich-Stolle, L.K.; Schlager, J.J.; Dai, L.; Hussain, S.M. Can silver nanoparticles be useful as potential biological labels? Nanotechnology 2008, 19, 235104–235116. [Google Scholar]
- Dhar, S.; Reddy, E.M.; Shiras, A.; Pokharkar, V.; Prasad, B.L.V. Natural gum reduced/stabilized gold nanoparticles for drug delivery formulations. Chem. Eur. J 2008, 14, 10244–10250. [Google Scholar]
- Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys 1985, 57, 783–826. [Google Scholar]
- Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl. Mater. Interfaces 2011, 3, 218–228. [Google Scholar]
- Jensen, T.R.; Malinsky, M.D.; Haynes, C.L.; van Duyne, R.P. Nanosphere lithography: Tunable localized surface Plasmon resonance spectra of silver nanoparticles. J. Phys. Chem. B 2000, 104, 10549–10556. [Google Scholar]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 2010, 518, 3127–3150. [Google Scholar]
- Sau, T.K.; Rogach, A.L. Nonspherical noble metal nanoparticles: Colloid-chemical synthesis and morphology control. Adv. Mater 2010, 22, 1781–1804. [Google Scholar]
- Zhang, Q.; Xie, J.; Yu, Y.; Lee, J.Y. Monodispersity control in the synthesis of monometallic and bimetallic quasi-spherical gold and silver nanoparticles. Nanoscale 2010, 2, 1962–1975. [Google Scholar]
- Kumar, A.; Joshi, H.; Pasricha, R.; Mandale, A.B.; Sastry, M. Phase transfer of silver nanoparticles from aqueous to organic solutions using fatty amine molecules. J. Colloid Interface Sci 2003, 264, 396–401. [Google Scholar]
- Pal, A.; Shah, S.; Devi, S. Synthesis of Au, Ag and Au–Ag alloy nanoparticles in aqueous polymer solution. Colloids Surf. A 2007, 302, 51–57. [Google Scholar]
- Rodríguez-Sánchez, L.; Blanco, M.C.; López-Quintela, M.A. Electrochemical Synthesis of Silver Nanoparticles. J. Phys. Chem. B 2000, 104, 9683–9688. [Google Scholar]
- Rivas, L.; Sanchez-cartos, S.; Garcia-Ramos, J.V.; Marcillo, G. Growth of silver colloidal particles obtained by citrate reduction to increase the Raman enhancement factor. Langmuir 2001, 17, 574–577. [Google Scholar]
- Pastoriza-Santos, I.; Liz-Marzan, L.M. Reduction of silver nanoparticles in DMF. Formation of monolayers and stable colloids. Pure Appl. Chem 2000, 72, 83–90. [Google Scholar]
- Chaudhari, V.R.; Haram, S.K.; Kulshreshtha, S.K. Micelle assisted morphological evolution of nanoparticles. Colloids Surf. A 2007, 301, 475–480. [Google Scholar]
- Hirai, H.; Nakao, Y.; Toshima, N. Preparation of Colloidal Transition Metals in Polymers by Reduction with Alcohols or Ethers. J. Macromol. Sci. Chem 1979, 13, 633–649. [Google Scholar]
- Silvert, P.Y.; Vijaykrishnan, V.; Vibert, P.; Herrera-Urbina, R.; Elhsissen, K.T. Synthesis and characterization of nanoscale Ag–Pd alloy particles. Nanostruct. Mater 1996, 7, 611–618. [Google Scholar]
- Zhou, Y.; Yu, S.H.; Wang, C.Y.; Li, X.G.; Zhu, Y.R.; Dendrites, Z.Y. A novel ultraviolet irradiation photoreduction technique for the preparation of single-crystal Ag nanorods and Ag dendrites. Adv. Mater 1999, 11, 850–852. [Google Scholar]
- Sudeep, P.K.; Kamat, P.V. Photosensitized Growth of Silver Nanoparticles under Visible Light Irradiation: A Mechanistic Investigation. Chem. Mater 2005, 17, 5404–5410. [Google Scholar]
- Tu, W.; Liu, H. Continuous synthesis of colloidal metal nanoclusters by microwave irradiation. J. Chem. Mater 2000, 12, 564–567. [Google Scholar]
- Vasileva, P.; Donkova, B.; Karadjova, I.; Dushkin, C. Synthesis of starch-stabilized silver nanoparticles and their application as a surface plasmon resonance-based sensor of hydrogen peroxide. Colloids Surf. A Physicochem. Eng. Aspects 2011, 382, 203–210. [Google Scholar]
- Mafune, F.; Kohno, J.Y.; Takeda, Y.; Kondow, T. Structure and stability of silver nanoparticles in aqueous solution produced by laser ablation. J. Phys. Chem. B 2000, 104, 8333–8337. [Google Scholar]
- Rao, Y.N.; Banerjee, D.; Datta, A. Gamma irradiation route to synthesis of highly re-dispersible natural polymer capped silver nanoparticles. Radiat. Phys. Chem 2010, 79, 1240–1246. [Google Scholar]
- Temgire, M.K.; Joshi, S.S. Optical and structural studies of silver nanoparticles. Radiat. Phys. Chem 2004, 71, 1039–1044. [Google Scholar]
- Wu, W.T.; Wang, Y.; Shi, L.; Zhu, Q.; Pang, W.; Xu, G.; Lu, F. Fabrication of silver/cross-linked poly(vinyl alcohol) cable-like nanostructures under γ-ray irradiation. Nanotechnology 2005, 16, 3017–3022. [Google Scholar]
- Kumara, M.; Varshney, L.; Francis, S. Radiolytic formation of Ag clusters in aqueous polyvinyl alcohol solution and hydrogel matrix. Radiat. Phys. Chem 2005, 73, 21–27. [Google Scholar]
- Ramnani, S.P.; Biswal, J.; Sabharwal, S. Synthesis of silver nanoparticles supported on silica aerogel using gamma radiolysis. Radiat. Phys. Chem 2007, 76, 1290–1294. [Google Scholar]
- Naghavi, K.; Saion, E.; Rezaee, K.; Yunus, W.M. Influence of dose on particle size of colloidal silver nanoparticles synthesized by gamma radiation. Radiat. Phys. Chem 2010, 79, 1203–1208. [Google Scholar]
- Jovanovi, Z.; Krkljes, A.; Stojkovska, J.; Tomic, S.; Obradovic, B.; Miskovic-Stankovic, V.; Kacarevic-Popovic, Z. Synthesis and characterization of silver/poly(n-vinyl-2-pyrrolidone) hydrogel nanocomposite obtained by in situ radiolytic method. Radiat. Phys. Chem 2011, 80, 1208–1215. [Google Scholar]
- Biswal, J.; Misra, M.; Borde, L.C.; Sabharwal, S. Synthesis of silver nanoparticles in methacrylic acid solution by gamma radiolysis and their application for estimation of dopamine at low concentration. Radiat. Phys. Chem 2012. [Google Scholar] [CrossRef]
- Juby, K.A.; Dwivedi, C.; Kumar, M.; Kota, S.; Misra, H.S.; Bajaj, P.N. Silver nanoparticle-load PVA/gum acacia hydrogel: Synthesis, characterization and antibacterial study. Carbohydr. Polym 2012, 89, 906–913. [Google Scholar]
- Lai, T.; Park, H.G.; Choi, S.H. γ-Irradiation-induced preparation of Ag and Au nanoparticles and their characterizations. Mater. Chem. Phys 2007, 105, 325–330. [Google Scholar]
- Belloni, J. Nucleation, growth and properties of nanoclusters studied by radiation chemistry application to catalysis. Catal. Today 2006, 113, 141–156. [Google Scholar]
- Mallicka, K.; Witcombb, M.J.; Scurrella, M.S. Self-assembly of silver nanoparticles in a polymer solvent: Formation of a nanochain through nanoscale soldering. Mater. Chem. Phys 2005, 90, 221–224. [Google Scholar]
- Zhiqiang, L.; Xiaobin, L.; Zhihong, P. The mechanism of agglomeration and control in the process of ultrafine powder prepared by wet chemical method. Chemistry 1999, 7, 54–57. [Google Scholar]
- Ulanski, P.; Bothe, E.; Rosiak, J.M.; von Sonntag, C. OH radical induced crosslinking and strand breakage of poly(vinyl alcohol) in aqueous solution in the absence and presence of oxygen. A pulse radiolysis and product study. Macromol. Chem. Phys 1994, 195, 1443–1461. [Google Scholar]
- Abargues, R.; Gradess, R.; Canet-Ferrer, J.; Abderrafi, K.; Valdes, J.L.; Martinez-Pastor, J. Scalable heterogeneous synthesis of metallic nanoparticles and aggregate with polyvinyl alcohol. New J. Chem 2009, 33, 913–917. [Google Scholar]
- Zhou, F.; Zhou, R.; Hao, X.; Wu, X.; Rao, W.; Chen, Y.; Gao, D. Influences of surfactant (PVA) concentration and pH on the preparation of copper nano-particles by electron beam irradiation. Radiat. Phys. Chem 2008, 77, 169–173. [Google Scholar]
- Geers, H.; Witt, W. Direct Calculation of the Volume Based Particle Size Distribution from PCS or PCCS Measurements. Proceedings of Particulate Systems Analysis 2008, Stratford-upon-Avon, UK, 21–23 September 2008.
- Abedini, A.; Larki, F.; Saion, E.; Zakaria, A.; Hussein, M.Z. Influence of dose and ion concentration on formation of binary Al–Ni alloy nanoclusters. Radiat. Phys. Chem 2012, 81, 1653–1658. [Google Scholar]
- Mie, G. Contributions to the optics of turbid media, particularly of colloidal metal solutions. J. Ann. Phys 1908, 25, 377–445. [Google Scholar]
- Noguez, C. Optical properties of isolated and supported metal nanoparticles. Opt. Mater 2005, 27, 1204–1211. [Google Scholar]
- Okamoto, T.; Yamaguchi, I. Optical absorption study of the surface Plasmon resonance in gold by self-assembly technique. J. Phys. Chem. B 2003, 38, 10321–10324. [Google Scholar]
- Renteria, V.M.; Garcia-Macedo, J. Modeling of optical absorption of silver prolate nanoparticles stabilized by Gemini surfactant. Colloids Surf. A 2006, 273, 1–3. [Google Scholar]
- Gharibshahi, E.; Saion, E. Quantum mechanical calculation of optical absorption of silver and gold nanoparticles by density functional theory. Phys. Int 2010, 1, 57–64. [Google Scholar]
- Saion, E.; Gharibshahi, E. On the theory of metal nanoparticles based on quantum mechanical calculation. J. Fund. Sci 2011, 7, 6–11. [Google Scholar]
- Gharibshahi, E.; Saion, E. Influence of dose on particle size and optical properties of colloidal platinum nanoparticles. Int. J. Mol. Sci 2012, 13, 14723–14741. [Google Scholar]
- Thomas, L.H. The calculation of atomic fields. Math. Proc. Camb. Philos. Soc 1927, 23, 542–548. [Google Scholar]
- Fermi, E. Un metodo statistico per la determinazione di alcune priorieta dell’atome. Rend. Accad. Naz. Lincei 1927, 6, 602–607. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev 1964, 136, 864–871. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev 1965, 140, 1133–1138. [Google Scholar]
- Dirac, P.A.M. Note on exchange phenomena in the Thomas atom. Math. Proc. Camb. Philos. Soc 1930, 26, 376–385. [Google Scholar]
- Von Weizsacker, C.F. Zur Theorie der Kernmassen. Zeitschrift fur Phys 1935, 96, 431–458. [Google Scholar]
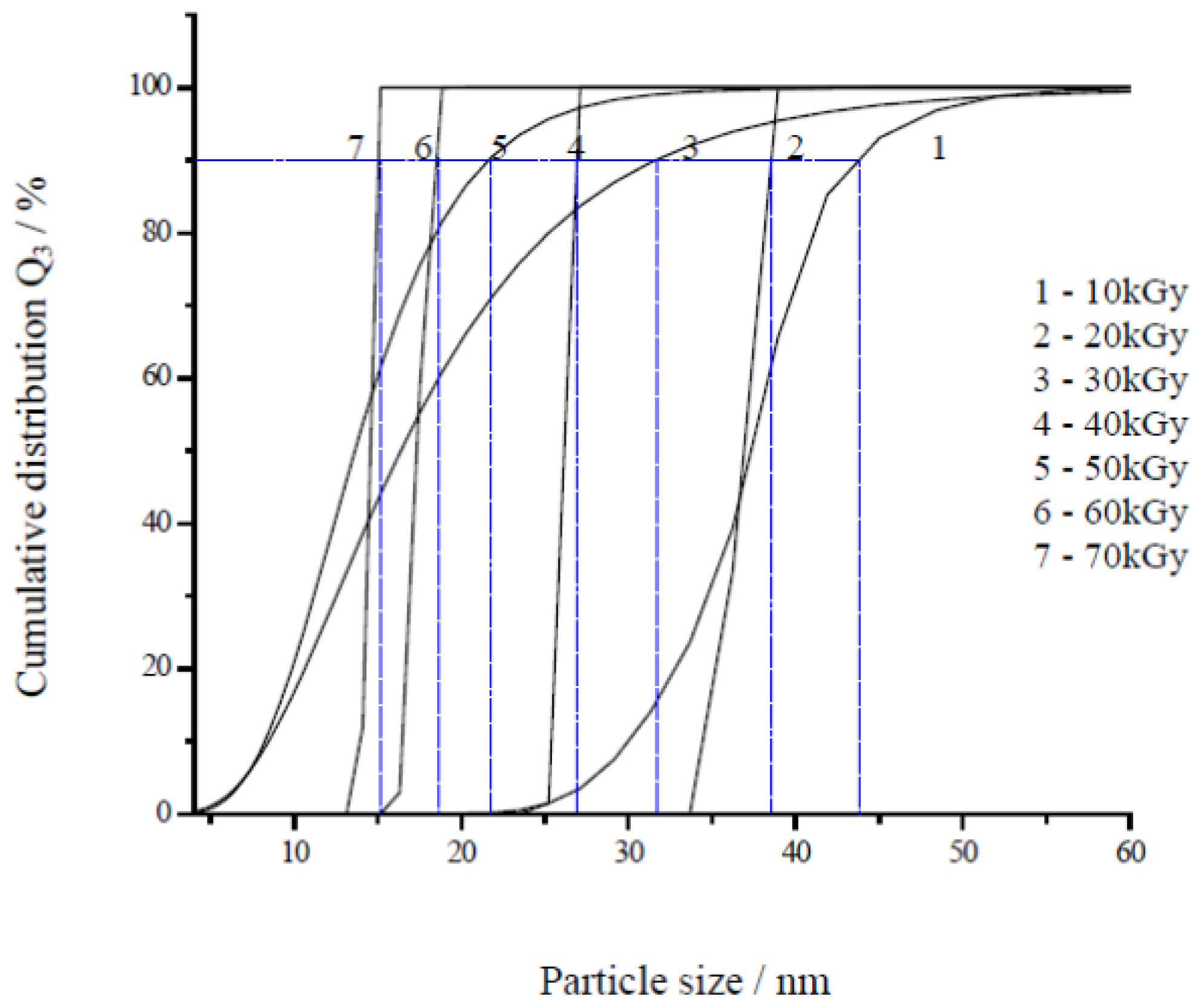
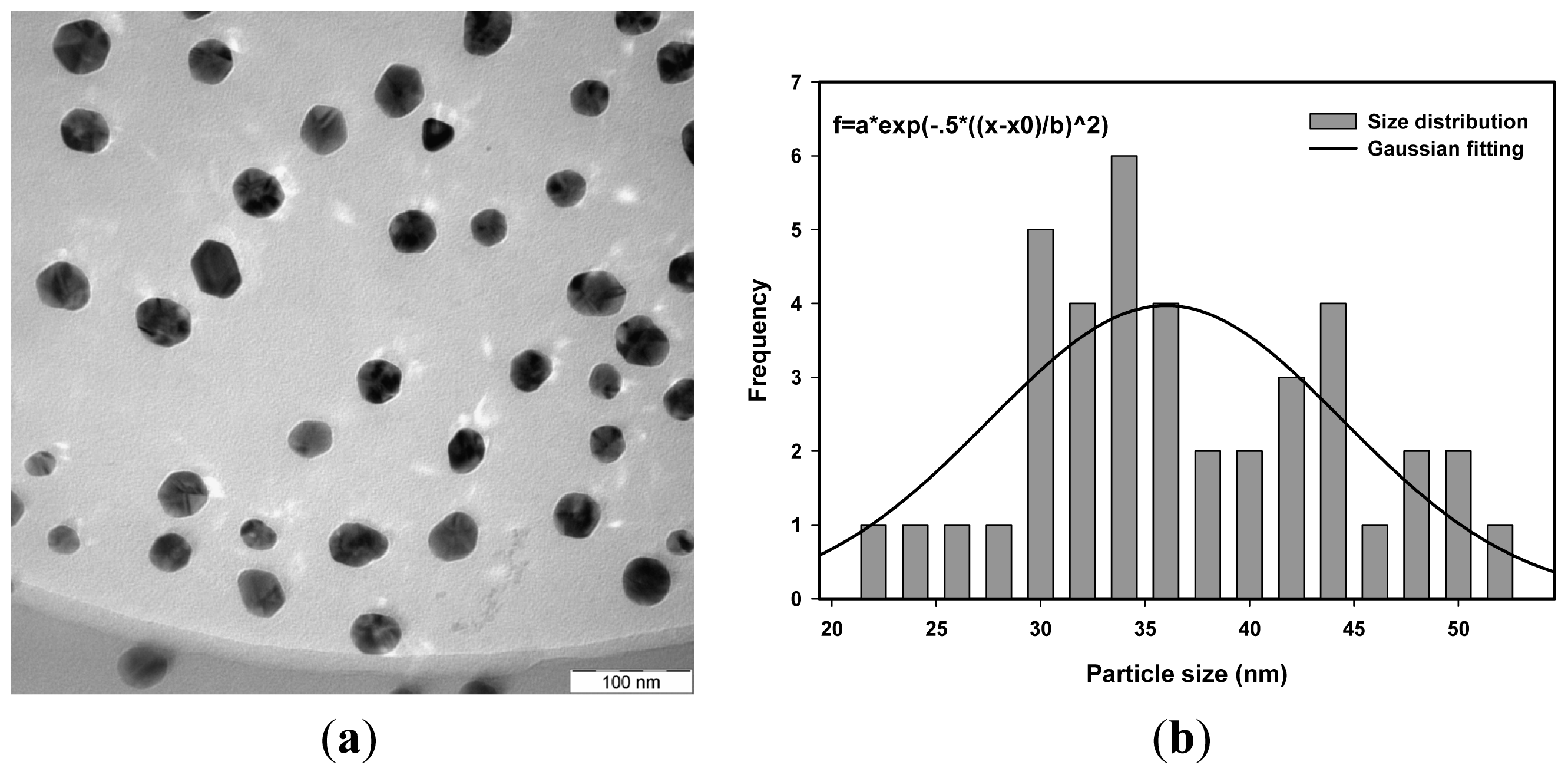
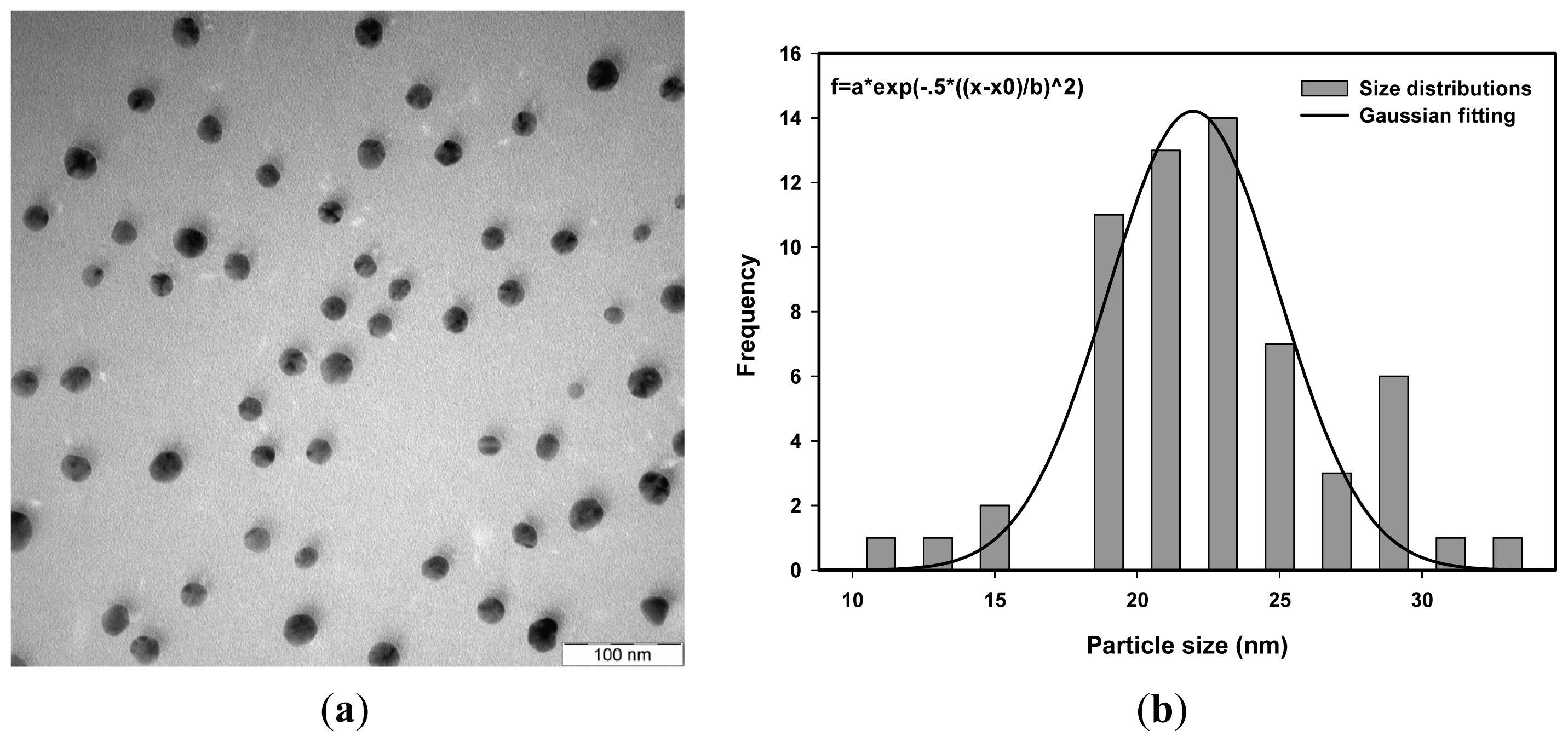
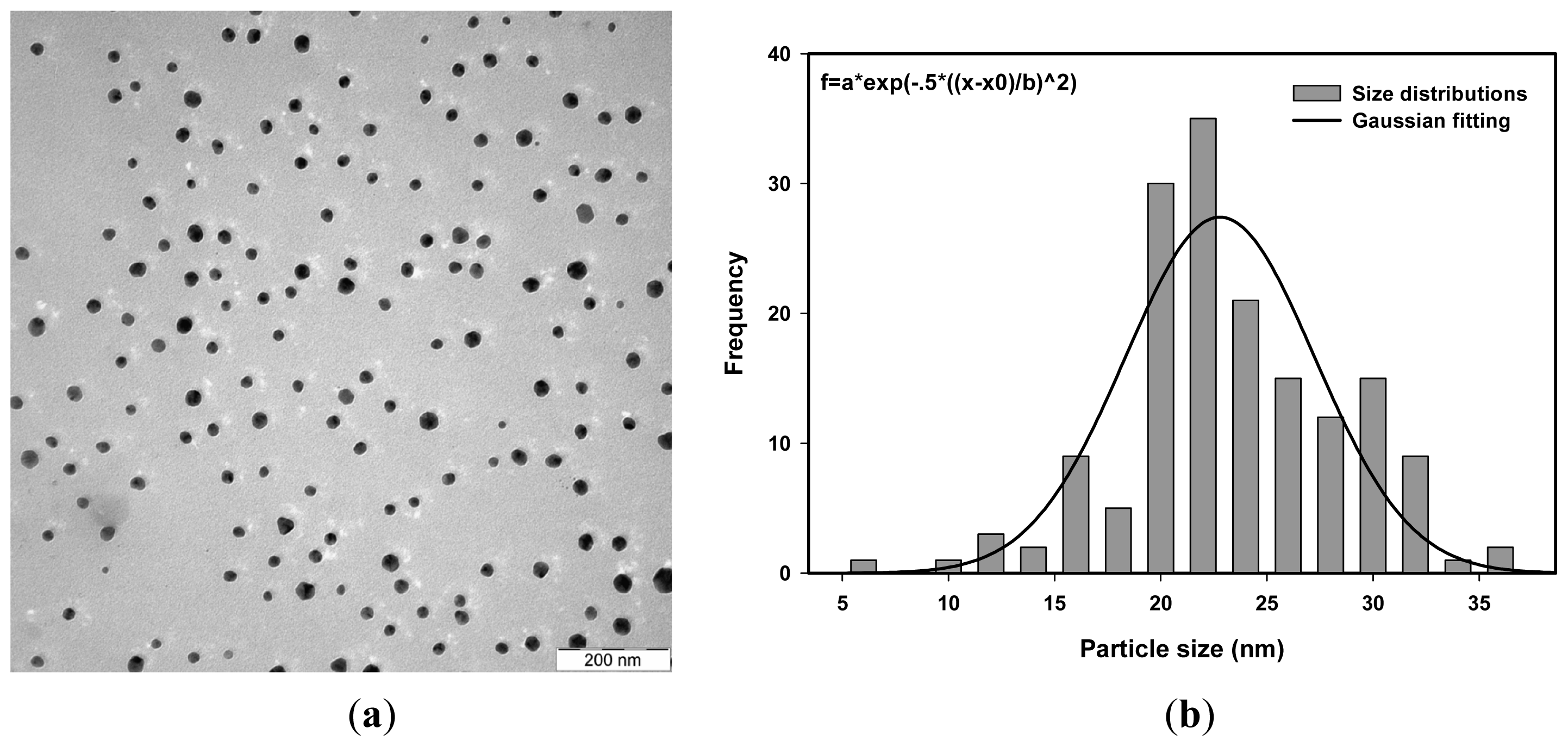

| Dose (kGy) | Average size measurement using TEM (nm) | Average size measurement using PCCS (nm) |
|---|---|---|
| 20 | 36.0 | 37.8 |
| 40 | 25.0 | 26.9 |
| 50 | 22.5 | 21.5 |
| Dose (kGy) | Particle size (nm) | Experiment λmax (nm) | Theory λmax (nm) | Conduction band (eV) |
|---|---|---|---|---|
| 10 | 43.9 | 410.0 | 410.12 | 3.023 |
| 20 | 37.8 | 409.5 | 409.54 | 3.027 |
| 30 | 31.6 | 409.0 | 409.12 | 3.031 |
| 40 | 26.9 | 408.5 | 408.46 | 3.034 |
| 50 | 21.5 | 408.0 | 408.07 | 3.038 |
| 60 | 18.1 | 407.5 | 407.52 | 3.041 |
| 70 | 15.0 | 407.0 | 406.80 | 3.045 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saion, E.; Gharibshahi, E.; Naghavi, K. Size-Controlled and Optical Properties of Monodispersed Silver Nanoparticles Synthesized by the Radiolytic Reduction Method. Int. J. Mol. Sci. 2013, 14, 7880-7896. https://doi.org/10.3390/ijms14047880
Saion E, Gharibshahi E, Naghavi K. Size-Controlled and Optical Properties of Monodispersed Silver Nanoparticles Synthesized by the Radiolytic Reduction Method. International Journal of Molecular Sciences. 2013; 14(4):7880-7896. https://doi.org/10.3390/ijms14047880
Chicago/Turabian StyleSaion, Elias, Elham Gharibshahi, and Kazem Naghavi. 2013. "Size-Controlled and Optical Properties of Monodispersed Silver Nanoparticles Synthesized by the Radiolytic Reduction Method" International Journal of Molecular Sciences 14, no. 4: 7880-7896. https://doi.org/10.3390/ijms14047880




