An Ultrasensitive Electrochemiluminescence Immunoassay for Carbohydrate Antigen 19-9 in Serum Based on Antibody Labeled Fe3O4 Nanoparticles as Capture Probes and Graphene/CdTe Quantum Dot Bionanoconjugates as Signal Amplifiers
Abstract
:1. Introduction
2. Results and Discussion
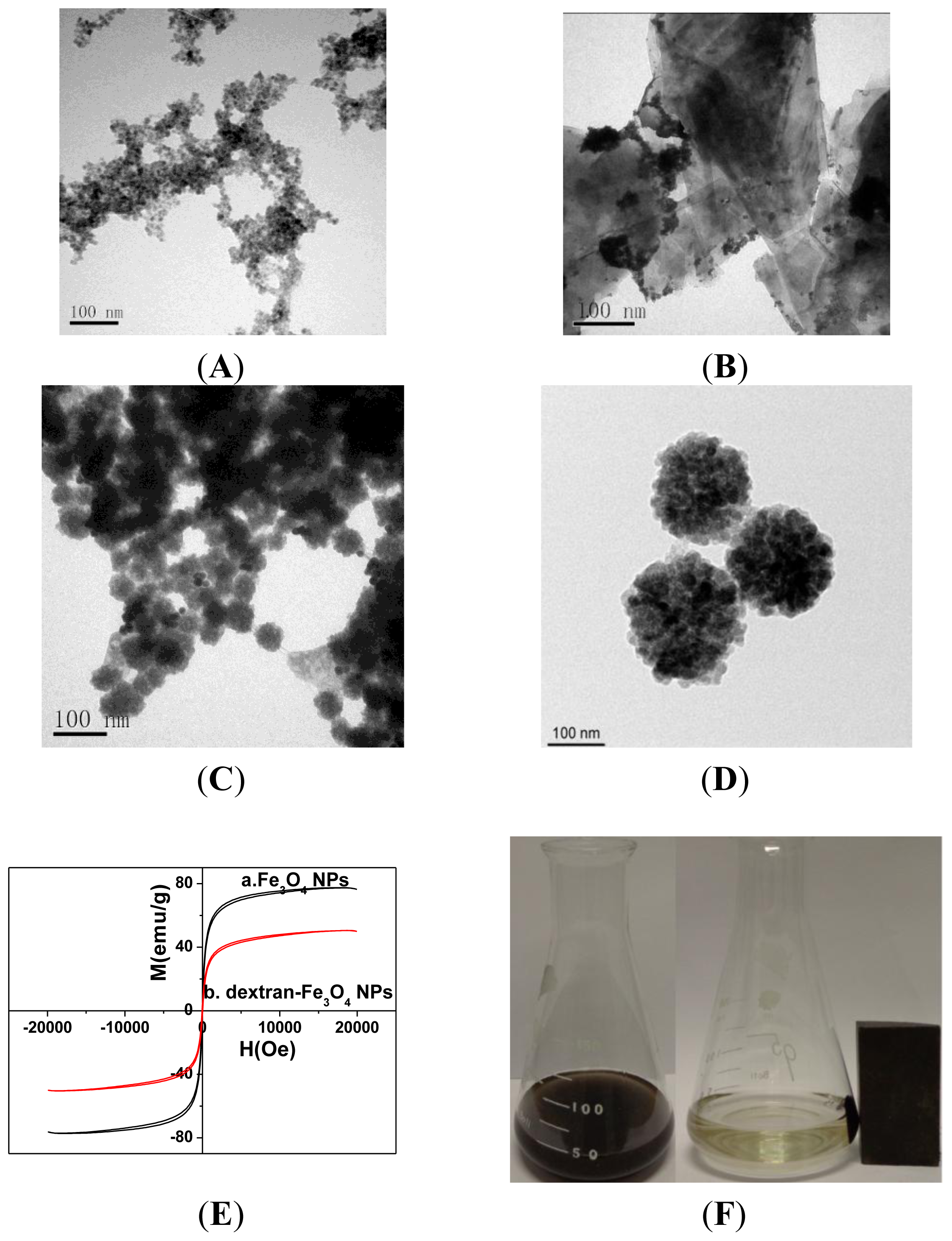
2.1. Spectral Characterization of the CdTe-G and Dextran-Fe3O4 Nanocomposite
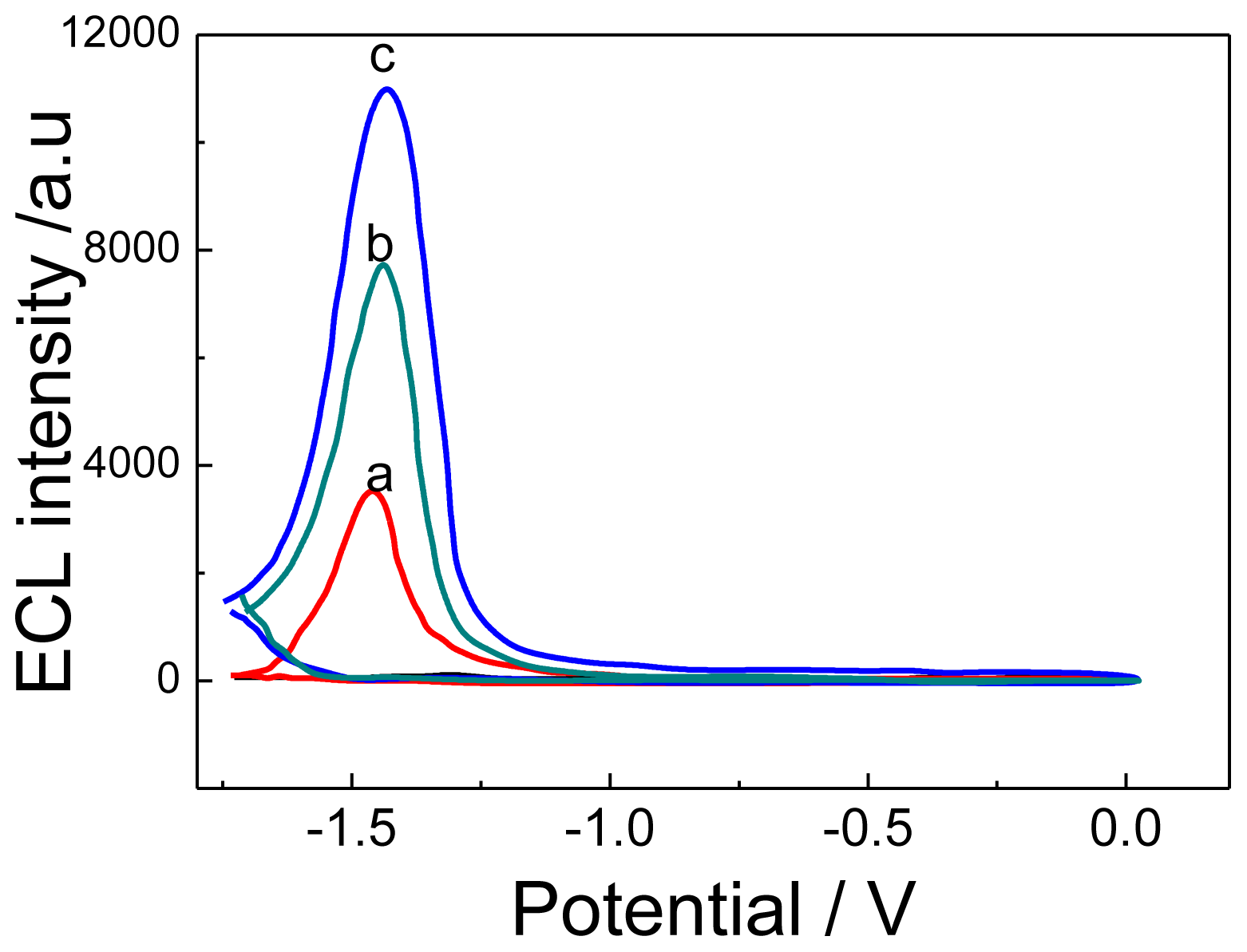
2.2. ECL Characterization of CdTe, CdTe-G and CA 19-9 Ab2/CdTe-G Signal Tag
2.3. Characterization of the ECL Immunosensor
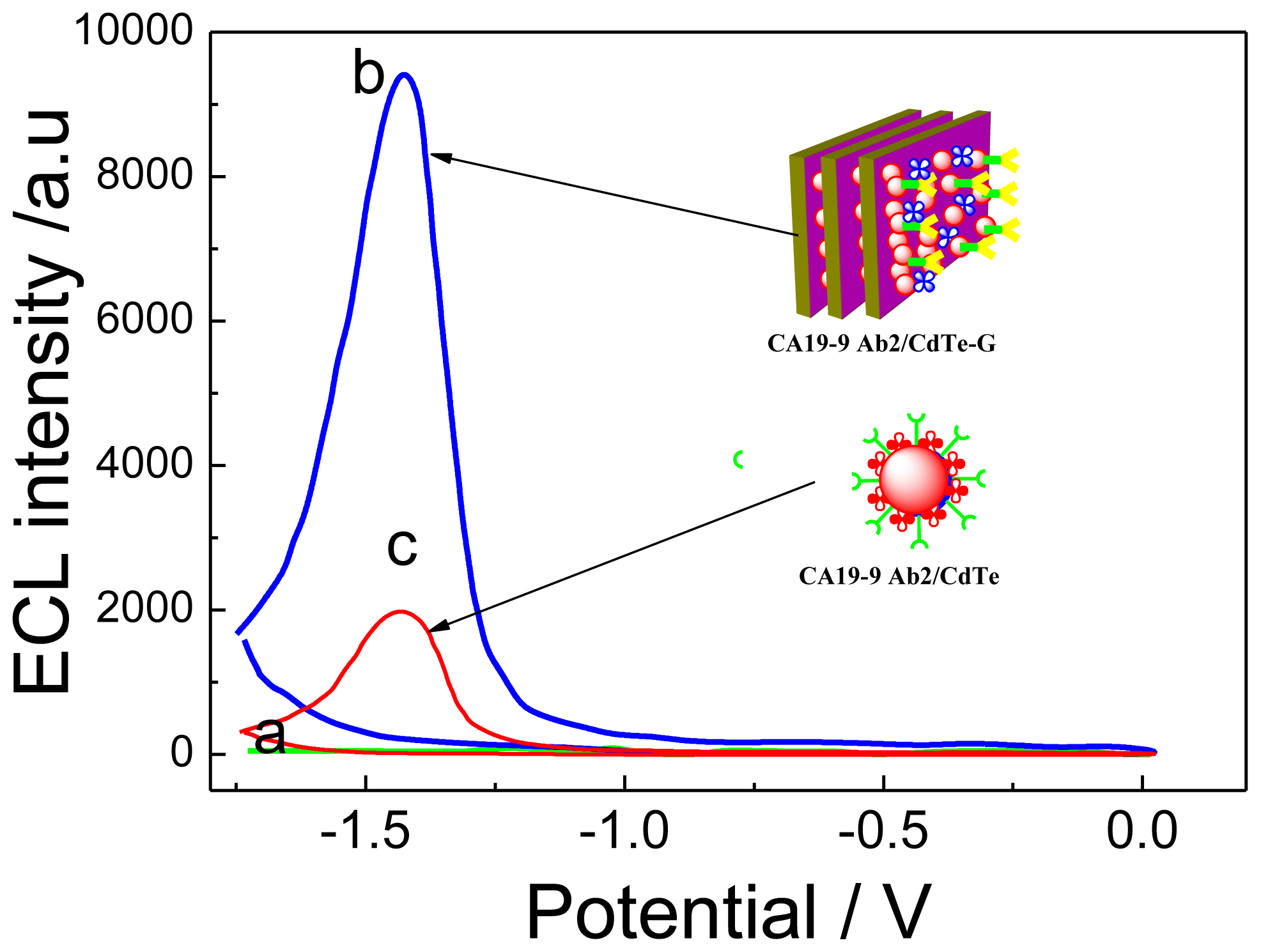
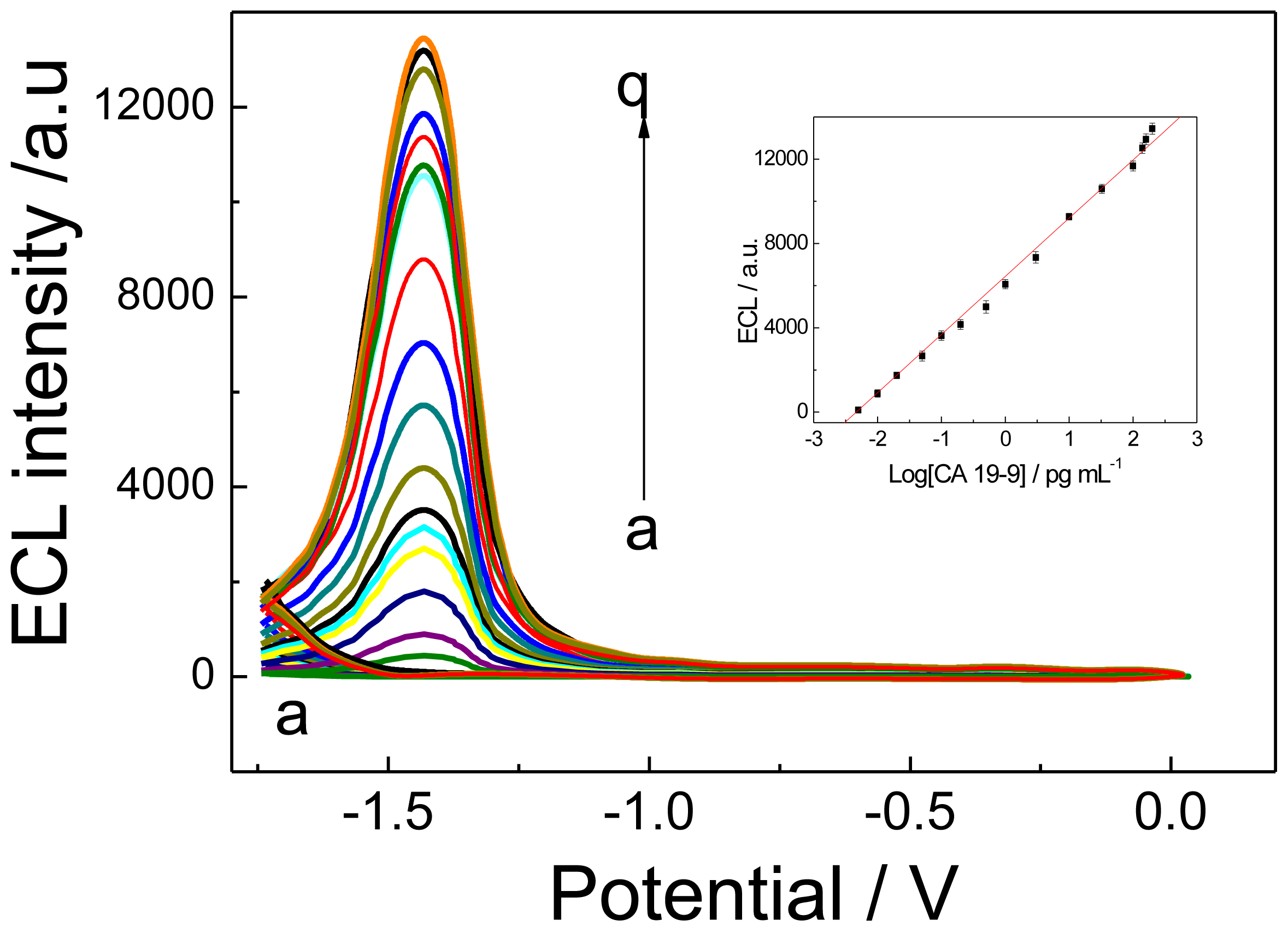
2.3.1. ECL Behavior
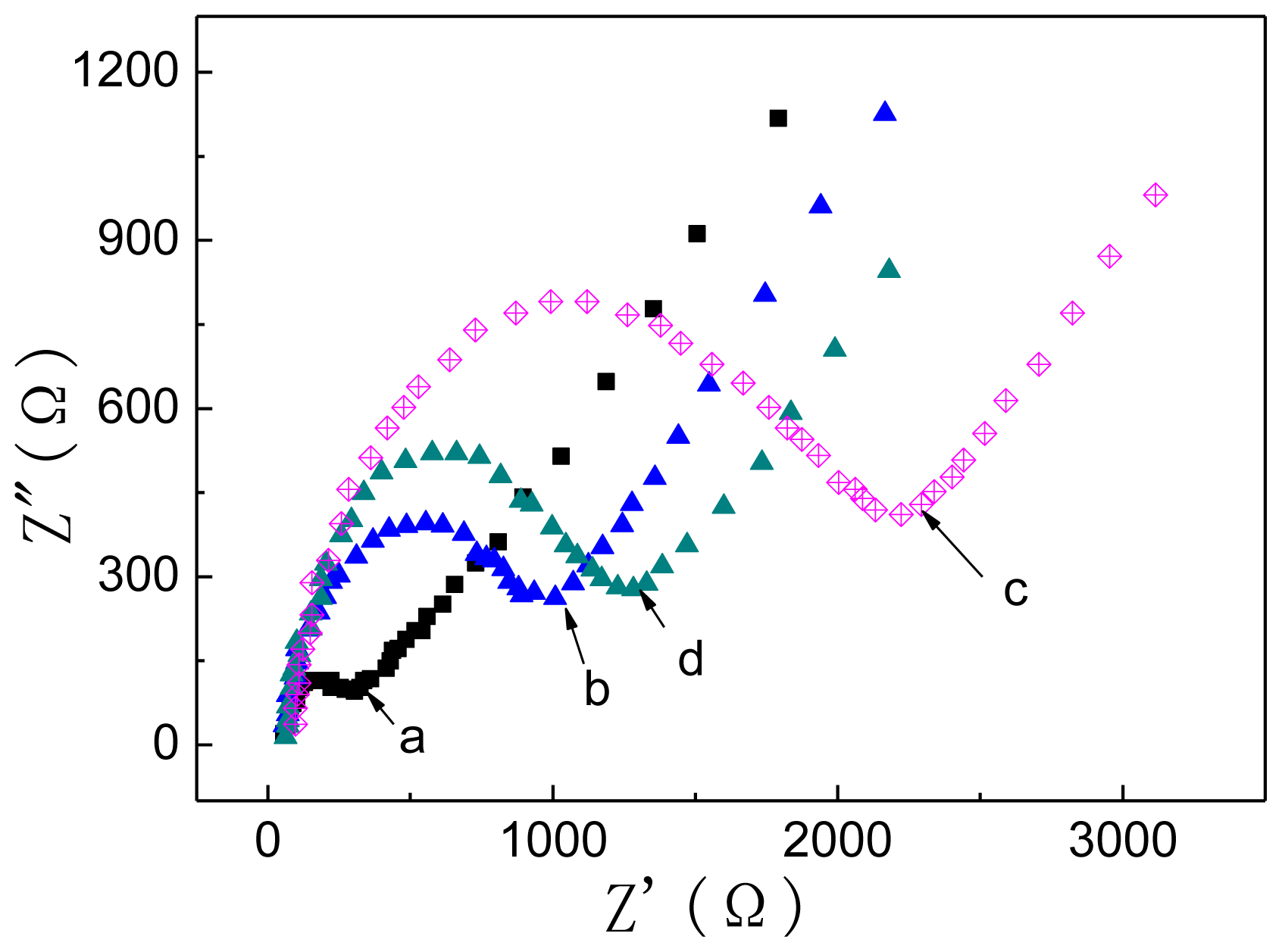
2.3.2. Electrochemical Impedance (EIS) Behavior
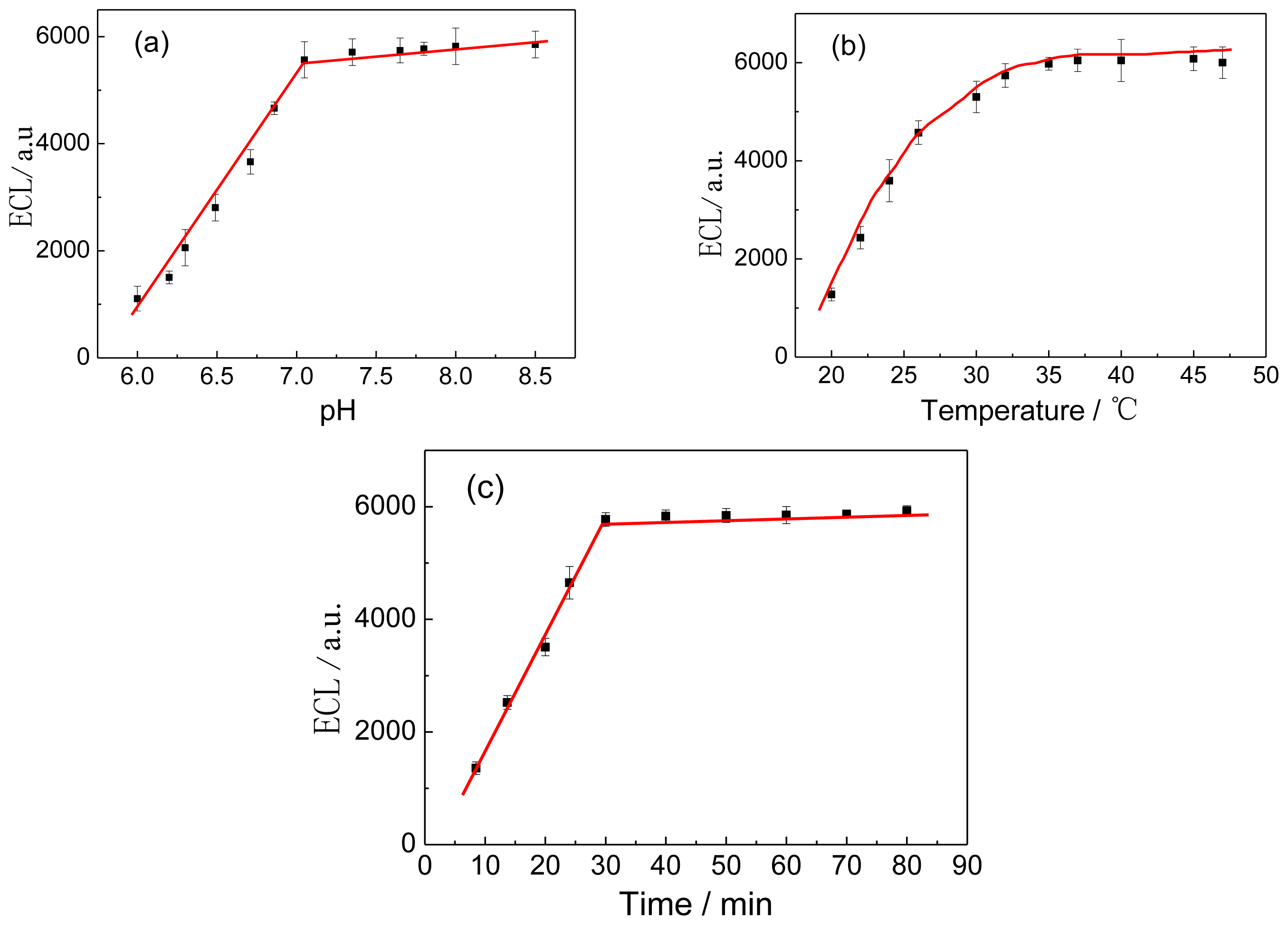
2.3.3. Optimization of Experimental Conditions
2.4. Analytical Performance
2.5. Specificity, Reproducibility and Stability of the ECL Immunosensor
2.6. Application of the Immunosensor in Serum Samples
3. Experimental Section
3.1. Reagents and Chemicals
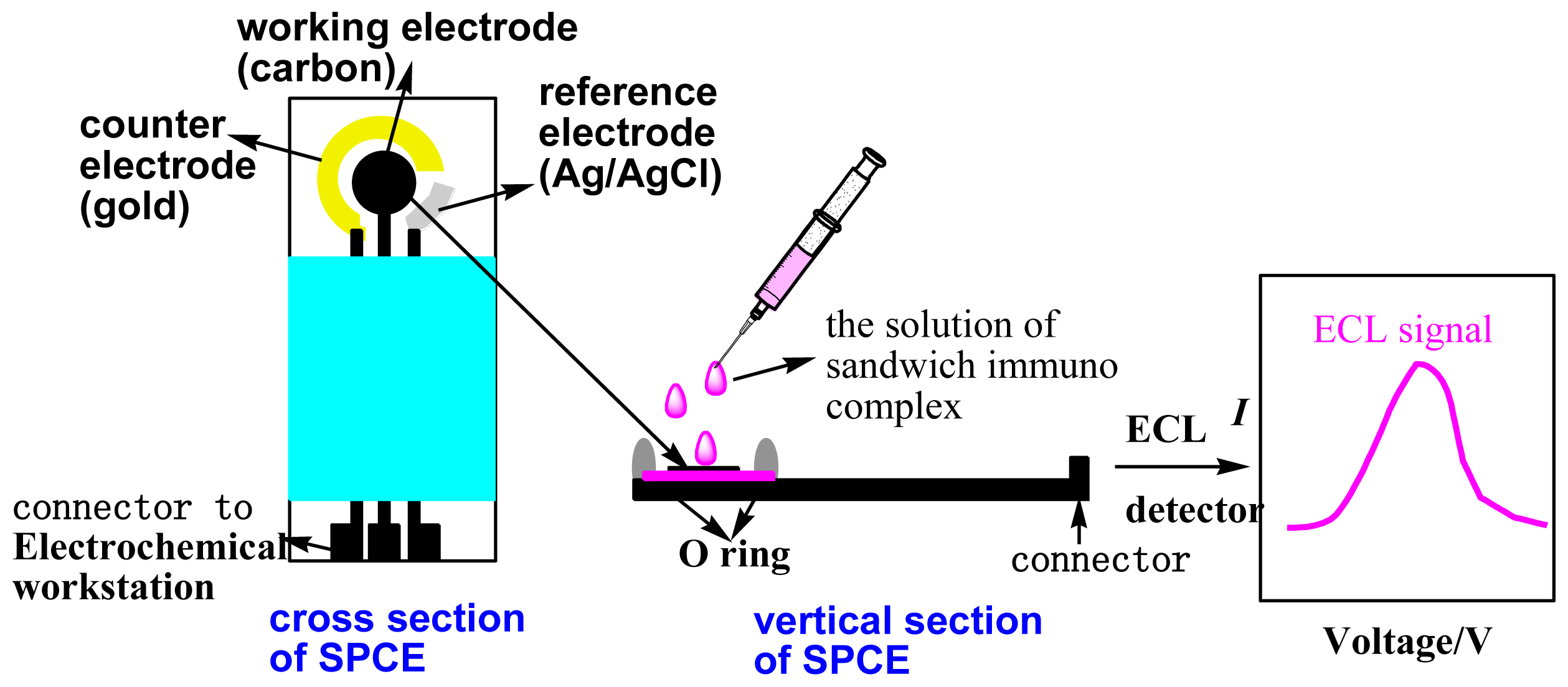
3.2. Apparatus
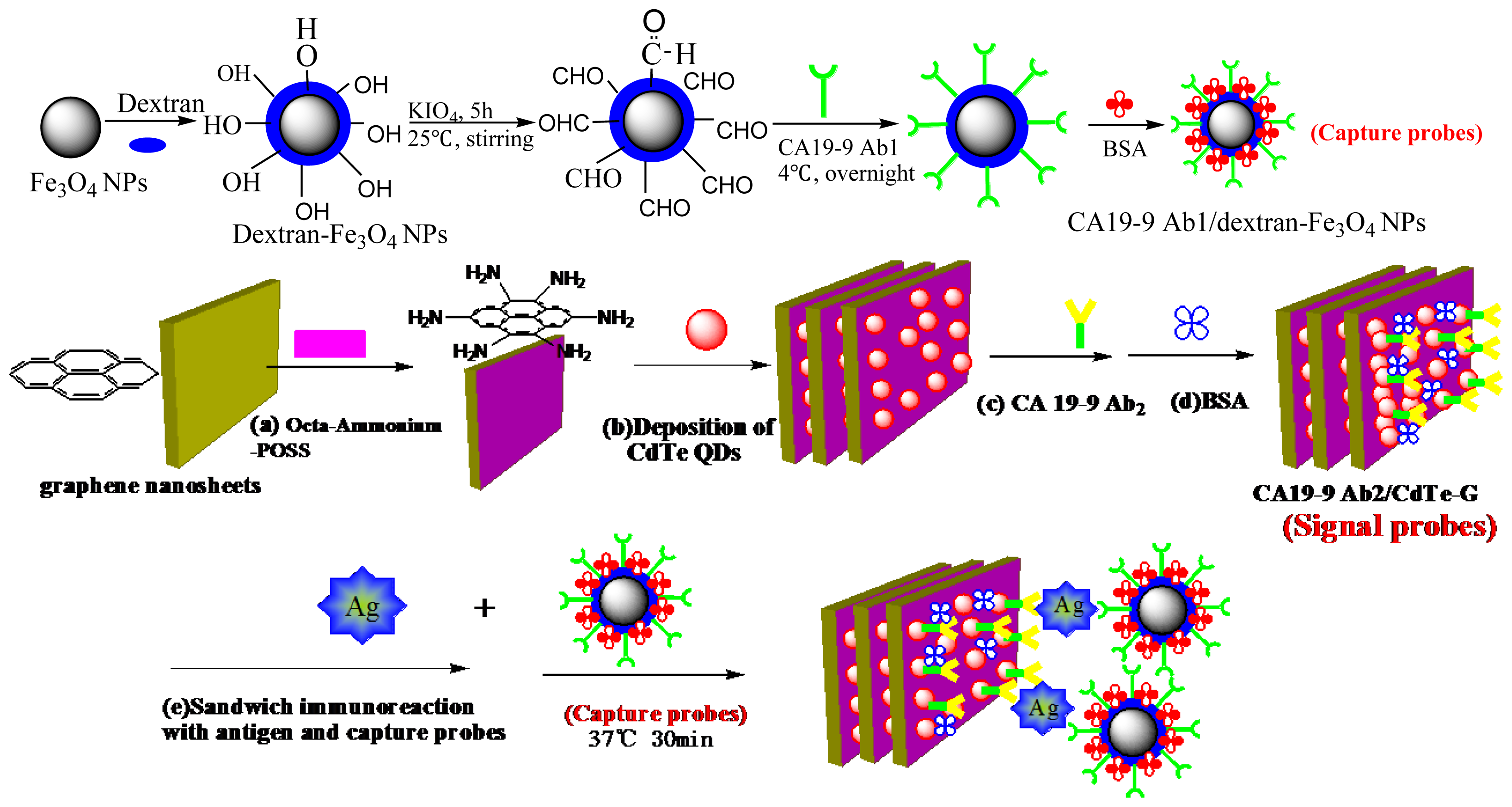
3.3. Synthesis of Dextran-Fe3O4 Magnetic Nanoparticles and CdTe-G
3.3.1. Dextran-Fe3O4 Magnetic Nanoparticles
3.3.2. CdTe-G
3.4. Immobilization of CA 19-9 Secondary Antibodies to CdTe-G (CA 19-9 Ab2/CdTe-G)
3.5. Immobilization of the Primary Antibody of CA 19-9 on the Surface of the Magnetic Dextran-Fe3O4 Nanoparticles (CA 19-9 Ab1/Dextran- Fe3O4)
3.6. ECL Immunoassay for CA 19-9
4. Conclusions
Acknowledgments
References
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S.; et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med 2007, 356, 227–236. [Google Scholar]
- Opstal-van Winden, A.W.J.; Rodenburg, W.; Pennings, J.L.A.; van Oostrom, C.T.M.; Beijnen, J.H.; Peeters, P.H.; van Gils, C.H.; de Vries, A. A bead-based multiplexed immunoassay to evaluate breast cancer biomarkers for early detection in pre-diagnostic serum. Int. J. Mol. Sci 2012, 13, 13587–13604. [Google Scholar]
- Pitteri, S.J.; Amon, L.M.; Busald, B.T.; Zhang, Y.; Johnson, M.M.; Chin, A.; Kennedy, J.; Wong, C.H.; Zhang, Q.; Wang, H.; et al. Detection of elevated plasma levels of epidermal growth factor receptor before breast cancer diagnosis among hormone therapy users. Cancer Res 2010, 70, 8598–8606. [Google Scholar]
- Park, I.J.; Choi, G.S.; Jun, S.H. Prognostic value of serum tumor antigen CA 19-9 after curative resection of colorectal cancer. Anticancer Res 2009, 29, 4303–4308. [Google Scholar]
- Karsan, A.; Eigl, B.J.; Flibotte, S.; Gelmon, K.; Switzer, P.; Hassell, P.; Harrison, D.; Law, J.; Hayes, M.; Stillwell, M.; et al. Analytical and preanalytical biases in serum proteomic pattern analysis for breast cancer diagnosis. Clin. Chem 2005, 51, 1525–1528. [Google Scholar]
- Li, X.Z.; Wang, L.; Zhou, C.; Guan, T.T.; Li, J.; Zhang, Y.H. Preliminary studies of application of CdTe nanocrystals and dextran-Fe3O4 magnetic nanoparticles in sandwich immunoassay. Clin. Chim. Acta 2007, 378, 168–174. [Google Scholar]
- Chu, H.-H.; Yan, J.-L.; Tu, Y.-F. Study on a Luminol-based Electrochemiluminescent Sensor for Label-Free DNA Sensing. Sensors 2010, 10, 9481–9492. [Google Scholar]
- Qi, H.; Peng, Y.; Gao, Q.; Zhang, C. Applications of nanomaterials in electrogenerated chemiluminescence biosensors. Sensors 2009, 9, 674–695. [Google Scholar]
- Gan, N.; Hou, J.; Hu, F.; Cao, Y.; Li, T.; Guo, Z.; Wang, J. A renewable and ultrasensitive electrochemiluminescence immunosenor based on magnetic RuL@SiO2-Au~RuL-Ab2 sandwich-type nano-immunocomplexes. Sensors 2011, 11, 7749–7762. [Google Scholar]
- Fujii, T.; Taguchi, Y.; Saiki, T.; Nagasaka, Y. A Fusion-spliced near-field optical fiber probe using photonic crystal fiber for nanoscale thermometry based on fluorescence-lifetime measurement of quantum dots. Sensors 2011, 11, 8358–8369. [Google Scholar]
- Gaponik, N.; Hickey, S.G.; Dorfs, D.; Rogach, A.L.; Eychmüller, A. Progress in the light emission of colloidal semiconductor nanocrystals. Small 2010, 6, 1364–1378. [Google Scholar]
- Parak, W.J.; Pellegrino, T.; Plank, C. Labelling of cells with quantum dots. Nanotechnology 2005, 16. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, C.Y.; Cao, X.D.; Liu, S.Q. Versatile immunosensor using a quantum dot coated silica nanosphere as a label for signal amplification. Anal. Chem 2010, 82, 6422–6429. [Google Scholar]
- Zhou, Y.; Chiu, C.-W.; Liang, H. Interfacial structures and properties of organic materials for biosensors: An overview. Sensors 2012, 12, 15036–15062. [Google Scholar]
- Zeng, Q.; Cheng, J.; Tang, L.; Liu, X.; Liu, Y.; Li, J.; Jiang, J. Self-assembled grapheme-enzyme hierarchical nanostructures for electrochemical biosensing. Adv. Funct. Mater 2010, 20, 3366–3372. [Google Scholar]
- Mioslav, P.; Petr, S. Electrochemical biosensors-principles and applications. J. Appl. Biomed 2008, 6, 57–64. [Google Scholar]
- Song, W.; Li, D.-W.; Li, Y.-T.; Li, Y.; Long, Y.-T. Disposable biosensor based on graphene oxide conjugated with tyrosinase assembled gold nanoparticles. Biosens. Bioelectron 2011, 26, 3181–3186. [Google Scholar]
- Tang, D.P.; Yuan, R.; Chai, Y.Q. Magnetic core-shell Fe3O4@Ag nanoparticle-coated carbon paste interface for studies of carcinoembryonic antigen in clinical immunoassay. J. Phys. Chem. B 2006, 110, 11640–11646. [Google Scholar]
- Wei, Q.; Xiang, Z.; He, J.; Wang, G.L.; Li, H.; Qian, Z.Y.; Yang, M.H. Dumbbell-like Au-Fe3O4 nanoparticles as label for the preparation of electrochemical immunosensors. Biosens. Bioelectron 2010, 26, 627–631. [Google Scholar]
- Limban, C.; Grumezescu, A.M.; Saviuc, C.; Voicu, G.; Predan, G.; Sakizlian, R.; Chifiriuc, M.C. Optimized anti-pathogenic agents based on core/shell nanostructures and 2-((4-Ethylphenoxy)ethyl)-N-(substituted-phenylcarbamothioyl)-benzamides. Int. J. Mol. Sci 2012, 13, 12584–12597. [Google Scholar]
- Moore, A.; Weissleder, R.; Bogdanov, A., Jr. Uptake of dextran-coated monocrystalline iron oxides in tumor cells and macrophages. J. Magn. Reson. Imaging 1997, 7, 1140–1145. [Google Scholar]
- Setterington, E.B.; Alocilja, E.C. Electrochemical biosensor for rapid and sensitive detection of magnetically extracted bacterial pathogens. Biosensors 2012, 2, 15–31. [Google Scholar]
- Choi, H.N.; Yoon, S.H.; Lyu, Y.K.; Lee, W.Y. Electrogenerated chemiluminescence ethanol biosensor based on carbon nanotube-titania-nafion composite film. Electroanalysis 2007, 19, 459–465. [Google Scholar]
- Garcher, C.; Brona, A.; Baudouin, C.; Bildstein, L.; Barac, J. CA 19-9 ELISA test: A new method for studying mucus changes in tears. Br. J. Ophthalmol 1998, 82, 88–90. [Google Scholar]
- Tao, K.; Dou, H.J.; Sun, K. Combined investigation of experimental characterization and theoretic calculation on the structure of dextran-Fe3O4 clusters. Coll. Surf. A 2006, 290, 70–76. [Google Scholar]
- Wang, Y.; Lu, J.; Tang, L.H.; Chang, H.X.; Li, J.H. Graphene oxide amplified electrogenerated chemiluminescence of quantum dots and its selective sensing for glutathione from thiol-containing compounds. Anal. Chem 2009, 81, 9710–9715. [Google Scholar]
- Zhang, X.; Zhou, X.P.; Ni, S.Y.; Dai, G.-L.; Wang, Y.-S. Preparation and optical properties of CdTe/CdO·nH2O core/shell nano-composites in aqueous solution. Chin. J. Chem. Phys 2007, 20, 779–783. [Google Scholar]
- Gan, N.; Zhou, J.; Xiong, P.; Hu, F.; Cao, Y.; Li, T.; Jiang, Q. An ultrasensitive electrochemiluminescent immunoassay for Aflatoxin M1 in milk, based on extraction by magnetic graphene and detection by antibody-labeled CdTe quantumn dots-carbon nanotubes nanocomposite. Toxins 2013, 5, 865–883. [Google Scholar]
| Sample | Added (pg/mL) | Found by the method | Recovery (%) | Time-resolved fluorescence assay, TRFA |
|---|---|---|---|---|
| Serum 1 | 0.002 | 0.0022 ± 0.0001 | 101 | 0.002 |
| Serum 2 | 0.02 | 0.017 ± 0.003 | 98.5 | 0.019 |
| Serum 3 | 0.2 | 0.22 ± 0.01 | 100 | 0.22 |
| Serum 4 | 2 | 1.82 ± 0.02 | 90.0 | 1.93 |
| Serum 5 | 20 | 19.6 ± 0.7 | 98.0 | 20.4 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gan, N.; Zhou, J.; Xiong, P.; Li, T.; Jiang, S.; Cao, Y.; Jiang, Q. An Ultrasensitive Electrochemiluminescence Immunoassay for Carbohydrate Antigen 19-9 in Serum Based on Antibody Labeled Fe3O4 Nanoparticles as Capture Probes and Graphene/CdTe Quantum Dot Bionanoconjugates as Signal Amplifiers. Int. J. Mol. Sci. 2013, 14, 10397-10411. https://doi.org/10.3390/ijms140510397
Gan N, Zhou J, Xiong P, Li T, Jiang S, Cao Y, Jiang Q. An Ultrasensitive Electrochemiluminescence Immunoassay for Carbohydrate Antigen 19-9 in Serum Based on Antibody Labeled Fe3O4 Nanoparticles as Capture Probes and Graphene/CdTe Quantum Dot Bionanoconjugates as Signal Amplifiers. International Journal of Molecular Sciences. 2013; 14(5):10397-10411. https://doi.org/10.3390/ijms140510397
Chicago/Turabian StyleGan, Ning, Jing Zhou, Ping Xiong, Tianhua Li, Shan Jiang, Yuting Cao, and Qianli Jiang. 2013. "An Ultrasensitive Electrochemiluminescence Immunoassay for Carbohydrate Antigen 19-9 in Serum Based on Antibody Labeled Fe3O4 Nanoparticles as Capture Probes and Graphene/CdTe Quantum Dot Bionanoconjugates as Signal Amplifiers" International Journal of Molecular Sciences 14, no. 5: 10397-10411. https://doi.org/10.3390/ijms140510397




