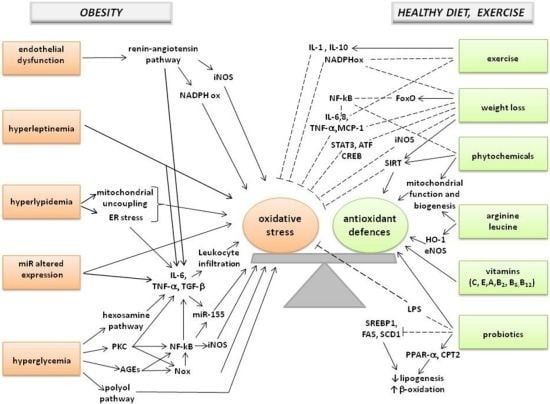
Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State
Abstract
:1. Introduction
2. Redox Balance in Obesity
2.1. Assessment of Redox State
2.2. Association between Oxidative Stress and Obesity
2.3. Conditions Generating ROS/RNS
3. Oxidative Stress in Obesity-Associated Diseases
3.1. Non-Alcoholic Fatty Liver Disease and Steatohepatitis
3.2. Metabolic Syndrome
3.3. Type 2 Diabetes
3.4. Cardiovascular Disease
3.5. Obstructive Sleep Apnea
3.6. Cancer
4. Potential Strategies to Reduce Oxidative Stress in Obesity
4.1. Weight Loss and Physical Activity
4.2. Dietary Pattern and Macronutrients
4.3. Vitamins and Phytochemicals
4.4. Aminoacids
4.5. Gut Microbiota
5. Conclusions
Conflict of Interest
References
- WHO. World Health Organization Fact Sheet for World Wide Prevalence of Obesity. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/indexhtml (accessed on 11 February 2013).
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci 2011, 12, 3117–3132. [Google Scholar]
- Nishida, C.; Ko, G.T.; Kumanyika, S. Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO expert consultation on waist circumference and waist-hip ratio. Eur. J. Clin. Nutr 2010, 64, 2–5. [Google Scholar]
- Lear, S.A.; James, P.T.; Ko, G.T.; Kumanyika, S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur. J. Clin. Nutr 2010, 64, 42–61. [Google Scholar]
- Pérez-Escamilla, R.; Obbagy, J.E.; Altman, J.M.; Essery, E.V.; McGrane, M.M.; Wong, Y.P.; Spahn, J.M.; Williams, C.L. Dietary energy density and body weight in adults and children: A systematic review. J. Acad. Nutr. Diet 2012, 112, 671–684. [Google Scholar]
- Coutinho, G.V.; Coutinho, F.R.; Faiad, J.Z.; Takii, M.S.; de Limareis, S.R.; Ignácio-Souza, L.M.; Paiva, A.A.; Latorraca, M.Q.; Gomes-da-Silva, M.H.; Martins, M.S. Intrauterine protein restriction combined with early postnatal overfeeding was not associated with adult-onset obesity but produced glucose intolerance by pancreatic dysfunction. Nutr. Metab 2013, 10, 5. [Google Scholar]
- Winter, Y.; Sankowski, R.; Back, T. Genetic determinants of obesity and related vascular diseases. Vitam. Horm 2013, 91, 29–48. [Google Scholar]
- Drewnowski, A. Obesity, diets, and social inequalities. Nutr. Rev 2009, 67, S36–S39. [Google Scholar]
- Delrue, M.A.; Michaud, J.L. Fat chance: Genetic syndromes with obesity. Clin. Genet 2004, 66, 83–93. [Google Scholar]
- Stratakis, C.A. Cushing syndrome in pediatrics. Endocrinol. Metab. Clin. North. Am 2012, 41, 793–803. [Google Scholar]
- Hasnain, M.W.; Victor, R.V.; Hollett, B. Weight gain and glucose dysregulation with second-generation antipsychotics and antidepressants: A review for primary care physicians. Postgrad. Med 2012, 124, 154–167. [Google Scholar]
- Warolin, J.; Coenen, K.R.; Kantor, J.L.; Whitaker, L.E.; Wang, L.; Acra, S.A.; Roberts, L.J., 2nd; Buchowski, M.S. The relationship of oxidative stress, adiposity and metabolic risk factors in healthy Black and White American youth. Pediatr. Obes. 2013. [Google Scholar] [CrossRef]
- Tran, B.; Oliver, S.; Rosa, J.; Galassetti, P. Aspects of inflammation and oxidative stress in pediatric obesity and type 1 diabetes: An overview of ten years of studies. Exp. Diabetes Res. 2012. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Patryn, E.; Boehm, D.; Berdowska, I.; Zielinski, B.; Noczynska, A. Advanced oxidation protein products (AOPPs) in juvenile overweight and obesity prior to and following weight reduction. Clin. Biochem 2008, 41, 943–949. [Google Scholar]
- Codoñer-Franch, P.; Tavárez-Alonso, S.; Murria-Estal, R.; Tortajada-Girbés, M.; Simó-Jordá, R.; Alonso-Iglesias, E. Elevated advanced oxidation protein products (AOPPs) indicate metabolic risk in severely obese children. Nutr. Metab. Cardiovasc. Dis 2012, 22, 237–243. [Google Scholar]
- Hermsdorff, H.H.; Barbosa, K.B.; Volp, A.C.; Puchau, B.; Bressan, J.; Zulet, M.A.; Martínez, J.A. Gender-specific relationships between plasma oxidized low-density lipoprotein cholesterol, total antioxidant capacity, and central adiposity indicators. Eur. J. Prev. Cardiol. 2012. [Google Scholar] [CrossRef]
- Karaouzene, N.; Merzouk, H.; Aribi, M.; Merzouk, S.A.; Berrouiguet, A.Y.; Tessier, C.; Narce, M. Effects of the association of aging and obesity on lipids, lipoproteins and oxidative stress biomarkers: A comparison of older with young men. Nutr. Metab. Cardiovasc. Dis 2011, 21, 792–799. [Google Scholar]
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, dietary and postprandial oxidative stress. J. Nutr 2005, 135, 969–972. [Google Scholar]
- Dandona, P.; Ghanim, H.; Chaudhuri, A.; Dhindsa, S.; Kim, S.S. Macronutrient intake induces oxidative and inflammatory stress: Potential relevance to atherosclerosis and insulin resistance. Exp. Mol. Med 2010, 42, 245–253. [Google Scholar]
- Serra, D.; Mera, P.; Malandrino, M.I.; Mir, J.F.; Herrero, L. Mitochondrial fatty acid oxidation in obesity. Antioxid. Redox Signal. 2012. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest 2004, 114, 1752–1761. [Google Scholar]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem 2009, 284, 10601–10609. [Google Scholar]
- Higuchi, M.; Dusting, G.J.; Peshavariya, H.; Jiang, F.; Hsiao, S.T.; Chan, E.C.; Liu, G.S. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and forkhead box o1 mediated upregulation of antioxidant enzymes. Stem. Cells Dev 2013, 22, 878–888. [Google Scholar]
- Horvath, T.L.; Andrews, Z.B.; Diano, S. Fuel utilization by hypothalamic neurons: Roles for ROS. Trends Endocrinol. Metab 2009, 20, 78–87. [Google Scholar]
- Mlinar, B.; Marc, J. New insights into adipose tissue dysfunction in insulin resistance. Clin. Chem. Lab. Med 2011, 49, 1925–1935. [Google Scholar]
- Patel, C.; Ghanim, H.; Ravishankar, S.; Sia, C.L.; Viswanathan, P.; Mohanty, P.; Dandona, P. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J. Clin. Endocrinol. Metab 2007, 92, 4476–4479. [Google Scholar]
- Bełtowski, J. Leptin and the regulation of endothelial function in physiological and pathological conditions. Clin. Exp. Pharmacol. Physiol 2012, 39, 168–178. [Google Scholar]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem 2012, 68, 701–711. [Google Scholar]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Skoumas, I.; Papademetriou, L.; Economou, M.; Stefanadis, C. The implication of obesity on total antioxidant capacity in apparently healthy men and women: The ATTICA study. Nutr. Metab. Cardiovasc. Dis 2007, 17, 590–597. [Google Scholar]
- Strauss, R.S. Comparison of serum concentrations of -tocopherol and -carotene in a cross-sectional sample of obese and nonobese children (NHANES III). J. Pediatr 1999, 134, 160–165. [Google Scholar]
- Bryan, S.; Baregzay, B.; Spicer, D.; Singal, P.K.; Khaper, N. Redox-inflammatory synergy in the metabolic syndrome. Can. J. Physiol. Pharmacol 2013, 91, 22–30. [Google Scholar]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar]
- Crujeiras, A.B.; Díaz-Lagares, A.; Carreira, M.C.; Amil, M.; Casanueva, F.F. Oxidative stress associated to dysfunctional adipose tissue: A potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radic. Res 2013, 47, 243–256. [Google Scholar]
- Tzanetakou, I.P.; Katsilambros, N.L.; Benetos, A.; Mikhailidis, D.P.; Perrea, D.N. “Is obesity linked to aging?”: Adipose tissue and the role of telomeres. Ageing Res. Rev 2012, 11, 220–229. [Google Scholar]
- Bigornia, S.J.; Mott, M.M.; Hess, D.T.; Apovian, C.M.; McDonnell, M.E.; Duess, M.A.; Kluge, M.A.; Fiscale, A.J.; Vita, J.A.; Gokce, N. Long-term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity 2010, 18, 754–759. [Google Scholar]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr 2010, 92, 1189–1196. [Google Scholar]
- Arora, T.; Singh, S.; Sharma, R.K. Probiotics: Interaction with gut microbiome and antiobesity potential. Nutrition 2013, 29, 591–596. [Google Scholar]
- Shen, J.; Obin, M.S.; Zhao, L. The gut microbiota, obesity and insulin resistance. Mol. Aspects Med 2013, 34, 39–58. [Google Scholar]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med 2009, 169, 659–669. [Google Scholar]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr 2011, 106, S5–S78. [Google Scholar]
- González-Castejón, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol. Res 2011, 64, 438–455. [Google Scholar]
- Chae, C.U.; Albert, C.M.; Moorthy, M.V.; Lee, I.M.; Buring, J.E. Vitamin E supplementation and the risk of heart failure in women. Circ. Heart Fail 2012, 5, 176–182. [Google Scholar]
- Lin, J.; Cook, N.R.; Albert, C.; Zaharris, E.; Gaziano, J.M.; van Denburgh, M.; Buring, J.E.; Manson, J.E. Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial. J. Natl. Cancer Inst 2009, 10, 14–23. [Google Scholar]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev 2012, 70, 257–265. [Google Scholar]
- Pinchuk, I.; Shoval, H.; Dotan, Y.; Lichtenberg, D. Evaluation of antioxidants: Scope, limitations and relevance of assays. Chem. Phys. Lipids 2012, 165, 638–647. [Google Scholar]
- Rindler, P.M.; Plafker, S.M.; Szweda, L.I.; Kinter, M. High dietary fat selectively increases catalase expression within cardiac mitochondria. J. Biol. Chem 2013, 288, 1979–1990. [Google Scholar]
- Lee, M.C. Assessment of oxidative stress and antioxidant property using electron spin resonance (ESR) spectroscopy. J. Clin. Biochem. Nutr 2013, 52, 1–8. [Google Scholar]
- Khoo, N.K.; Cantu-Medellin, N.; Devlin, J.E.; St. Croix, C.M.; Watkins, S.C.; Fleming, A.M.; Champion, H.C.; Mason, R.P.; Freeman, B.A.; Kelley, E.E. Obesity-induced tissue free radical generation: An in vivo immuno-spin trapping study. Free Radic. Biol. Med 2012, 52, 2312–2319. [Google Scholar]
- Komosinska-Vassev, K.; Olczyk, P.; Winsz-Szczotka, K.; Klimek, K.; Olczyk, K. Plasma biomarkers of oxidative and AGE-mediated damage of proteins and glycosaminoglycans during healthy ageing: A possible association with ECM metabolism. Mech. Ageing Dev 2012, 133, 538–548. [Google Scholar]
- Dorjgochoo, T.; Gao, Y.T.; Chow, W.H.; Shu, X.O.; Yang, G.; Cai, Q.; Rothman, N.; Cai, H.; Li, H.; Deng, X.; et al. Major metabolite of F2-isoprostane in urine may be a more sensitive biomarker of oxidative stress than isoprostane itself. Am. J. Clin. Nutr 2012, 96, 405–414. [Google Scholar]
- D’Archivio, M.; Annuzzi, G.; Varì, R.; Filesi, C.; Giacco, R.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Rivellese, A.A.; Masella, R. Predominant role of obesity/insulin resistance in oxidative stress development. Eur. J. Clin. Invest 2012, 42, 70–78. [Google Scholar]
- Keaney, J.F., Jr; Larson, M.G.; Vasan, R.S.; Wilson, P.W.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Framingham Study. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol 2003, 23, 434–439. [Google Scholar]
- Ferretti, G.; Bacchetti, T.; Masciangelo, S.; Bicchiega, V. HDL-paraoxonase and membrane lipid peroxidation: A comparison between healthy and obese subjects. Obesity 2010, 18, 1079–1084. [Google Scholar]
- Aslan, M.; Horoz, M.; Sabuncu, T.; Celik, H.; Selek, S. Serum paraoxonase enzyme activity and oxidative stress in obese subjects. Pol. Arch. Med. Wewn 2011, 121, 181–186. [Google Scholar]
- Il’yasova, D.; Wang, F.; Spasojevic, I.; Base, K.; D’Agostino, R.B., Jr; Wagenknecht, L.E. Urinary F2-isoprostanes, obesity, and weight gain in the IRAS cohort. Obesity 2012, 20, 1915–1921. [Google Scholar]
- Kanaya, A.M.; Wassel, C.L.; Stoddard, P.J.; Harris, T.B.; Cummings, S.R.; Kritchevsky, S.B.; Goodpaster, B.H.; Green, C.; Satterfield, S.; Gross, M.D. F2-isoprostanes and adiposity in older adults. Obesity 2011, 19, 861–867. [Google Scholar]
- Freeman, L.R.; Zhang, L.; Nair, A.; Dasuri, K.; Francis, J.; Fernandez-Kim, S.O.; Bruce-Keller, A.J.; Keller, J.N. Obesity increases cerebrocortical reactive oxygen species and impairs brain function. Free Radic. Biol. Med 2013, 56, 226–233. [Google Scholar]
- Yuzefovych, L.V.; Musiyenko, S.I.; Wilson, G.L.; Rachek, L.I. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One 2013, 8, e54059. [Google Scholar]
- Wang, S.; Kaufman, R.J. The impact of the unfolded protein response on human disease. J. Cell. Biol 2012, 197, 857–867. [Google Scholar]
- Pou, K.M.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; Maurovich-Horvat, P.; Larson, M.G.; Keaney, J.F., Jr; Meigs, J.B.; Lipinska, I.; Kathiresan, S.; et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation 2007, 116, 1234–1241. [Google Scholar]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar]
- Al-Aubaidy, H.A.; Jelinek, H.F. Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur. J. Endocrinol 2011, 164, 899–904. [Google Scholar]
- Kilhovd, B.K.; Juutilainen, A.; Lehto, S.; Rönnemaa, T.; Torjesen, P.A.; Hanssen, K.F.; Laakso, M. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: A population-based 18 year follow-up study. Diabetologia 2007, 50, 1409–1417. [Google Scholar]
- Nikolaidis, M.G.; Kerksick, C.M.; Lamprecht, M.; McAnulty, S.R. Redox biology of exercise. Oxid. Med. Cell Longev 2012. [Google Scholar] [CrossRef]
- Gutierrez-Lopez, L.; Garcia-Sanchez, J.R.; Rincon-Viquez Mde, J.; Lara-Padilla, E.; Sierra-Vargas, M.P.; Olivares-Corichi, I.M. Hypocaloric diet and regular moderate aerobic exercise is an effective strategy to reduce anthropometric parameters and oxidative stress in obese patients. Obes. Facts 2012, 5, 12–22. [Google Scholar]
- Brown, L.A.; Kerr, C.J.; Whiting, P.; Finer, N.; McEneny, J.; Ashton, T. Oxidant stress in healthy normal-weight, overweight, and obese individuals. Obesity 2009, 17, 460–466. [Google Scholar]
- Stefanović, A.; Kotur-Stevuljević, J.; Spasić, S.; Bogavac-Stanojević, N.; Bujisić, N. The influence of obesity on the oxidative stress status and the concentration of leptin in type 2 diabetes mellitus patients. Diabetes Res. Clin. Pract 2008, 79, 156–163. [Google Scholar]
- Viroonudomphol, D.; Pongpaew, P.; Tungtrongchitr, R.; Phonrat, B.; Supawan, V.; Vudhivai, N.; Schelp, F.P. Erythrocyte antioxidant enzymes and blood pressure in relation to overweight and obese Thai in Bangkok. Southeast Asian J. Trop. Med. Public Health 2000, 31, 325–334. [Google Scholar]
- Mittal, P.C.; Kant, R. Correlation of increased oxidative stress to body weight in disease-free post menopausal women. Clin. Biochem 2009, 42, 1007–1011. [Google Scholar]
- Olivares-Corichi, I.M.; Viquez, M.J.; Gutierrez-Lopez, L.; Ceballos-Reyes, G.M.; Garcia-Sanchez, J.R. Oxidative stress present in the blood from obese patients modifies the structure and function of insulin. Horm. Metab. Res 2011, 43, 748–753. [Google Scholar]
- Bougoulia, M.; Triantos, A.; Koliakos, G. Plasma interleukin-6 levels, glutathione peroxidase and isoprostane in obese women before and after weight loss. Association with cardiovascular risk factors. Hormones 2006, 5, 192–199. [Google Scholar]
- Tinahones, F.J.; Murri-Pierri, M.; Garrido-Sánchez, L.; García-Almeida, J.M.; García-Serrano, S.; García-Arnés, J.; García-Fuentes, E. Oxidative stress in severely obese persons is greater in those with insulin resistance. Obesity 2009, 17, 240–246. [Google Scholar]
- Lim, S.; Won, H.; Kim, Y.; Jang, M.; Jyothi, K.R.; Kim, Y.; Dandona, P.; Ha, J.; Kim, S.S. Antioxidant enzymes induced by repeated intake of excess energy in the form of high-fat, high-carbohydrate meals are not sufficient to block oxidative stress in healthy lean individuals. Br. J. Nutr 2011, 106, 1544–1551. [Google Scholar]
- Adachi, T.; Toishi, T.; Wu, H.; Kamiya, T.; Hara, H. Expression of extracellular superoxide dismutase during adipose differentiation in 3T3-L1 cells. Redox Rep 2009, 14, 34–40. [Google Scholar]
- Via, M. The malnutrition of obesity: Micronutrient deficiencies that promote diabetes. ISRN Endocrinol. 2012. [Google Scholar] [CrossRef]
- Ortega, R.M.; Rodríguez-Rodríguez, E.; Aparicio, A.; Jiménez-Ortega, A.I.; Palmeros, C.; Perea, J.M.; Navia, B.; López-Sobaler, A.M. Young children with excess of weight show an impaired selenium status. Int. J. Vitam. Nutr. Res 2012, 82, 121–129. [Google Scholar]
- Weisstaub, G.; Hertrampf, E.; López de Romaña, D.; Salazar, G.; Bugueño, C.; Castillo-Duran, C. Plasma zinc concentration, body composition and physical activity in obese preschool children. Biol. Trace Elem. Res 2007, 118, 167–174. [Google Scholar]
- Kaidar-Person, O.; Person, B.; Szomstein, S.; Rosenthal, R.J. Nutritional deficiencies in morbidly obese patients: A new form of malnutrition? Part B: Minerals. Obes. Surg 2008, 18, 1028–1034. [Google Scholar]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr 2009, 90, 1252–1263. [Google Scholar]
- Harnroongroj, T.; Jintaridhi, P.; Vudhivai, N.; Pongpaew, P.; Tungtrongchitr, R.; Phonrat, B.; Changbumrung, S.; Schelp, F.P. B vitamins, vitamin C and hematological measurements in overweight and obese Thais in Bangkok. J. Med. Assoc. Thai 2002, 85, 17–25. [Google Scholar]
- Reitman, A.; Friedrich, I.; Ben-Amotz, A.; Levy, Y. Low plasma antioxidants and normal plasma B vitamins and homocysteine in patients with severe obesity. Isr. Med. Assoc. J 2002, 4, 590–593. [Google Scholar]
- Kaidar-Person, O.; Person, B.; Szomstein, S.; Rosenthal, R.J. Nutritional deficiencies in morbidly obese patients: A new form of malnutrition? Part A: Vitamins. Obes. Surg 2008, 18, 870–876. [Google Scholar]
- Andersen, L.F.; Jacobs, D.R., Jr; Gross, M.D.; Schreiner, P.J.; Dale Williams, O.; Lee, D.H. Longitudinal associations between body mass index and serum carotenoids: The CARDIA study. Br. J. Nutr. 2006, 95, 358–365. [Google Scholar]
- Aasheim, E.T.; Bøhmer, T. Low preoperative vitamin levels in morbidly obese patients: A role of systemic inflammation. Surg. Obes. Relat. Dis 2008, 4, 779–780. [Google Scholar]
- Canoy, D.; Wareham, N.; Welch, A.; Bingham, S.; Luben, R.; Day, N.; Khaw, K.T. Plasma ascorbic acid concentrations and fat distribution in 19,068 British men and women in the European Prospective Investigation into Cancer and Nutrition Norfolk cohort study. Am. J. Clin. Nutr 2005, 82, 1203–1209. [Google Scholar]
- Coen, P.M.; Goodpaster, B.H. Role of intramyocelluar lipids in human health. Trends Endocrinol. Metab 2012, 23, 391–398. [Google Scholar]
- Amati, F. Revisiting the diacylglycerol-induced insulin resistance hypothesis. Obes. Rev 2012, 13, S40–S50. [Google Scholar]
- Diaz-Meco, M.T.; Moscat, J. The atypical PKCs in inflammation: NF-κB and beyond. Immunol. Rev 2012, 246, 154–167. [Google Scholar]
- Piperi, C.; Adamopoulos, C.; Dalagiorgou, G.; Diamanti-Kandarakis, E.; Papavassiliou, A.G. Crosstalk between advanced glycation and endoplasmic reticulum stress: Emerging therapeutic targeting for metabolic diseases. J. Clin. Endocrinol. Metab 2012, 97, 2231–2242. [Google Scholar]
- Boldin, M.P.; Baltimore, D. MicroRNAs, new effectors and regulators of NF-κB. Immunol. Rev 2012, 246, 205–220. [Google Scholar]
- Williams, M.D.; Mitchell, G.M. MicroRNAs in insulin resistance and obesity. Exp. Diabetes Res. 2012. [Google Scholar] [CrossRef]
- Hulsmans, M.; de Keyzer, D.; Holvoet, P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J 2011, 25, 2515–2527. [Google Scholar]
- Surmi, B.K.; Hasty, A.H. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul. Pharmacol 2010, 52, 27–36. [Google Scholar]
- Xue, P.; Hou, Y.; Chen, Y.; Yang, B.; Fu, J.; Zheng, H.; Yarborough, K.; Woods, C.G.; Liu, D.; Yamamoto, M.; et al. Adipose deficiency of Nrf2 in ob/ob mice results in severe metabolic syndrome. Diabetes 2012, 62, 845–854. [Google Scholar]
- Whaley-Connell, A.; Sowers, J.R. Oxidative stress in the cardiorenal metabolic syndrome. Curr. Hypertens. Rep 2012, 14, 360–365. [Google Scholar]
- Guo, F.; Chen, X.L.; Wang, F.; Liang, X.; Sun, Y.X.; Wang, Y.J. Role of angiotensin II type 1 receptor in angiotensin II-induced cytokine production in macrophages. J. Interferon Cytokine Res 2011, 31, 351–361. [Google Scholar]
- Ceci, R.; Sabatini, S.; Duranti, G.; Savini, I.; Avigliano, L.; Rossi, A. Acute, but not chronic, leptin treatment induces acyl-CoA oxidase in C2C12 myotubes. Eur. J. Nutr 2007, 46, 364–368. [Google Scholar]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol 2006, 6, 772–783. [Google Scholar]
- Rahimi, R.S.; Landaverde, C. Nonalcoholic Fatty liver disease and the metabolic syndrome: Clinical implications and treatment. Nutr. Clin. Pract 2013, 28, 40–51. [Google Scholar]
- Harman-Boehm, I.; Bluher, M.; Redel, H.; Sion-Vardy, N.; Ovadia, S.; Avinoach, E.; Shai, I.; Kloting, N.; Stumvoll, M.; Bashan, N.; et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: Effect of regional adiposity and the co-morbidities of obesity. J. Clin. Endocrinol. Metab 2007, 92, 2240–2247. [Google Scholar]
- Fujita, K.; Nishizawa, H.; Funahashi, T.; Shimomura, I.; Shimabukuro, M. Systemic oxidative stress is associated with visceral fat accumulation and the metabolic syndrome. Circ. J 2006, 70, 1437–1442. [Google Scholar]
- Styskal, J.; van Remmen, H.; Richardson, A.; Salmon, A.B. Oxidative stress and diabetes: What can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic. Biol. Med 2012, 52, 46–58. [Google Scholar]
- Chetboun, M.; Abitbol, G.; Rozenberg, K.; Rozenfeld, H.; Deutsch, A.; Sampson, S.R.; Rosenzweig, T. Maintenance of redox state and pancreatic beta-cell function: Role of leptin and adiponectin. J. Cell. Biochem 2012, 113, 1966–1976. [Google Scholar]
- Coutinho, T.; Goel, K.; Corrêa de Sá, D.; Carter, R.E.; Hodge, D.O.; Kragelund, C.; Kanaya, A.M.; Zeller, M.; Park, J.S.; Kober, L.; et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: Role of “normal weight central obesity”. J. Am. Coll. Cardiol 2013, 61, 553–560. [Google Scholar]
- Mandavia, C.H.; Pulakat, L.; de Marco, V.; Sowers, J.R. Over-nutrition and metabolic cardiomyopathy. Metabolism 2012, 61, 1205–1210. [Google Scholar]
- Standl, E. Dysglycemia and abdominal obesity. Curr. Vasc. Pharmacol 2012, 10, 678–679. [Google Scholar]
- Abhijit, S.; Bhaskaran, R.; Narayanasamy, A.; Chakroborty, A.; Manickam, N.; Dixit, M.; Mohan, V.; Balasubramanyam, M. Hyperinsulinemia-induced vascular smooth muscle cell (VSMC) migration and proliferation is mediated by converging mechanisms of mitochondrial dysfunction and oxidative stress. Mol. Cell Biochem 2013, 373, 95–105. [Google Scholar]
- Anfossi, G.; Russo, I.; Massucco, P.; Mattiello, L.; Doronzo, G.; de Salve, A.; Trovati, M. Impaired synthesis and action of antiaggregating cyclic nucleotides in platelets from obese subjects: Possible role in platelet hyperactivation in obesity. Eur. J. Clin. Invest 2004, 34, 482–489. [Google Scholar]
- Declercq, V.; Enns, J.; Yeganeh, A.; Taylor, C.; Zahradka, P. Modulation of cardiovascular function by adipokines. Cardiovasc. Hematol. Disord. Drug. Targets 2012, 13, 59–72. [Google Scholar]
- Shen, W.; Tian, C.; Chen, H.; Yang, Y.; Zhu, D.; Gao, P.; Liu, J. Oxidative stress mediates chemerin-induced autophagy in endothelial cells. Free Radic. Biol. Med 2013, 55, 73–82. [Google Scholar]
- Yang, Z.; Ming, X.F. mTOR signalling: The molecular interface connecting metabolic stress, aging and cardiovascular diseases. Obes. Rev 2012, 13, 58–68. [Google Scholar]
- Bailey-Downs, L.C.; Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Sonntag, W.E.; Csiszar, A.; Ungvari, Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: A paracrine mechanism contributing to vascular redox dysregulation and inflammation. J. Gerontol. A 2012. [Google Scholar] [CrossRef]
- Lee, S.D.; Ju, G.; Choi, J.A.; Kim, J.W.; Yoon, I.Y. The association of oxidative stress with central obesity in obstructive sleep apnea. Sleep Breath 2012, 16, 511–517. [Google Scholar]
- Jelic, S.; Lederer, D.J.; Adams, T.; Padeletti, M.; Colombo, P.C.; Factor, P.H.; Le Jemtel, T.H. Vascular inflammation in obesity and sleep apnea. Circulation 2010, 121, 1014–1021. [Google Scholar]
- Dyugovskaya, L.; Lavie, P.; Lavie, L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am. J. Respir. Crit. Care Med 2002, 165, 934–939. [Google Scholar]
- Fujihara, S.; Mori, H.; Kobara, H.; Nishiyama, N.; Kobayashi, M.; Oryu, M.; Masaki, T. Metabolic syndrome, obesity, and gastrointestinal cancer. Gastroenterol. Res. Pract 2012. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. Adults. N. Engl. J. Med 2003, 348, 1625–1638. [Google Scholar]
- Vucenik, I.; Stains, J.P. Obesity and cancer risk: Evidence, mechanisms, and recommendations. Ann. N. Y. Acad. Sci 2012, 1271, 37–43. [Google Scholar]
- Gillett, M.; Royle, P.; Snaith, A.; Scotland, G.; Poobalan, A.; Imamura, M.; Black, C.; Boroujerdi, M.; Jick, S.; Wyness, L.; et al. Non-pharmacological interventions to reduce the risk of diabetes in people with impaired glucose regulation: A systematic review and economic evaluation. Health Technol. Assess 2012, 16, 1–236. [Google Scholar]
- Pendyala, S.; Neff, L.M.; Suárez-Fariñas, M.; Holt, P.R. Diet-induced weight loss reduces colorectal inflammation: Implications for colorectal carcinogenesis. Am. J. Clin. Nutr 2011, 93, 234–242. [Google Scholar]
- Solá, E.; Navarro, S.; Medina, P.; Vayá, A.; Estellés, A.; Hernández-Mijares, A.; España, F. Activated protein C levels in obesity and weight loss influence. Thromb. Res 2009, 123, 697–700. [Google Scholar]
- Crujeiraseiras, A.B.; Parra, D.; Milagro, F.I.; Goyenechea, E.; Larrarte, E.; Margareto, J.; Martínez, J.A. Differential expression of oxidative stress and inflammation related genes in peripheral blood mononuclear cells in response to a low-calorie diet: A nutrigenomics study. OMICS 2008, 12, 251–261. [Google Scholar]
- Crujeiraseiras, A.B.; Parra, D.; Goyenechea, E.; Abete, I.; Martínez, J.A. Tachyphylaxis effects on postprandial oxidative stress and mitochondrial-related gene expression in overweight subjects after a period of energy restriction. Eur. J. Nutr 2009, 48, 341–347. [Google Scholar]
- Dandona, P.; Mohanty, P.; Ghanim, H.; Aljada, A.; Browne, R.; Hamouda, W.; Prabhala, A.; Afzal, A.; Garg, R. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J. Clin. Endocrinol. Metab 2001, 86, 355–362. [Google Scholar]
- Kuennen, M.R. Interaction between race and weight loss intervention strategy: Effect on markers of inflammation and fat distribution in overweight women. Obesity 2012, 20, 1335–1336. [Google Scholar]
- You, J.S.; Park, J.Y.; Zhao, X.; Jeong, J.S.; Choi, M.J.; Chang, K.J. Relationship among serum taurine, serum adipokines, and body composition during 8-week human body weight control program. Adv. Exp. Med. Biol 2013, 776, 113–120. [Google Scholar]
- Crujeiraseiras, A.B.; Parra, D.; Goyenechea, E.; Martínez, J.A. Sirtuin gene expression in human mononuclear cells is modulated by caloric restriction. Eur. J. Clin. Invest 2008, 38, 672–678. [Google Scholar]
- Gallí, M.; van Gool, F.; Leo, O. Sirtuins and inflammation: Friends or foes? Biochem. Pharmacol 2011, 81, 569–576. [Google Scholar]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med 2011, 89, 667–676. [Google Scholar]
- Rector, R.S.; Warner, S.O.; Liu, Y.; Hinton, P.S.; Sun, G.Y.; Cox, R.H.; Stump, C.S.; Laughlin, M.H.; Dellsperger, K.C.; Thomas, T.R. Exercise and diet induced weight loss improves measures of oxidative stress and insulin sensitivity in adults with characteristics of the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab 2007, 293, E500–E506. [Google Scholar]
- Montero, D.; Walther, G.; Perez-Martin, A.; Roche, E.; Vinet, A. Endothelial dysfunction, inflammation, and oxidative stress in obese children and adolescents: Markers and effect of lifestyle intervention. Obes. Rev 2012, 13, 441–455. [Google Scholar]
- Strasser, B. Physical activity in obesity and metabolic syndrome. Ann. N. Y. Acad. Sci. 2012. [Google Scholar] [CrossRef]
- Feairheller, D.L.; Brown, M.D.; Park, J.Y.; Brinkley, T.E.; Basu, S.; Hagberg, J.M.; Ferrell, R.E.; Fenty-Stewart, N.M. Exercise training, NADPH oxidase p22phox gene polymorphisms, and hypertension. Med. Sci. Sports Exerc 2009, 41, 1421–1428. [Google Scholar]
- Hopps, E.; Canino, B.; Caimi, G. Effects of exercise on inflammation markers in type 2 diabetic subjects. Acta Diabetol 2011, 48, 183–189. [Google Scholar]
- De Lemos, E.T.; Oliveira, J.; Pinheiro, J.P.; Reis, F. Regular physical exercise as a strategy to improve antioxidant and anti-inflammatory status: Benefits in type 2 diabetes mellitus. Oxid. Med. Cell. Longev 2012. [Google Scholar] [CrossRef]
- Mellor, K.; Ritchie, R.H.; Meredith, G.; Woodman, O.L.; Morris, M.J.; Delbridge, L.M. High-fructose diet elevates myocardial superoxide generation in mice in the absence of cardiac hypertrophy. Nutrition 2010, 26, 842–848. [Google Scholar]
- Cummings, B.P.; Stanhope, K.L.; Graham, J.L.; Evans, J.L.; Baskin, D.G.; Griffen, S.C.; Havel, P.J. Dietary fructose accelerates the development of diabetes in UCD-T2DM rats: Amelioration by the antioxidant, alpha-lipoic acid. Am. J. Physiol. Regul. Integr. Comp. Physiol 2010, 298, R1343–R1350. [Google Scholar]
- Leamy, A.K.; Egnatchik, R.A.; Young, J.D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid. Res 2013, 52, 165–174. [Google Scholar]
- Buckland, G.; Travier, N.; Cottet, V.; González, C.A.; Luján-Barroso, L.; Agudo, A.; Trichopoulou, A.; Lagiou, P.; Trichopoulos, D.; Peeters, P.H.; et al. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int. J. Cancer 2012, 26. [Google Scholar] [CrossRef]
- Agnoli, C.; Grioni, S.; Sieri, S.; Palli, D.; Masala, G.; Sacerdote, C.; Vineis, P.; Tumino, R.; Giurdanella, M.C.; Pala, V.; et al. Italian mediterranean index and risk of colorectal cancer in the Italian section of the EPIC cohort. Int. J. Cancer 2013, 132, 1404–1411. [Google Scholar]
- Demarin, V.; Lisak, M.; Morović, S. Mediterranean diet in healthy lifestyle and prevention of stroke. Acta. Clin. Croat 2011, 50, 67–77. [Google Scholar]
- Samieri, C.; Okereke, O.I.; Devore, E.E.; Grodstein, F. Long-term adherence to the mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J. Nutr. 2013. [Google Scholar] [CrossRef]
- Van Dijk, S.J.; Feskens, E.J.; Bos, M.B.; de Groot, L.C.; de Vries, J.H.; Müller, M.; Afman, L.A. Consumption of a high monounsaturated fat diet reduces oxidative phosphorylation gene expression in peripheral blood mononuclear cells of abdominally overweight men and women. J. Nutr 2012, 142, 1219–1225. [Google Scholar]
- Ortega-Azorín, C.; Sorlí, J.V.; Asensio, E.M.; Coltell, O.; Martínez-González, M.Á.; Salas-Salvadó, J.; Covas, M.I.; Arós, F.; Lapetra, J.; Serra-Majem, L.; et al. Associations of the FTO rs9939609 and the MC4R rs17782313 polymorphisms with type 2 diabetes are modulated by diet, being higher when adherence to the Mediterranean diet pattern is low. Cardiovasc. Diabetol 2012, 11, 137. [Google Scholar]
- Bradlee, M.L.; Singer, M.R.; Qureshi, M.M.; Moore, L.L. Food group intake and central obesity among children and adolescents in the Third National Health and Nutrition Examination Survey (NHANES III). Public. Health Nutr 2010, 22, 1–9. [Google Scholar]
- Liu, S.; Manson, J.E.; Lee, I.M.; Cole, S.R.; Hennekens, C.H.; Willett, W.C.; Buring, J.E. Fruit and vegetable intake and risk of cardiovascular disease: The Women’s Health Study. Am. J. Clin. Nutr 2000, 72, 922–928. [Google Scholar]
- Potter, A.S.; Foroudi, S.; Stamatikos, A.; Patil, B.S.; Deyhim, F. Drinking carrot juice increases total antioxidant status and decreases lipid peroxidation in adults. Nutr. J 2011, 10, 96. [Google Scholar]
- O’Neil, C.E.; Keast, D.R.; Nicklas, T.A.; Fulgoni, V.L., 3rd. Out-of-hand nut consumption is associated with improved nutrient intake and health risk markers in US children and adults: National Health and Nutrition Examination Survey 1999–2004. Nutr. Res 2012, 32, 185–194. [Google Scholar]
- O’Neil, C.E.; Nicklas, T.A.; Rampersaud, G.C.; Fulgoni, V.L., III. 100% Orange juice consumption is associated with better diet quality, improved nutrient adequacy, decreased risk for obesity, and improved biomarkers of health in adults. Nutr. J 2012, 11, 107. [Google Scholar]
- Crujeiraseiras, A.B.; Parra, M.D.; Rodríguez, M.C.; Martínez de Morentin, B.E.; Martínez, J.A. A role for fruit content in energy-restricted diets in improving antioxidant status in obese women during weight loss. Nutrition 2006, 22, 593–599. [Google Scholar]
- Balakumar, P.; Taneja, G. Fish oil and vascular endothelial protection: Bench to bedside. Free Radic. Biol. Med 2012, 53, 271–279. [Google Scholar]
- Wang, Y.; Crawford, M.A.; Chen, J.; Li, J.; Ghebremeskel, K.; Campbell, T.C.; Fan, W.; Parker, R.; Leyton, J. Fish consumption, blood docosahexaenoic acid and chronic diseases in Chinese rural populations. Comp. Biochem. Physiol. A 2003, 136, 127–140. [Google Scholar]
- Hayakawa, S.; Yoshikawa, D.; Ishii, H.; Tanaka, M.; Kumagai, S.; Matsumoto, M.; Hayashi, M.; Sugiura, T.; Hayashi, K.; Ando, H.; et al. Association of plasma ω-3 to ω-6 polyunsaturated fatty acid ratio with complexity of coronary artery lesion. Intern. Med 2012, 51, 1009–1014. [Google Scholar]
- Nyby, M.D.; Matsumoto, K.; Yamamoto, K.; Abedi, K.; Eslami, P.; Hernandez, G.; Smutko, V.; Berger, M.E.; Tuck, M.L. Dietary fish oil prevents vascular dysfunction and oxidative stress in hyperinsulinemic rats. Am. J. Hypertens 2005, 18, 213–219. [Google Scholar]
- Wang, T.M.; Chen, C.J.; Lee, T.S.; Chao, H.Y.; Wu, W.H.; Hsieh, S.C.; Sheu, H.H.; Chiang, A.N. Docosahexaenoic acid attenuates VCAM-1 expression and NF-κB activation in TNF-α-treated human aortic endothelial cells. J. Nutr. Biochem 2011, 22, 187–194. [Google Scholar]
- Sjoberg, N.J.; Milte, C.M.; Buckley, J.D.; Howe, P.R.; Coates, A.M.; Saint, D.A. Dose-dependent increases in heart rate variability and arterial compliance in overweight and obese adults with DHA-rich fish oil supplementation. Br. J. Nutr 2010, 103, 243–248. [Google Scholar]
- McDonald, D.M.; O’Kane, F.; McConville, M.; Devine, A.B.; McVeigh, G.E. Platelet redox balance in diabetic patients with hypertension improved by n-3 fatty acids. Diabetes Care 2012. [Google Scholar] [CrossRef]
- Rhee, Y.; Brunt, A. Flaxseed supplementation improved insulin resistance in obese glucose intolerant people: A randomized crossover design. Nutr. J 2011, 10, 44. [Google Scholar]
- Kusunoki, C.; Yang, L.; Yoshizaki, T.; Nakagawa, F.; Ishikado, A.; Kondo, M.; Morino, K.; Sekine, O.; Ugi, S.; Nishio, Y.; et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun 2013, 430, 225–230. [Google Scholar]
- Dilzer, A.; Park, Y. Implication of conjugated linoleic acid (CLA) in human health. Crit. Rev. Food Sci. Nutr 2012, 52, 488–513. [Google Scholar]
- Kim, J.; Paik, H.D.; Shin, M.J.; Park, E. Eight weeks of conjugated linoleic acid supplementation has no effect on antioxidant status in healthy overweight/obese Korean individuals. Eur. J. Nutr 2012, 51, 135–141. [Google Scholar]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar]
- Hermsdorff, H.H.; Puchau, B.; Volp, A.C.; Barbosa, K.B.; Bressan, J.; Zulet, M.Á.; Martínez, J.A. Dietary total antioxidant capacity is inversely related to central adiposity as well as to metabolic and oxidative stress markers in healthy young adults. Nutr. Metab 2011, 22, 8–59. [Google Scholar]
- Del Rio, D.; Agnoli, C.; Pellegrini, N.; Krogh, V.; Brighenti, F.; Mazzeo, T.; Masala, G.; Bendinelli, B.; Berrino, F.; Sieri, S.; et al. Total antioxidant capacity of the diet is associated with lower risk of ischemic stroke in a large Italian cohort. J. Nutr 2011, 141, 118–123. [Google Scholar]
- Ghanim, H.; Sia, C.L.; Upadhyay, M.; Korzeniewski, K.; Viswanathan, P.; Abuaysheh, S.; Mohanty, P.; Dandona, P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am. J. Clin. Nutr 2010, 91, 940–949. [Google Scholar]
- Codoñer-Franch, P.; López-Jaén, A.B.; de la Mano-Hernández, A.; Sentandreu, E.; Simó-Jordá, R.; Valls-Bellés, V. Oxidative markers in children with severe obesity following low-calorie diets supplemented with mandarin juice. Acta Paediatr 2010, 99, 1841–1846. [Google Scholar]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Hedayati, M.; Hosseinpour-Niazi, S.; Azizi, F. Broccoli sprouts reduce oxidative stress in type 2 diabetes: A randomized double-blind clinical trial. Eur. J. Clin. Nutr 2011, 65, 972–977. [Google Scholar]
- Khaw, K.T.; Bingham, S.; Welch, A.; Luben, R.; Wareham, N.; Oakes, S.; Day, N. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: A prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet 2001, 357, 657–663. [Google Scholar]
- Sutherland, W.H.; Manning, P.J.; Walker, R.J.; de Jong, S.A.; Ryalls, A.R.; Berry, E.A. Vitamin E supplementation and plasma 8-isoprostane and adiponectin in overweight subjects. Obesity 2007, 15, 386–391. [Google Scholar]
- Wang, Q.; Sun, Y.; Ma, A.; Li, Y.; Han, X.; Liang, H. Effects of vitamin E on plasma lipid status and oxidative stress in Chinese women with metabolic syndrome. Int. J. Vitam. Nutr. Res 2010, 80, 178–187. [Google Scholar]
- Greenlee, H.; Kwan, M.L.; Kushi, L.H.; Song, J.; Castillo, A.; Weltzien, E.; Quesenberry, C.P., Jr; Caan, B.J. Antioxidant supplement use after breast cancer diagnosis and mortality in the Life After Cancer Epidemiology (LACE) cohort. Cancer 2012, 118, 2048–2058. [Google Scholar]
- Klein, E.A.; Thompson, I.M., Jr; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar]
- Suksomboon, N.; Poolsup, N.; Sinprasert, S. Effects of vitamin E supplementation on glycaemic control in type 2 diabetes: Systematic review of randomized controlled trials. J. Clin. Pharm. Ther 2011, 36, 53–63. [Google Scholar]
- Lonn, E.; Bosch, J.; Yusuf, S.; Sheridan, P.; Pogue, J.; Arnold, J.M.; Ross, C.; Arnold, A.; Sleight, P.; Probstfield, J.; et al. HOPE and HOPE-TOO Trial Investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA 2005, 293, 1338–1347. [Google Scholar]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar]
- Harding, A.H.; Wareham, N.J.; Bingham, S.A.; Khaw, K.; Luben, R.; Welch, A.; Forouhi, N.G. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: The European prospective investigation of cancer—Norfolk prospective study. Arch. Intern. Med 2008, 168, 1493–1499. [Google Scholar]
- Boekholdt, S.M.; Meuwese, M.C.; Day, N.E.; Luben, R.; Welch, A.; Wareham, N.J.; Khaw, K.T. Plasma concentrations of ascorbic acid and C-reactive protein, and risk of future coronary artery disease, in apparently healthy men and women: The EPIC-Norfolk prospective population study. Br. J. Nutr 2006, 96, 516–522. [Google Scholar]
- Myint, P.K.; Luben, R.N.; Welch, A.A.; Bingham, S.A.; Wareham, N.J.; Khaw, K.T. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer Norfolk prospective population study. Am. J. Clin. Nutr 2008, 87, 64–69. [Google Scholar]
- Myint, P.K.; Luben, R.N.; Wareham, N.J.; Khaw, K.T. Association between plasma vitamin C concentrations and blood pressure in the European prospective investigation into cancer-Norfolk population-based study. Hypertension 2011, 58, 372–379. [Google Scholar]
- Pfister, R.; Sharp, S.J.; Luben, R.; Wareham, N.J.; Khaw, K.T. Plasma vitamin C predicts incident heart failure in men and women in European Prospective Investigation into Cancer and Nutrition-Norfolk prospective study. Am. Heart J 2011, 162, 246–253. [Google Scholar]
- Lawlor, D.A.; Davey Smith, G.; Kundu, D.; Bruckdorfer, K.R.; Ebrahim, S. Those confounded vitamins: What can we learn from the differences between observational versus randomised trial evidence? Lancet 2004, 363, 1724–1727. [Google Scholar]
- Song, Y.; Xu, Q.; Park, Y.; Hollenbeck, A.; Schatzkin, A.; Chen, H. Multivitamins, individual vitamin and mineral supplements, and risk of diabetes among older U.S. adults. Diabetes Care 2011, 34, 108–114. [Google Scholar]
- Juraschek, S.P.; Guallar, E.; Appel, L.J.; Miller, E.R., 3rd. Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr 2012, 95, 1079–1088. [Google Scholar]
- Gaziano, J.M.; Glynn, R.J.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Sesso, H.D.; Buring, J.E. Vitamins E and C in the prevention of prostate and total cancer in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2009, 301, 52–62. [Google Scholar]
- Suzuki, K.; Inoue, T.; Hioki, R.; Ochiai, J.; Kusuhara, Y.; Ichino, N.; Osakabe, K.; Hamajima, N.; Ito, Y. Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population. Clin. Nutr 2006, 25, 780–789. [Google Scholar]
- Gaziano, J.M.; Manson, J.E.; Branch, L.G.; Colditz, G.A.; Willett, W.C.; Buring, J.E. A prospective study of consumption of carotenoids in fruits and vegetables and decreased cardiovascular mortality in the elderly. Ann. Epidemiol 1995, 5, 255–260. [Google Scholar]
- Agarwal, M.; Parameswari, R.P.; Vasanthi, H.R.; Das, D.K. Dynamic action of carotenoids in cardioprotection and maintenance of cardiac health. Molecules 2012, 17, 4755–4769. [Google Scholar]
- Arunkumar, E.; Bhuvaneswari, S.; Anuradha, C.V. An intervention study in obese mice with astaxanthin, a marine carotenoid—Effects on insulin signaling and pro-inflammatory cytokines. Food Funct 2012, 3, 120–126. [Google Scholar]
- Hozawa, A.; Jacobs, D.R., Jr; Steffes, M.W.; Gross, M.D.; Steffen, L.M.; Lee, D.H. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: The Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clin. Chem 2007, 53, 447–455. [Google Scholar]
- Hozawa, A.; Jacobs, D.R., Jr; Steffes, M.W.; Gross, M.D.; Steffen, L.M.; Lee, D.H. Circulating carotenoid concentrations and incident hypertension: The Coronary Artery Risk Development in Young Adults (CARDIA) study. J. Hypertens 2009, 27, 237–242. [Google Scholar]
- Czernichow, S.; Vergnaud, A.C.; Galan, P.; Arnaud, J.; Favier, A.; Faure, H.; Huxley, R.; Hercberg, S.; Ahluwalia, N. Effects of long-term antioxidant supplementation and association of serum antioxidant concentrations with risk of metabolic syndrome in adults. Am. J. Clin. Nutr 2009, 90, 329–335. [Google Scholar]
- Choi, H.D.; Kim, J.H.; Chang, M.J.; Kyu-Youn, Y.; Shin, W.G. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother. Res 2011, 25, 1813–1818. [Google Scholar]
- Iwamoto, M.; Imai, K.; Ohta, H.; Shirouchi, B.; Sato, M. Supplementation of highly concentrated β-cryptoxanthin in a satsuma mandarin beverage improves adipocytokine profiles in obese Japanese women. Lipids Health Dis 2012, 11, 52. [Google Scholar]
- Engelhard, Y.N.; Gazer, B.; Paran, E. Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: A double-blind, placebo-controlled pilot study. Am. Heart. J 2006, 151, 100. [Google Scholar]
- Valero, M.A.; Vidal, A.; Burgos, R.; Calvo, F.L.; Martínez, C.; Luengo, L.M.; Cuerda, C. Meta-analysis on the role of lycopene in type 2 diabetes mellitus. Nutr. Hosp 2011, 26, 1236–1241. [Google Scholar]
- Pérez-Jiménez, J.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary intake of 337 polyphenols in French adults. Am. J. Clin. Nutr 2011, 93, 1220–1228. [Google Scholar]
- Pedret, A.; Valls, R.M.; Fernández-Castillejo, S.; Catalán, Ú.; Romeu, M.; Giralt, M.; Lamuela-Raventós, R.M.; Medina-Remón, A.; Arija, V.; Aranda, N.; et al. Polyphenol-rich foods exhibit DNA antioxidative properties and protect the glutathione system in healthy subjects. Mol. Nutr. Food Res 2012, 56, 1025–1033. [Google Scholar]
- Sies, H.; Hollman, P.C.; Grune, T.; Stahl, W.; Biesalski, H.K.; Williamson, G. Protection by flavanol-rich foods against vascular dysfunction and oxidative damage: 27th Hohenheim Consensus Conference. Adv. Nutr 2012, 3, 217–221. [Google Scholar]
- Baret, P.; Septembre-Malaterre, A.; Rigoulet, M.; Lefebvre d’Hellencourt, C.; Priault, M.; Gonthier, M.P.; Devin, A. Dietary polyphenols preconditioning protects 3T3-L1 preadipocytes from mitochondrial alterations induced by oxidative stress. Int. J. Biochem. Cell Biol 2013, 45, 167–174. [Google Scholar]
- De la Garza, A.L.; Milagro, F.I.; Boque, N.; Campión, J.; Martínez, J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med 2011, 77, 773–785. [Google Scholar]
- Bolca, S.; van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol 2013, 24, 220–225. [Google Scholar]
- Hollman, P.C.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr 2011, 141, S989–S1009. [Google Scholar]
- Leiherer, A.; Mündlein, A.; Drexel, H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascul. Pharmacol 2013, 58, 3–20. [Google Scholar]
- Hokayem, M.; Blond, E.; Vidal, H.; Lambert, K.; Meugnier, E.; Feillet-Coudray, C.; Coudray, C.; Pesenti, S.; Luyton, C.; Lambert-Porcheron, S.; et al. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2012. [Google Scholar] [CrossRef]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Effect of oryzanol and ferulic acid on the glucose metabolism of mice fed with a high-fat diet. J. Food Sci 2011, 76, H7–H10. [Google Scholar]
- De Groote, D.; van Belleghem, K.; Devière, J.; van Brussel, W.; Mukaneza, A.; Amininejad, L. Effect of the intake of resveratrol, resveratrol phosphate, and catechin-rich grape seed extract on markers of oxidative stress and gene expression in adult obese subjects. Ann. Nutr. Metab 2012, 61, 15–24. [Google Scholar]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; Lemieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem 2013, 24, 613–623. [Google Scholar]
- Rayalam, S.; Yang, J.Y.; Ambati, S.; Della-Fera, M.A.; Baile, C.A. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother. Res 2008, 22, 1367–1371. [Google Scholar]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell. Metab 2011, 14, 612–622. [Google Scholar]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr 2011, 106, 383–389. [Google Scholar]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res 2012, 32, 537–541. [Google Scholar]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther 2013, 27, 37–48. [Google Scholar]
- Tomé-Carneiro, J.; Larrosa, M.; Yáñez-Gascón, M.J.; Dávalos, A.; Gil-Zamorano, J.; Gonzálvez, M.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; Espín, J.C.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res 2013, 72, 69–82. [Google Scholar]
- Ghanim, H.; Sia, C.L.; Abuaysheh, S.; Korzeniewski, K.; Patnaik, P.; Marumganti, A.; Chaudhuri, A.; Dandona, P. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J. Clin. Endocrinol. Metab 2010, 95, E1–E8. [Google Scholar]
- Elamin, E.; Masclee, A.; Juuti-Uusitalo, K.; van Ijzendoorn, S.; Troost, F.; Pieters, H.J.; Dekker, J.; Jonkers, D. Fatty acid ethyl esters induce intestinal epithelial barrier dysfunction via a reactive oxygen species-dependent mechanism in a three-dimensional cell culture model. PLoS One 2013. [Google Scholar] [CrossRef]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res 2010, 70, 9003–9011. [Google Scholar]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar]
- Anhê, G.F.; Okamoto, M.M.; Kinote, A.; Sollon, C.; Lellis-Santos, C.; Anhê, F.F.; Lima, G.A.; Hirabara, S.M.; Velloso, L.A.; Bordin, S.; et al. Quercetin decreases inflammatory response and increases insulin action in skeletal muscle of ob/ob mice and in L6 myotubes. Eur. J. Pharmacol 2012, 689, 285–293. [Google Scholar]
- Chuang, C.C.; Martinez, K.; Xie, G.; Kennedy, A.; Bumrungpert, A.; Overman, A.; Jia, W.; McIntosh, M.K. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-α-mediated inflammation and insulin resistance in primary human adipocytes. Am. J. Clin. Nutr 2010, 92, 1511–1521. [Google Scholar]
- Egert, S.; Boesch-Saadatmandi, C.; Wolffram, S.; Rimbach, G.; Müller, M.J. Serum lipid and blood pressure responses to quercetin vary in overweight patients by apolipoprotein E genotype. J. Nutr 2010, 140, 278–284. [Google Scholar]
- Roghani, M.; Baluchnejadmojarad, T. Hypoglycemic and hypolipidemic effect and antioxidant activity of chronic epigallocatechin-gallate in streptozotocin-diabetic rats. Pathophysiology 2010, 17, 55–59. [Google Scholar]
- Si, H.; Fu, Z.; Babu, P.V.; Zhen, W.; Leroith, T.; Meaney, M.P.; Voelker, K.A.; Jia, Z.; Grange, R.W.; Liu, D. Dietary epicatechin promotes survival of obese diabetic mice and Drosophila melanogaster. J. Nutr 2011, 141, 1095–1100. [Google Scholar]
- Nagao, T.; Meguro, S.; Hase, T.; Otsuka, K.; Komikado, M.; Tokimitsu, I.; Yamamoto, T.; Yamamoto, K.A. catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity 2009, 17, 310–317. [Google Scholar]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res 2012, 32, 421–427. [Google Scholar]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr 2010, 29, 31–40. [Google Scholar]
- De Amicis, F.; Russo, A.; Avena, P.; Santoro, M.; Vivacqua, A.; Bonofiglio, D.; Mauro, L.; Aquila, S.; Tramontano, D.; Fuqua, S.A.; et al. In vitromechanism for downregulation of ER-α expression by epigallocatechin gallate in ER+/PR+ human breast cancer cells. Mol. Nutr. Food Res 2013. [Google Scholar] [CrossRef]
- Cimino, S.; Sortino, G.; Favilla, V.; Castelli, T.; Madonia, M.; Sansalone, S.; Russo, G.I.; Morgia, G. Polyphenols: Key issues involved in chemoprevention of prostate cancer. Oxid. Med. Cell. Longev. 2012. [Google Scholar] [CrossRef]
- Eden, J.A. Phytoestrogens for menopausal symptoms: A review. Maturitas 2012, 72, 157–159. [Google Scholar]
- Hurt, R.T.; Wilson, T. Geriatric obesity: Evaluating the evidence for the use of flavonoids to promote weight loss. J. Nutr. Gerontol. Geriatr 2012, 31, 269–289. [Google Scholar]
- Behloul, N.; Wu, G. Genistein: A promising therapeutic agent for obesity and diabetes treatment. Eur. J. Pharmacol 2013, 698, 31–38. [Google Scholar]
- Goodman-Gruen, D.; Kritz-Silverstein, D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J. Nutr 2001, 131, 1202–1206. [Google Scholar]
- Goodman-Gruen, D.; Kritz-Silverstein, D. Usual dietary isoflavone intake and body composition in postmenopausal women. Menopause 2003, 10, 427–432. [Google Scholar]
- Erba, D.; Casiraghi, M.C.; Martinez-Conesa, C.; Goi, G.; Massaccesi, L. Isoflavone supplementation reduces DNA oxidative damage and increases O-β-N-acetyl-d-glucosaminidase activity in healthy women. Nutr. Res 2012, 32, 233–240. [Google Scholar]
- Clerici, C.; Nardi, E.; Battezzati, P.M.; Asciutti, S.; Castellani, D.; Corazzi, N.; Giuliano, V.; Gizzi, S.; Perriello, G.; di Matteo, G.; et al. Novel soy germ pasta improves endothelial function, blood pressure, and oxidative stress in patients with type 2 diabetes. Diabetes Care 2011, 34, 1946–1948. [Google Scholar]
- Shehzad, A.; Khan, S.; Sup Lee, Y. Curcumin molecular targets in obesity and obesity-related cancers. Future Oncol 2012, 8, 179–190. [Google Scholar]
- Zingg, J.M.; Hasan, S.T.; Meydani, M. Molecular mechanisms of hypolipidemic effects of curcumin. Biofactors 2013. [Google Scholar] [CrossRef]
- Shao, W.; Yu, Z.; Chiang, Y.; Yang, Y.; Chai, T.; Foltz, W.; Lu, H.; Fantus, I.G.; Jin, T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One 2012, 7, e28784. [Google Scholar]
- Bradford, P.G. Curcumin and obesity. Biofactors 2013. [Google Scholar] [CrossRef]
- He, H.J.; Wang, G.Y.; Gao, Y.; Ling, W.H.; Yu, Z.W.; Jin, T.R. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J. Diabetes 2012, 3, 94–104. [Google Scholar]
- Bachmeier, B.E.; Mirisola, V.; Romeo, F.; Generoso, L.; Esposito, A.; Dell’eva, R.; Blengio, F.; Killian, P.H.; Albini, A.; Pfeffer, U. Reference profile correlation reveals estrogen-like trancriptional activity of Curcumin. Cell Physiol. Biochem 2010, 26, 471–482. [Google Scholar]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol 2011, 650, 1–7. [Google Scholar]
- Whiting, S.; Derbyshire, E.; Tiwari, B.K. Capsaicinoids and capsinoids. A potential role for weight management? A systematic review of the evidence. Appetite 2012, 59, 341–348. [Google Scholar]
- Choi, S.E.; Kim, T.H.; Yi, S.A.; Hwang, Y.C.; Hwang, W.S.; Choe, S.J.; Han, S.J.; Kim, H.J.; Kim, D.J.; Kang, Y.; Lee, K.W. Capsaicin attenuates palmitate-induced expression of macrophage inflammatory protein 1 and interleukin 8 by increasing palmitate oxidation and reducing c-Jun activation in THP-1 (human acute monocytic leukemia cell) cells. Nutr. Res 2011, 31, 468–478. [Google Scholar]
- Kang, J.H.; Tsuyoshi, G.; Le Ngoc, H.; Kim, H.M.; Tu, T.H.; Noh, H.J.; Kim, C.S.; Choe, S.Y.; Kawada, T.; Yoo, H.; et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J. Med. Food 2011, 14, 310–315. [Google Scholar]
- Mandard, S.; Patsouris, D. Nuclear control of the inflammatory response in mammals by peroxisome proliferator-activated receptors. PPAR Res. 2013. [Google Scholar] [CrossRef]
- Tan, B.; Li, X.; Yin, Y.; Wu, Z.; Liu, C.; Tekwe, C.D.; Wu, G. Regulatory roles for l-arginine in reducing white adipose tissue. Front. Biosci 2012, 17, 2237–2246. [Google Scholar]
- Zemel, M.B.; Bruckbauer, A. Effects of a leucine and pyridoxine-containing nutraceutical on fat oxidation, and oxidative and inflammatory stress in overweight and obese subjects. Nutrients 2012, 4, 529–541. [Google Scholar]
- Vrieze, A.; van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar]
- Diamant, M.; Blaak, E.E.; de Vos, W.M. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes. Rev 2011, 12, 272–281. [Google Scholar]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr 2011, 94, 58–65. [Google Scholar]
- Park, D.Y.; Ahn, Y.T.; Huh, C.S.; McGregor, R.A.; Choi, M.S. Dual probiotic strains suppress high fructose-induced metabolic syndrome. World J. Gastroenterol 2013, 19, 274–283. [Google Scholar]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr. Microbiol 2011, 62, 1689–1696. [Google Scholar]
- Kullisaar, T.; Songisepp, E.; Mikelsaar, M.; Zilmer, K.; Vihalemm, T.; Zilmer, M. Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Br. J. Nutr 2003, 90, 449–456. [Google Scholar]
- WHO: Facts and figures. The challenge of obesity—Quick statistics. Available online: http://www.euro.who.int/en/what-we-do/health-topics/noncommunicable-diseases/obesity/factsand-figures (accessed on 12 February 2013).
- Maric-Bilkan, C. Obesity and diabetic kidney disease. Med. Clin. North Am 2013, 97, 59–74. [Google Scholar]
- Ballard-Barbash, R.; Berrigan, D.; Potischman, N.; Dowling, E. Obesity and Cancer Epidemiology. In Cancer and Energy Balance, Epidemiology and Overview; Berger, N.A., Ed.; Springer-Verlag New York, LLC: New York, NY, USA, 2010. [Google Scholar]
- Curti, M.L.; Jacob, P.; Borges, M.C.; Rogero, M.M.; Ferreira, S.R. Studies of gene variants related to inflammation, oxidative stress, dyslipidemia, and obesity: Implications for a nutrigenetic approach. J. Obes. 2011. [Google Scholar] [CrossRef]
- Estadella, D.; da Penha Oller do Nascimento, C.M.; Oyama, L.M.; Ribeiro, E.B.; Dâmaso, A.R.; de Piano, A. Lipotoxicity: Effects of dietary saturated and transfatty acids. Mediators Inflamm 2013. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Savini, I.; Catani, M.V.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State. Int. J. Mol. Sci. 2013, 14, 10497-10538. https://doi.org/10.3390/ijms140510497
Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State. International Journal of Molecular Sciences. 2013; 14(5):10497-10538. https://doi.org/10.3390/ijms140510497
Chicago/Turabian StyleSavini, Isabella, Maria Valeria Catani, Daniela Evangelista, Valeria Gasperi, and Luciana Avigliano. 2013. "Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State" International Journal of Molecular Sciences 14, no. 5: 10497-10538. https://doi.org/10.3390/ijms140510497