Melatonin-Based Therapeutics for Neuroprotection in Stroke
Abstract
:1. Introduction
2. Glial Cell Protection by Melatonin in Ischemic Brain
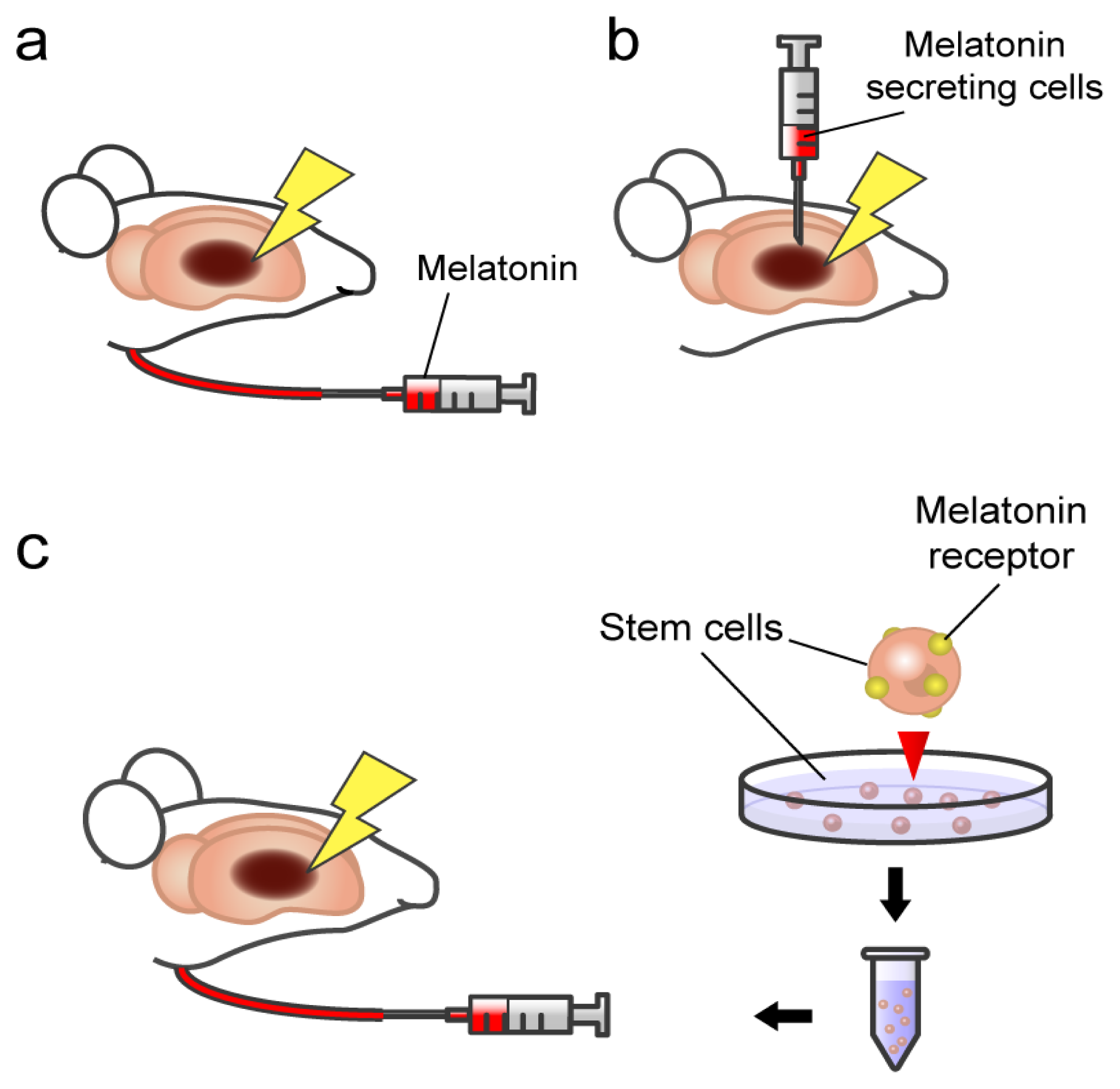
3. Melatonin and Stroke: Transplantation of Pineal Gland in Experimental Stroke Animals
4. Melatonin Action on Stem Cells: Involvement of Specificmelatonin Receptor
5. Towards Clinical Applications of Melatonin-Based Therapeutics
6. Conclusions
Acknowledgements
Conflict of Interest
References
- Lesnikov, V.A.; Pierpaoli, W. Pineal Cross-Transplantation (Old-to-Young and Vice Versa) as Evidence for an Endogenous Aging Clock. In The Aging Clock: The Pineal Gland and Other Pacemakers in the Progression of Aging and Carcinogenesis: Third Stromboli Conference on Aging and Cancer; Pierpaoli, W., Regelson, W., Fabris, N., Eds.; New York Academy of Sciences: New York, NY, USA, 1994; Volume 719, pp. 456–460. [Google Scholar]
- Palaoglu, S.; Palaoglu, O.; Akarsu, E.S.; Ayhan, I.H.; Ozgen, T.; Erbengi, A. Behavioral assessment of pinealectomy and fetal pineal-gland transplantation in rats: Part II. Acta Neurochir 1994, 128, 8–12. [Google Scholar]
- Pierpaoli, W.; Regelson, W. Pineal control of aging: Effect of melatonin and pineal grafting on aging mice. Proc. Natl. Acad. Sci. USA 1994, 91, 787–791. [Google Scholar]
- Poulos, S.G.; Borlongan, C.V. Artificial lighting conditions and melatonin alter motor performance in adult rats. Neurosci. Lett 2000, 280, 33–36. [Google Scholar]
- Reiter, R.J.; Maestroni, G.J.M. Melatonin in relation to the antioxidative defense and immune systems: Possible implications for cell and organ transplantation. J. Mol. Med 1999, 77, 36–39. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Qi, W.B.; Manchester, L.C.; Karbownik, M.; Calvo, J.R. Pharmacology and physiology of melatonin in the reduction of oxidative stress in vivo. Biol. Signals Recept 2000, 9, 160–171. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Kim, S.J.; El-Sokkary, G.H. Melatonin protects hippocampal neurons in vivo against kainic acid-induced damage in mice. J. Neurosci. Res 1998, 54, 382–389. [Google Scholar]
- Tan, D.-X.; Reiter, R.J.; Manchester, L.C.; Yan, M.-T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Topics Med. Chem 2002, 2, 181–197. [Google Scholar]
- Du, C.; Hu, R.; Csernansky, C.A.; Hsu, C.Y.; Choi, D.W. Very delayed infarction after mild focal cerebral ischemia: A role for apoptosis? J. Cereb. Blood Flow Metab 1996, 16, 195–201. [Google Scholar]
- Lee, J.M.; Zipfel, G.J.; Choi, D.W. The changing landscape of ischaemic brain injury mechanisms. Nature 1999, 399, A7–A14. [Google Scholar]
- Nakao, N.; Frodl, E.M.; Widner, H.; Carlson, E.; Eggerding, F.A.; Epstein, C.J.; Brundin, P. Overexpressing Cu/Zn superoxide-dismutase enhances survival of transplanted neurons in a rat model of Parkinsons-disease. Nat. Med 1995, 1, 226–231. [Google Scholar]
- Leker, R.R.; Teichner, A.; Lavie, G.; Shohami, E.; Lamensdorf, I.; Ovadia, H. The nitroxide antioxidant tempol is cerebroprotective against focal cerebral ischemia in spontaneously hypertensive rats. Exp. Neurol 2002, 176, 355–363. [Google Scholar]
- Suzuki, M.; Tabuchi, M.; Ikeda, M.; Tomita, T. Concurrent formation of peroxynitrite nitric oxide synthase in the brain with the expression of inducible during middle cerebral artery occlusion and reperfusion in rats. Brain Res 2002, 951, 113–120. [Google Scholar]
- Qi, J.; Hong, Z.Y.; Xin, H.; Zhu, Y.Z. Neuroprotective Effects of leonurine on ischemia/reperfusion-induced mitochondrial dysfunctions in rat cerebral cortex. Biol. Pharm. Bull 2010, 33, 1958–1964. [Google Scholar]
- Loh, K.P.; Qi, J.; Tan, B.K.H.; Liu, X.H.; Wei, B.G.; Zhu, Y.Z. Leonurine protects middle cerebral artery occluded rats through antioxidant effect and regulation of mitochondrial function. Stroke 2010, 41, 2661–2668. [Google Scholar]
- Thaakur, S.; Sravanthi, R. Neuroprotective effect of Spirulina in cerebral ischemia-reperfusion injury in rats. J. Neural Transm 2010, 117, 1083–1091. [Google Scholar]
- Khan, M.M.; Ishrat, T.; Ahmad, A.; Hoda, M.N.; Khan, M.B.; Khuwaja, G.; Srivastava, P.; Raza, S.S.; Islam, F.; Ahmad, S. Sesamin attenuates behavioral, biochemical and histological alterations induced by reversible middle cerebral artery occlusion in the rats. Chem. Biol. Interact 2010, 183, 255–263. [Google Scholar]
- He, S.; Yang, J.H.; Wu, B.; Pan, Y.J.; Wan, H.T.; Wang, Y.; Du, Y.G.; Wang, S.D. Neuroprotective effect of parthenocissin A, a natural antioxidant and free radical scavenger, in focal cerebral ischemia ofrRats. Phytother. Res 2010, 24, S63–S70. [Google Scholar]
- Simao, F.; Matte, A.; Matte, C.; Soares, F.M.S.; Wyse, A.T.S.; Netto, C.A.; Salbego, C.G. Resveratrol prevents oxidative stress and inhibition of Na+K+-ATPase activity induced by transient global cerebral ischemia in rats. J. Nutr. Biochem 2011, 22, 921–928. [Google Scholar]
- Zhang, G.L.; Deng, J.P.; Wang, B.H.; Zhao, Z.W.; Li, J.; Gao, L.; Liu, B.L.; Xong, J.R.; Guo, X.D.; Yan, Z.Q.; et al. Gypenosides improve cognitive impairment induced by chronic cerebral hypoperfusion in rats by suppressing oxidative stress and astrocytic activation. Behav. Pharmacol 2011, 22, 633–644. [Google Scholar]
- Gaur, V.; Aggarwal, A.; Kumar, A. Possible nitric oxide mechanism in the protective effect of hesperidin against ischemic reperfusion cerebral injury in rats. Indian J. Exp. Biol 2011, 49, 609–618. [Google Scholar]
- Ahmad, A.; Khan, M.M.; Hoda, M.N.; Raza, S.S.; Khan, M.B.; Javed, H.; Ishrat, T.; Ashafaq, M.; Ahmad, M.E.; Safhi, M.M.; et al. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem. Res 2011, 36, 1360–1371. [Google Scholar]
- Tai, S.H.; Hung, Y.C.; Lee, E.J.; Lee, A.C.; Chen, T.Y.; Shen, C.C.; Chen, H.Y.; Lee, M.Y.; Huang, S.Y.; Wu, T.S. Melatonin protects against transient focal cerebral ischemia in both reproductively active and estrogen-deficient female rats: The impact of circulating estrogen on its hormetic dose-response. J. Pineal Res 2011, 50, 292–303. [Google Scholar]
- Jung, H.W.; Mahesh, R.; Bae, H.S.; Kim, Y.H.; Kang, J.S.; Park, Y.K. The antioxidant effects of Joongpoongtang 05 on brain injury after transient focal cerebral ischemia in rats. J. Nat. Med 2011, 65, 322–329. [Google Scholar]
- Suzuki, S.; Kawamata, T.; Okada, Y.; Kobayashi, T.; Nakamura, T.; Hori, T. Filtrate of Phellinus linteusbroth culture reduces infarct size significantly in a rat model of permanent focal cerebral ischemia. Evid.-Based Complement Altern. Med. 2011. [Google Scholar] [CrossRef]
- Silachev, D.N.; Isaev, N.K.; Pevzner, I.B.; Zorova, L.D.; Stelmashook, E.V.; Novikova, S.V.; Plotnikov, E.Y.; Skulachev, V.P.; Zorov, D.B. The mitochondria-targeted antioxidants and remote kidney preconditioning ameliorate brain damage through kidney-to-brain cross-talk. PLoS One 2012, 7, e51553. [Google Scholar]
- Li, S.J.; Wu, C.H.; Zhu, L.; Gao, J.; Fang, J.; Li, D.F.; Fu, M.H.; Liang, R.X.; Wang, L.; Cheng, M.; et al. By improving regional cortical blood flow, attenuating mitochondrial dysfunction and sequential apoptosis galangin acts as a potential neuroprotective agent after acute ischemic stroke. Molecules 2012, 17, 13403–13423. [Google Scholar]
- Park, J.; Park, H.H.; Choi, H.; Kim, Y.S.; Yu, H.J.; Lee, K.Y.; Lee, Y.J.; Kim, S.H.; Koh, S.H. Coenzyme Q10 protects neural stem cells against hypoxia by enhancing survival signals. Brain Res 2012, 1478, 64–73. [Google Scholar]
- Gundimeda, U.; McNeill, T.H.; Elhiani, A.A.; Schiffman, J.E.; Hinton, D.R.; Gopalakrishna, R. Green tea polyphenols precondition against cell death induced by oxygen-glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase C epsilon. J. Biol. Chem 2012, 287, 34694–34708. [Google Scholar]
- Huang, H.F.; Guo, F.; Cao, Y.Z.; Shi, W.; Xia, Q. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics: Antioxidant effect and oxidative stress regulation in acute experimental stroke. CNS Neurosci. Ther 2012, 18, 811–818. [Google Scholar]
- Chen, L.Y.; Wang, L.N.; Zhang, X.J.; Cui, L.L.; Xing, Y.X.; Dong, L.P.; Liu, Z.J.; Li, Y.H.; Zhang, X.L.; Wang, C.H.; et al. The protection by Octreotide against experimental ischemic stroke: Up-regulated transcription factor Nrf2, HO-1 and down-regulated NF-κB expression. Brain Res 2012, 1475, 80–87. [Google Scholar]
- Qian, Y.S.; Guan, T.; Huang, M.H.; Cao, L.X.; Li, Y.M.; Cheng, H.; Jin, H.X.; Yu, D.Y. Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochem. Int 2012, 60, 759–767. [Google Scholar]
- Sakata, H.; Niizuma, K.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Katsu, M.; Narasimhan, P.; Maier, C.M.; Nishiyama, Y.; Chan, P.H. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J. Neurosci 2012, 32, 3462–3473. [Google Scholar]
- Connell, B.J.; Saleh, T.M. Co-administration of apocynin with lipoic acid enhances neuroprotection in a rat model of ischemia/reperfusion. Neurosci. Lett 2012, 507, 43–46. [Google Scholar]
- Bae, O.N.; Serfozo, K.; Baek, S.H.; Lee, K.Y.; Dorrance, A.; Rumbeiha, W.; Fitzgerald, S.D.; Farooq, M.U.; Naravelta, B.; Bhatt, A.; et al. Safety and efficacy evaluation of carnosine, an endogenous neuroprotective agent for ischemic stroke. Stroke 2013, 44, 205–212. [Google Scholar]
- Shuaib, A.; Lees, K.R.; Lyden, P.; Grotta, J.; Davalos, A.; Davis, S.M.; Diener, H.; Ashwood, T.; Wasiewski, W.W.; Emeribe, U. NXY-059 for the treatment of acute ischemic stroke. N. Engl. J. Med 2007, 357, 562–571. [Google Scholar]
- Shinohara, Y.; Saito, I.; Kobayashi, S.; Uchiyama, S. Edaravone (radical scavenger) versus sodium ozagrel (antiplatelet agent) in acute noncardioembolic ischemic stroke (EDO trial). Cerebrovasc. Dis 2009, 27, 485–492. [Google Scholar]
- Minematsu, K.; Yamaguchi, T.; Origasa, H.; Hashi, K.; Kobayashi, S.; Ezura, M.; Nagao, T.; Kimura, K.; Okada, Y.; Hashimoto, Y. Edaravone in combination with Argatroban for the treatment of acute atherothrombotic brain infarction: The Edaravone Argatroban Stroke Therapy (EAST) study. Stroke 2009, 40, E106. [Google Scholar]
- Manev, H.; Uz, T.; Kharlamov, A.; Joo, J.Y. Increased brain damage after stroke or excitotoxic seizures in melatonin-deficient rats. FASEB J 1996, 10, 1546–1551. [Google Scholar]
- Kilic, E.; Ozdemir, Y.G.; Bolay, H.; Kelestimur, H.; Dalkara, T. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J. Cereb. Blood Flow Metab 1999, 19, 511–516. [Google Scholar]
- Pei, Z.; Pang, S.F.; Cheung, R.T. Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke 2003, 34, 770–775. [Google Scholar]
- Sinha, K.; Degaonkar, M.N.; Jagannathan, N.R.; Gupta, Y.K. Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur. J. Pharmacol 2001, 428, 185–192. [Google Scholar]
- Lee, E.J.; Lee, M.Y.; Chen, H.Y.; Hsu, Y.S.; Wu, T.S.; Chen, S.T.; Chang, G.L. Melatonin attenuates gray and white matter damage in a mouse model of transient focal cerebral ischemia. J. Pineal Res 2005, 38, 42–52. [Google Scholar]
- Lee, M.Y.; Kuan, Y.H.; Chen, H.Y.; Chen, T.Y.; Chen, S.T.; Huang, C.C.; Yang, I.P.; Hsu, Y.S.; Wu, T.S.; Lee, E.J. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J. Pineal Res 2007, 42, 297–309. [Google Scholar]
- Kondoh, T.; Uneyama, H.; Nishino, H.; Torii, K. Melatonin reduces cerebral edema formation caused by transient forebrain ischemia in rats. Life Sci 2002, 72, 583–590. [Google Scholar]
- Chen, T.Y.; Lee, M.Y.; Chen, H.Y.; Kuo, Y.L.; Lin, S.C.; Wu, T.S.; Lee, E.J. Melatonin attenuates the postischemic increase in blood-brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J. Pineal Res 2006, 40, 242–250. [Google Scholar]
- Kilic, E.; Kilic, U.; Bacigaluppi, M.; Guo, Z.; Ben Abdallah, N.; Wolfer, D.P.; Reiter, R.J.; Hermann, D.M.; Bassetti, C.L. Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery, and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J. Pineal Res 2008, 45, 142–148. [Google Scholar] [Green Version]
- Brzezinski, A. Melatonin in humans. N. Engl. J. Med 1997, 336, 186–195. [Google Scholar]
- Pei, Z.; Ho, H.T.S.; Cheung, R.T.F. Pre-treatment with melatonin reduces volume of cerebral infarction in a permanent middle cerebral artery occlusion stroke model in the rat. Neurosci. Lett 2002, 318, 141–144. [Google Scholar]
- Pei, Z.; Pang, S.F.; Cheung, R.T.F. Pretreatment with melatonin reduces volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. J. Pineal Res 2002, 32, 168–172. [Google Scholar]
- Pei, Z.; Cheung, R.T.F. Pretreatment with melatonin exerts anti-inflammatory effects against ischemia/reperfusion injury in a rat middle cerebral artery occlusion stroke model. J. Pineal Res 2004, 37, 85–91. [Google Scholar]
- Borlongan, C.V.; Yamaoto, M.; Takei, N.; Kumazaki, M.; Ungsuparkorn, C.; Hida, H.; Sanberg, P.R.; Nishino, H. Glial cell survival is enhanced during melatonin-induced neuroprotection against cerebral ischemia. FASEB J 2000, 14, 1307–1317. [Google Scholar]
- Borlongan, C.V.; Sumaya, I.; Moss, D.; Kumazaki, M.; Sakurai, T.; Hida, H.; Nishino, H. Melatonin-secreting pineal gland: A novel tissue source for neural transplantation therapy in stroke. Cell Transplant 2003, 12, 225–234. [Google Scholar]
- Kaneko, Y.; Hayashi, T.; Yu, S.; Tajiri, N.; Bae, E.C.; Solomita, M.A.; Chheda, S.H.; Weinbren, N.L.; Parolini, O.; Borlongan, C.V. Human amniotic epithelial cells express melatonin receptor MT1, but not melatonin receptor MT2: A new perspective to neuroprotection. J. Pineal Res 2011, 50, 272–280. [Google Scholar]
- Vernadakis, A. Glia-neuron intercommunications and synaptic plasticity. Prog. Neurobiol 1996, 49, 185–214. [Google Scholar]
- Leroux, P.D.; Reh, T.A. Independent regulation of primary dendritic and axonal growth by maturing astrocytes in vitro. Neurosci. Lett 1995, 198, 5–8. [Google Scholar]
- Diamond, J.S.; Bergles, D.E.; Jahr, C.E. Glutamate release monitored with astrocyte transporter currents during LTP. Neuron 1998, 21, 425–433. [Google Scholar]
- Luscher, C.; Malenka, R.C.; Nicoll, R.A. Monitoring glutamate release during LTP with glial transporter currents. Neuron 1998, 21, 435–441. [Google Scholar]
- Blanc, E.M.; Bruce-Keller, A.J.; Mattson, M.P. Astrocytic gap junctional communication decreases neuronal vulnerability to oxidative stress-induced disruption of Ca2+ homeostasis and cell death. J. Neurochem 1998, 70, 958–970. [Google Scholar]
- Lin, J.H.C.; Weigel, H.; Cotrina, M.L.; Liu, S.J.; Bueno, E.; Hansen, A.J.; Hansen, T.W.; Goldman, S.; Nedergaard, M. Gap-junction-mediated propagation and amplification of cell injury. Nat. Neurosci 1998, 1, 494–500. [Google Scholar]
- Bronstein, D.M.; PerezOtano, I.; Sun, V.; Sawin, S.B.M.; Chan, J.; Wu, G.C.; Hudson, P.M.; Kong, L.Y.; Hong, J.S.; McMillian, M.K. Glia-dependent neurotoxicity and neuroprotection in mesencephalic cultures. Brain Res 1995, 704, 112–116. [Google Scholar]
- Langeveld, C.H.; Jongenelen, C.A.M.; Schepens, E.; Stoof, J.C.; Bast, A.; Drukarch, B. Cultured rat striatal and cortical astrocytes protect mesencephalic dopaminergic-neurons against hydrogen-peroxide toxicity independent of their effect on neuronal development. Neurosci. Lett 1995, 192, 13–16. [Google Scholar]
- Lin, L.F.H.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial-cell line derived neurotrophic factor for midbrain dopaminergic-neurons. Science 1993, 260, 1130–1132. [Google Scholar]
- Lehrmann, E.; Kiefer, R.; Christensen, T.; Toyka, K.V.; Zimmer, J.; Diemer, N.H.; Hartung, H.P.; Finsen, B. Microglia and macrophages are major sources of locally produced transforming growth factor beta(1) after transient middle cerebral artery occlusion in rats. Glia 1998, 24, 437–448. [Google Scholar]
- Ridet, J.L.; Malhotra, S.K.; Privat, A.; Gage, F.H. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci 1997, 20, 570–577. [Google Scholar]
- Hoffer, B.J.; Hoffman, A.; Bowenkamp, K.; Huettl, P.; Hudson, J.; Martin, D.; Lin, L.F.H.; Gerhardt, G.A. Glial-cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic-neurons in-vivo. Neurosci. Lett 1994, 182, 107–111. [Google Scholar]
- Isacson, O.; Deacon, T.W.; Pakzaban, P.; Galpern, W.R.; Dinsmore, J.; Burns, L.H. Transplanted xenogeneic neural cells in neurodegenerative disease-models exhibit remarkable axonal target specificity and distinct growth-patterns of glial and axonal fibers. Nat. Med 1995, 1, 1189–1194. [Google Scholar]
- Svendsen, C.N.; Caldwell, M.A.; Shen, J.; terBorg, M.G.; Rosser, A.E.; Tyers, P.; Karmiol, S.; Dunnett, S.B. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson’s disease. Exp. Neurol 1997, 148, 135–146. [Google Scholar]
- Deacon, T.; Schumacher, J.; Dinsmore, J.; Thomas, C.; Palmer, P.; Kott, S.; Edge, A.; Penney, D.; Kassissieh, S.; Dempsey, P.; et al. Histological evidence of fetal pig neural cell survival after transplantation into a patient with Parkinson's disease. Nat. Med 1997, 3, 350–353. [Google Scholar]
- Horellou, P.; Mallet, J. Gene therapy for Parkinson’s disease. Mol. Neurobiol 1997, 15, 241–256. [Google Scholar]
- White, H.S.; Chow, S.Y.; Yenchow, Y.C.; Woodbury, D.M. Effect of elevated potassium on the ion content of mouse astrocytes and neurons. Can. J. Physiol. Pharmacol 1992, 70, S263–S268. [Google Scholar]
- Hansson, E.; Ronnback, L. Astrocytes in glutamate neurotransmission. FASEB J 1995, 9, 343–350. [Google Scholar]
- Goldberg, M.P.; Choi, D.W. Combined oxygen and glucose deprivation in cortical cell-culture; calcium dependent and calcium-independent mechanisms of neuronal injury. J. Neurosci 1993, 13, 3510–3524. [Google Scholar]
- Sochocka, E.; Juurlink, B.H.J.; Code, W.E.; Hertz, V.; Peng, L.; Hertz, L. Cell-death inprimaly cultures of mouse neurons and astrocytes during exposure to and recovery from hypoxia, substrate deprivation and stimulated ischemia. Brain Res 1994, 638, 21–28. [Google Scholar]
- Petito, C.K.; Olarte, J.P.; Roberts, B.; Nowak, T.S.; Pulsinelli, W.A. Selective glial vulnerability following transient global ischemia in rat brain. J. Neuropathol. Exp. Neurol 1998, 57, 231–238. [Google Scholar]
- Pantoni, L.; Garcia, J.H.; Gutierrez, J.A. Cerebral white matter is highly vulnerable to ischemia. Stroke 1996, 27, 1641–1646. [Google Scholar]
- Fisher, M. Characterizing the target of acute stroke therapy. Stroke 1997, 28, 866–872. [Google Scholar]
- Siesjo, B.K. Pathophysiology and treatment of focal cerebral-ischemia. Part II: Mechanisms of damage and treatment. J. Neurosurg 1992, 77, 337–354. [Google Scholar]
- Borlongan, C.V.; Tajima, Y.; Trojanowski, J.Q.; Lee, V.M.Y.; Sanberg, P.R. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp. Neurol 1998, 149, 310–321. [Google Scholar]
- Aihara, N.; Mizukawa, K.; Koide, K.; Mabe, H.; Nishino, H. Striatal grafts in infarct striatopallidum increase GABA release, reorganize GABAA receptor and improve water-maze learning in the rat. Brain Res. Bull 1994, 33, 483–488. [Google Scholar]
- Nishino, H.; Czurko, Z.; Onizuka, K.; Fukuda, A.; Hida, H.; Ungsuparkorn, C.; Kunimatsu, M.; Sasaki, M.; Karadi, Z.; Lenard, L. Neuronal damage following transient cerebral ischemia and its restoration by neural transplant. Neurobiology 1994, 2, 223–234. [Google Scholar]
- Matuszak, Z.; Reszka, K.J.; Chignell, C.F. Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Radic. Biol. Med 1997, 23, 367–372. [Google Scholar]
- Iacovitti, L.; Stull, N.D.; Johnston, K. Melatonin rescues dopamine neurons from cell death in tissue culture models of oxidative stress. Brain Res 1997, 768, 317–326. [Google Scholar]
- Hirata, H.; Asanuma, M.; Cadet, J.L. Melatonin attenuates methamphetamine-induced toxic effects on dopamine and serotonin terminals in mouse brain. Synapse 1998, 30, 150–155. [Google Scholar]
- Giusti, P.; Gusella, M.; Lipartiti, M.; Milani, D.; Zhu, W.J.; Vicini, S.; Manev, H. Melatonin protects primary cultures of cerebellar granule neurons from kainate but not from N-methyl-d-aspartate excitotoxicity. Exp. Neurol 1995, 131, 39–46. [Google Scholar]
- Reiter, R.J. Oxidative damage in the central nervous system: Protection by melatonin. Prog. Neurobiol 1998, 56, 359–384. [Google Scholar]
- Cho, S.; Joh, T.H.; Baik, H.W.; Dibinis, C.; Volpe, B.T. Melatonin administration protects CA1 hippocampal neurons after transient forebrain ischemia in rats. Brain Res 1997, 755, 335–338. [Google Scholar]
- Joo, J.Y.; Uz, T.; Manev, H. Opposite effects of pinealectomy and melatonin administration on brain damage following cerebral focal ischemia in rat. Restor. Neurol. Neurosci 1998, 13, 185–191. [Google Scholar]
- Fiorina, P.; Lattuada, G.; Ponari, O.; Silvestrini, C.; DallAglio, P. Impaired nocturnal melatonin excretion and changes of immunological status in ischaemic stroke patients. Lancet 1996, 347, 692–693. [Google Scholar]
- Miller, J.W.; Selhub, J.; Joseph, J.A. Oxidative damage caused by free radicals produced during catecholamine autoxidation: Protective effects of O-methylation and melatonin. Free Radic. Biol. Med 1996, 21, 241–249. [Google Scholar]
- Cuzzocrea, S.; Costantino, G.; Caputi, A.P. Protective effect of melatonin on cellular energy depletion mediated by peroxynitrite and poly (ADP-ribose) synthetase activation in a non-septic shock model induced by zymosan in the rat. J. Pineal Res 1998, 25, 78–85. [Google Scholar]
- Zhang, H.W.; Squadrito, G.L.; Uppu, R.; Pryor, W.A. Reaction of peroxynitrite with melatonin: A mechanistic study. Chem. Res. Toxicol 1999, 12, 526–534. [Google Scholar]
- Cagnoli, C.M.; Atabay, C.; Kharlamova, E.; Manev, H. Melatonin protects neurons from singlet oxygen-induced apoptosis. J. Pineal Res 1995, 18, 222–226. [Google Scholar]
- Melchiorri, D.; Reiter, R.J.; Sewerynek, E.; Chen, L.D.; Nistico, G. Melatonin reduces kainate-induced lipid-peroxidation in homogenates of different brain-regions. FASEB J 1995, 9, 1205–1210. [Google Scholar]
- Mawatari, C.; Yasui, Y.; Sugitani, K.; Takadera, T.; Kato, S. Reactive oxygen species involved in the glutamate toxicity of C6 glioma cells via XC− antiporter system. Neuroscience 1996, 73, 201–208. [Google Scholar]
- Wang, Y.; Lin, S.Z.; Chiou, A.L.; Williams, L.R.; Hoffer, B.J. Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J. Neurosci 1997, 17, 4341–4348. [Google Scholar]
- Borlongan, C.V.; Sanberg, P.R. Neural transplantation for treatment of Parkinson’s disease. Drug Discov. Today 2002, 7, 674–682. [Google Scholar]
- Borlongan, C.V.; Sanberg, P.R. Neural transplantation in the new millenium. Cell Trans 2002, 11, 615–618. [Google Scholar]
- Isacson, O.; Deacon, T.W. Specific axon guidance factors persist in the adult brain as demonstrated by pig neuroblasts transplanted to the rat. Neuroscience 1996, 75, 827–837. [Google Scholar]
- Redmond, D.E. Cellular replacement therapy for Parkinson’s disease—Where we are today? Neuroscientist 2002, 8, 457–488. [Google Scholar]
- Grabowski, M.; Johansson, B.B.; Brundin, P. Fetal Neocortical Grafts Placed in Brain Infarcts do not Improve Paw-Reaching Deficits in Adult Spontaneously Hypertensive Rats. In Acta Neurochirurgica Supplement; Mechanisms of Secondary Brain Damage in Cerebral Ischemia and Trauma; Baethmann, A., Kempski, O., Plesnila, N., Staub, F., Eds.; Springer-Verlag: Vienna, Austria, New York, NY, USA, 1996; Volume 66, pp. 68–72. [Google Scholar]
- Sinden, J.D.; Stroemer, P.; Grigoryan, G.; Patel, S.; French, S.J.; Hodges, H. Functional Repair with Neural Stem Cells. In Neural Transplantation in Neurodegenerative Disease: Current Status and New Directions; Chadwick, D.J., Goode, J.A., Eds.; 2000; Volume 231, pp. 270–283. [Google Scholar]
- Kondziolka, D.; Wechsler, L.; Goldstein, S.; Meltzer, C.; Thulborn, K.R.; Gebel, J.; Jannetta, P.; DeCesare, S.; Elder, E.M.; McGrogan, M.; et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2000, 55, 565–569. [Google Scholar]
- Meltzer, C.C.; Kondziolka, D.; Villemagne, V.L.; Wechsler, L.; Goldstein, S.; Thulborn, K.R.; Gebel, J.; Elder, E.M.; DeCesare, S.; Jacobs, A. Serial [F-18]fluorodeoxyglucose positron emission tomography after human neuronal implantation for stroke. Neurosurgery 2001, 49, 586–591. [Google Scholar]
- Nelson, P.T.; Kondziolka, D.; Wechsler, L.; Goldstein, S.; Gebel, J.; DeCesare, S.; Elder, E.M.; Zhang, P.J.; Jacobs, A.; McGrogan, M.; et al. Clonal human (hNT) neuron grafts for stroke therapy: Neuropathology in a patient 27 months after implantation. Am. J. Pathol 2002, 160, 1201–1206. [Google Scholar]
- Savitz, S.I.; Misra, V.; Kasam, M.; Juneja, H.; Cox, C.S.; Alderman, S.; Aisiku, I.; Kar, S.; Gee, A.; Grotta, J.C. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann. Neurol 2011, 70, 59–69. [Google Scholar]
- Bang, O.Y.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol 2005, 57, 874–882. [Google Scholar]
- Bubenik, G.A.; Blask, D.E.; Brown, G.M.; Maestroni, G.J.M.; Pang, S.F.; Reiter, R.J.; Viswanathan, M.; Zisapel, N. Prospects of the clinical utilization of melatonin. Biol. Signals Recept 1998, 7, 195–219. [Google Scholar]
- Gupta, Y.K.; Chaudhary, G.; Sinha, K. Enhanced protection by melatonin and meloxicam combination in a middle cerebral artery occlusion model of acute ischemic stroke in rat. Can. J. Physiol. Pharmacol 2002, 80, 210–217. [Google Scholar]
- Skaper, S.D.; Ancona, B.; Facci, L.; Franceschini, D.; Giusti, P. Melatonin prevents the delayed death of hippocampal neurons induced by enhanced excitatory neurotransmission and the nitridergic pathway. FASEB J 1998, 12, 725–731. [Google Scholar]
- Kondoh, T.; Lee, S.H.; Low, W.C. Alterations in striatal dopamine release and reuptake under conditions of mild, moderate, and severe cerebral-ischemia. Neurosurgery 1995, 37, 948–954. [Google Scholar]
- Borlongan, C.V.; Cahill, D.W.; Sanberg, P.R. Locomotor and passive-avoidance deficits following occlusion of the middle cerebral-artery. Physiol. Behav 1995, 58, 909–917. [Google Scholar]
- Borlongan, C.V.; Martinez, R.; Shytle, R.D.; Freeman, T.B.; Cahill, D.W.; Sanberg, P.R. Striatal dopamine-mediated motor behavior is altered following occlusion of the middle cerebral-artery. Pharmacol. Biochem. Behav 1995, 52, 225–229. [Google Scholar]
- Borlongan, C.V.; Zhou, F.C.; Hayashi, T.; Su, T.P.; Hoffer, B.J.; Wang, Y. Involvement of GDNF in neuronal protection against 6-OHDA-induced parkinsonism following intracerebral transplantation of fetal kidney tissues in adult rats. Neurobiol. Dis 2001, 8, 636–646. [Google Scholar]
- Dillon-Carter, O.; Johnston, R.E.; Borlongan, C.V.; Truckenmiller, M.E.; Coggiano, M.; Freed, W.J. T155g-immortalized kidney cells produce growth factors and reduce sequelae of cerebral ischemia. Cell Transplant 2002, 11, 251–259. [Google Scholar]
- Johnston, R.E.; Dillon-Carter, O.; Freed, W.J.; Borlongan, C.V. Trophic factor secreting kidney cell lines: In vitro characterization and functional effects following transplantation in ischemic rats. Brain Res 2001, 900, 268–276. [Google Scholar]
- Boer, G.J.; Griffioen, H.A. Developmental and functional-aspects of grafting of the suprachiasmatic nucleus in the Brattleboro and the arrhythmic rat. Eur. J. Morphol 1990, 28, 330–345. [Google Scholar]
- Lu, S.Y.; Shipley, M.T.; Norman, A.B.; Sanberg, P.R. Striatal, ventral mesencephalic and cortical transplants into the intact rat striatum: A neuroanatomical study. Exp. Neurol 1991, 113, 109–130. [Google Scholar]
- Tonder, N.; Sorensen, T.; Zimmer, J.; Jorgensen, M.B.; Johansen, F.F.; Diemer, N.H. Neural grafting to ischemic lesions of the adult-rat hippocampus. Exp. Brain Res 1989, 74, 512–526. [Google Scholar]
- Grosse, D.; Davis, F.C. Melatonin entrains the restored circadian activity rhythms of syrian hamsters bearing fetal suprachiasmatic nucleus grafts. J. Neurosci 1998, 18, 8032–8037. [Google Scholar]
- Cai, H.; Li, Z.M.; Goette, A.; Mera, F.; Honeycutt, C.; Feterik, K.; Wilcox, J.N.; Dudley, S.C.; Harrison, D.G.; Langberg, J.J. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: Potential mechanisms for atrial thrombosis and stroke. Circulation 2002, 106, 2854–2858. [Google Scholar]
- Maier, C.M.; Chan, P.H. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist 2002, 8, 323–334. [Google Scholar]
- Wang, Y.; Chang, C.F.; Morales, M.; Chiang, Y.H.; Hoffer, J. Protective Effects of Glial Cell Line-Derived Neurotrophic Factor in Ischemic Brain Injury. In Nitric Oxide: Novel Actions, Deleterious Effects and Clinical Potential; Chiueh, C.C., Hong, J.S., Leong, S.K., Eds.; New York Academy of Sciences: New York, NY, USA, 2002; Volume 962, pp. 423–437. [Google Scholar]
- Kawashima, S.; Yamashita, T.; Miwa, Y.; Ozaki, M.; Namiki, M.; Hirase, T.; Inoue, N.; Hirata, K.; Yokoyama, M. HMG-CoA reductase inhibitor has protective effects against stroke events in stroke-prone spontaneously hypertensive rats. Stroke 2003, 34, 157–163. [Google Scholar]
- Takeda, H.; Spatz, M.; Ruetzler, C.; McCarron, R.; Becker, K.; Hallenbeck, J. Induction of mucosal tolerance to E-selectin prevents ischemic and hemorrhagic stroke in spontaneously hypertensive genetically stroke-prone rats. Stroke 2002, 33, 2156–2163. [Google Scholar]
- Borlongan, C.V.; Su, T.P.; Wang, Y. Delta opioid peptide augments functional effects and intrastriatal graft survival of rat fetal ventral mesencephalic cells. Cell Transplant 2001, 10, 53–58. [Google Scholar]
- Marquardt, L.; Ruf, A.; Mansmann, U.; Winter, R.; Schuler, M.; Buggle, F.; Mayer, H.; Grau, A.J. Course of platelet activation markers after ischemic stroke. Stroke 2002, 33, 2570–2574. [Google Scholar]
- Tabuchi, M.; Umegaki, K.; Ito, T.; Suzuki, M.; Tomita, I.; Ikeda, M.; Tomita, T. Fluctuation of serum NOx concentration at stroke onset in a rat spontaneous stroke model (M-SHRSP): Peroxynitrite formation in brain lesions. Brain Res 2002, 949, 147–156. [Google Scholar]
- Walder, C.E.; Green, S.P.; Darbonne, W.C.; Mathias, J.; Rae, J.; Dinauer, M.C.; Curnutte, J.T.; Thomas, G.R. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke 1997, 28, 2252–2258. [Google Scholar]
- Pierpaoli, W.; Dallara, A.; Pedrinis, E.; Regelson, W. The pineal control of aging. The effects of melatonin and pineal grafting on the survival of older mice. Ann. N.Y. Acad. Sci 1991, 621, 291–313. [Google Scholar]
- Wu, W.; Scott, D.E.; Reiter, R.J. No difference in day-night serum melatonin concentration after pineal grafting into the third cerebral ventricle of pinealectomized rats. J. Pineal Res 1991, 11, 70–74. [Google Scholar]
- Chiang, Y.H.; Lin, S.Z.; Borlongan, C.V.; Hoffer, B.J.; Morales, M.; Wang, Y. Transplantation of fetal kidney tissue reduces cerebral infarction induced by middle cerebral artery ligation. J. Cereb. Blood Flow Metab 1999, 19, 1329–1335. [Google Scholar]
- Chiang, Y.H.; Morales, M.; Zhou, F.C.; Borlongan, C.; Hoffer, B.J.; Wang, Y. Fetal intra-nigral ventral mesencephalon and kidney tissue bridge transplantation restores the nigrostriatal dopamine pathway in hemi-parkinsonian rats. Brain Res 2001, 889, 200–207. [Google Scholar]
- Parr, A.M.; Tator, C.H.; Keating, A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant 2007, 40, 609–619. [Google Scholar]
- Antonucci, I.; Iezzi, I.; Morizio, E.; Mastrangelo, F.; Pantalone, A.; Mattioli-Belmonte, M.; Gigante, A.; Salini, V.; Calabrese, G.; Tete, S.; et al. Isolation of osteogenic progenitors from human amniotic fluid using a single step culture protocol. BMC Biotechnol. 2009. [Google Scholar] [CrossRef]
- Antonucci, I.; Pantalone, A.; de Amicis, D.; D’Onofrio, S.; Stuppia, L.; Palka, G.; Salini, V. Human amniotic fluid stem cells culture onto titanium screws: A new perspective for bone engineering. J. Biol. Regul. Homeost. Agents 2009, 23, 277–279. [Google Scholar]
- Cargnoni, A.; Di Marcello, M.; Campagnol, M.; Nassuato, C.; Albertini, A.; Parolini, O. Amniotic membrane patching promotes ischemic rat heart repair. Cell Transplant 2009, 18, 1147–1159. [Google Scholar]
- Yu, S.J.; Soncini, M.; Kaneko, Y.; Hess, D.C.; Parolini, O.; Borlongan, C.V. Amnion: A potent graft source for cell therapy in stroke. Cell Transplant 2009, 18, 111–118. [Google Scholar]
- Shinya, M.; Komuro, H.; Saihara, R.; Urita, Y.; Kaneko, M.; Liu, Y. Neural differentiation potential of rat amniotic epithelial cells. Fetal Pediatr. Pathol 2010, 29, 133–143. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Leon, J.; Kilic, U.; Kilic, E. When melatonin gets on your nerves: Its beneficial actions in experimental models of stroke. Exp. Biol. Med 2005, 230, 104–117. [Google Scholar]
- Koh, P.-O. Melatonin regulates nitric oxide synthase expression in ischemic brain injury. J. Vet. Med. Sci 2008, 70, 747–750. [Google Scholar]
- Lee, C.H.; Yoo, K.-Y.; Choi, J.H.; Park, O.K.; Hwang, I.K.; Kwon, Y.-G.; Kim, Y.-M.; Won, M.-H. Melatonin’s protective action against ischemic neuronal damage is associated with up-regulation of the MT2 melatonin receptor. J. Neurosci. Res 2010, 88, 2630–2640. [Google Scholar]
- Lekic, T.; Hartman, R.; Rojas, H.; Manaenko, A.; Chen, W.; Ayer, R.; Tang, J.; Zhang, J.H. Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J. Neurotrauma 2010, 27, 627–637. [Google Scholar]
- Lin, H.-W.; Lee, E.J. Effects of melatonin in experimental stroke models in acute, sub-acute, and chronic stages. Neuropsychiatr. Dis. Treat 2009, 5, 157–162. [Google Scholar]
- Tocharus, J.; Khonthun, C.; Chongthammakun, S.; Govitrapong, P. Melatonin attenuates methamphetamine-induced overexpression of pro-inflammatory cytokines in microglial cell lines. J. Pineal Res 2010, 48, 347–352. [Google Scholar]
- Beni, S.M.; Kohen, R.; Reiter, R.J.; Tan, D.-X.; Shohami, E. Melatonin-induced neuroprotection after closed head injury is associated with increased brain antioxidants and attenuated late-phase activation of NF-κB and AP-1. FASEB J 2004, 18, 149–151. [Google Scholar]
- Ramirez-Rodriguez, G.; Klempin, F.; Babu, H.; Benitez-King, G.; Kempermann, G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 2009, 34, 2180–2191. [Google Scholar]
- Wang, X.; Figueroa, B.E.; Stavrovskaya, I.G.; Zhang, Y.; Sirianni, A.C.; Zhu, S.; Day, A.L.; Kristal, B.S.; Friedlander, R.M. Methazolamide and melatonin inhibit mitochondrial cytochrome c release and are neuroprotective in experimental models of ischemic injury. Stroke 2009, 40, 1877–1885. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Tamura, H. Melatonin defeats neurally-derived free radicals and reduces the associated neuromorphological and neurobehavioral damage. J. Physiol. Pharmacol 2007, 58, 5–22. [Google Scholar]
- Xu, S.-C.; He, M.-D.; Zhong, M.; Zhang, Y.-W.; Wang, Y.; Yang, L.; Yang, J.; Yu, Z.-P.; Zhou, Z. Melatonin protects against Nickel-induced neurotoxicity in vitro by reducing oxidative stress and maintaining mitochondrial function. J. Pineal Res 2010, 49, 86–94. [Google Scholar]
- Reiter, R.J.; Paredes, S.D.; Manchester, L.C.; Tan, D.-X. Reducing oxidative/nitrosative stress: A newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol 2009, 44, 175–200. [Google Scholar]
- Hardeland, R.; Tan, D.X.; Reiter, R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res 2009, 47, 109–126. [Google Scholar]
- Samantaray, S.; Das, A.; Thakore, N.P.; Matzelle, D.D.; Reiter, R.J.; Ray, S.K.; Banik, N.L. Therapeutic potential of melatonin in traumatic central nervous system injury. J. Pineal Res 2009, 47, 134–142. [Google Scholar]
- Moriya, T.; Horie, N.; Mitome, M.; Shinohara, K. Melatonin influences the proliferative and differentiative activity of neural stem cells. J. Pineal Res 2007, 42, 411–418. [Google Scholar]
- Rennie, K.; De Butte, M.; Pappas, B.A. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J. Pineal Res 2009, 47, 313–317. [Google Scholar]
- Sharma, R.; McMillan, C.R.; Niles, L.P. Neural stem cell transplantation and melatonin treatment in a 6-hydroxydopamine model of Parkinson’s disease. J. Pineal Res 2007, 43, 245–254. [Google Scholar]
- Niles, L.P.; Armstrong, K.J.; Castro, L.M.R.; Dao, C.V.; Sharma, R.; McMillan, C.R.; Doering, L.C.; Kirkham, D.L. Neural stem cells express melatonin receptors and neurotrophic factors: Colocalization of the MT1 receptor with neuronal and glial markers. BMC Neurosci. 2004, 5. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Li, X.; Cai, Z.; Yang, N.; Liu, Y.; Shu, J.; Pan, L.; Zuo, P. Melatonin regulates the viability and differentiation of rat midbrain neural stem cells. Cell. Mol. Neurobiol 2008, 28, 569–579. [Google Scholar]
- Kilic, E.; Kilic, U.; Reiter, R.J.; Bassetti, C.L.; Hermann, D.M. Prophylactic use of melatonin protects against focal cerebral ischemia in mice: Role of endothelin converting enzyme-1. J. Pineal Res 2004, 37, 247–251. [Google Scholar]
- Kilic, U.; Kilic, E.; Reiter, R.J.; Bassetti, C.L.; Hermann, D.M. Signal transduction pathways involved in melatonin-induced neuroprotection after focal cerebral ischemia in mice. J. Pineal Res 2005, 38, 67–71. [Google Scholar]
- Chen, H.-Y.; Hung, Y.-C.; Chen, T.-Y.; Huang, S.-Y.; Wang, Y.-H.; Lee, W.-T.; Wu, T.-S.; Lee, E.J. Melatonin improves presynaptic protein, SNAP-25, expression and dendritic spine density and enhances functional and electrophysiological recovery following transient focal cerebral ischemia in rats. J. Pineal Res 2009, 47, 260–270. [Google Scholar]
- Wang, X. The Antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci. Ther 2009, 15, 345–357. [Google Scholar]
- Romeu, L.R.G.; da Motta, E.L.A.; Maganhin, C.C.; Oshima, C.T.F.; Fonseca, M.C.; Barrueco, K.F.; Simoes, R.S.; Pellegrino, R.; Baracat, E.C.; Soares, J.M. Effects of melatonin on histomorphology and on the expression of steroid receptors, VEGF, and PCNA in ovaries of pinealectomized female rats. Fertil. Steril 2011, 95, 1379–1384. [Google Scholar]
- Imbesi, M.; Uz, T.; Manev, H. Role of melatonin receptors in the effects of melatonin on BDNF and neuroprotection in mouse cerebellar neurons. J. Neural Transm 2008, 115, 1495–1499. [Google Scholar]
- Hill, S.M.; Cheng, C.; Yuan, L.; Mao, L.L.; Jockers, R.; Dauchy, B.; Frasch, T.; Blask, D.E. Declining melatonin levels and MT1 receptor expression in aging rats is associated with enhanced mammary tumor growth and decreased sensitivity to melatonin. Breast Cancer Res. Treat 2011, 127, 91–98. [Google Scholar]
- Mao, L.L.; Cheng, Q.; Guardiola-Lemaitre, B.; Schuster-Klein, C.; Dong, C.M.; Lai, L.; Hill, S.M. In vitro and in vivo antitumor activity of melatonin receptor agonists. J. Pineal Res 2010, 49, 210–221. [Google Scholar]
- Mor, M.; Rivara, S.; Pala, D.; Bedini, A.; Spadoni, G.; Tarzia, G. Recent advances in the development of melatonin MT1 and MT2 receptor agonists. Expert Opin. Ther. Pat 2010, 20, 1059–1077. [Google Scholar]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin-A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol 2011, 93, 350–384. [Google Scholar]
- Chern, C.M.; Liao, J.F.; Wang, Y.H.; Shen, Y.C. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic. Biol. Med 2012, 52, 1634–1647. [Google Scholar]
- Hardeland, R.; Poeggeler, B.; Srinivasan, V.; Trakht, I.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonergic drugs in clinical practice. Arzneimittel-Forsch 2008, 58, 1–10. [Google Scholar]
- Simpson, D.; Curran, M.P. Ramelteon—A review of its use in insomnia. Drugs 2008, 68, 1901–1919. [Google Scholar]
- Albers, G.W.; Goldstein, L.B.; Hess, D.C.; Wechsler, L.R.; Furie, K.L.; Gorelick, P.B.; Hurn, P.; Liebeskind, D.S.; Nogueira, R.G.; Saver, J.L.; et al. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke 2011, 42, 2645–2650. [Google Scholar]
- Wechsler, L.; Steindler, D.; Borlongan, C.; Chopp, M.; Savitz, S.; Deans, R.; Caplan, L.; Hess, D.; Mays, R.W.; Participants, S. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): Bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke 2009, 40, 510–515. [Google Scholar]
- Savitz, S.I.; Chopp, M.; Deans, R.; Carmichael, S.T.; Phinney, D.; Wechsler, L. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke 2011, 42, 825–829. [Google Scholar]
| Author | Antioxidant | Model | Main finding |
|---|---|---|---|
| Qi et al. (2010) [14] | Leonurine | Rat/MCAo | Histological/functional improvement, inhibit ROS production |
| Loh et al. (2010) [15] | Leonurine | Rat/MCAo | Histological/functional improvement, inhibit ROS production |
| Thaakur et al. (2010) [16] | Spirulina | Rat/MCAo | Histological/functional improvement |
| Khan et al. (2010) [17] | Sesamin | Rat/MCAo | Functional improvement, reducing thiobarbituric acid reactive species and protein carbonyl |
| He et al. (2010) [18] | Parthenocissin A | Rat/MCAo | Histological/functional improvement, suppressing lipid peroxidation and restoring superoxide dismutase, inhibiting NO and NOS elevation |
| Simao et al. (2011) [19] | Resveratrol | Rat/Global cerebral ischemia | Reducing neuronal death and generation of ROS, lipid peroxidation and NO content |
| Zhang et al. (2011) [20] | Gypenosides | Rat/Chronic cerebral hypoperfusion | Improving cognitive function |
| Gaur et al. (2011) [21] | Hesperidin | Rat/Common carotid artery occlusion | Functional improvement, reducing oxidative damage |
| Ahmad et al. (2011) [22] | Quercetin dihydrate | Rat/MCAo | Histological/functional improvement |
| Tai et al. (2011) [23] | Melatonin | Primary neuron/OGD | Synergistic antioxidant and radical-scavenging actions with estradiol |
| Jung et al. (2011) [24] | Joongpoongtang 05 | Rat/MCAo | Histological improvement, a decrease in oxidants |
| Suzuki et al. (2011) [25] | Phellinus linteus broth culture | Rat/MCAo | Histological improvement |
| Silachev et al. (2012) [26] | SkQR1 | Rat/MCAo | Histological/functional improvement |
| Li et al. (2012) [27] | Galangin | Rat/MCAo | Histological/functional improvement, protective effect on the mitochondria |
| Park et al. (2012) [28] | Coenzyme Q10 | NSC/hypoxia | Cell protection |
| Gundimeda et al. (2012) [29] | Green tea polyphenols | PC12 cell/OGD | Cell protection |
| Huang et al. (2012) [30] | MnTm4PyP | Mouse/MCAo, Cortical neurons/H2O2 injury | Histological/functional improvement and increased cell viabillity |
| Chen et al. (2012) [31] | Octreotide | Rat/MCAo | Histological/functional improvement, upregulation of transcription factor Nrf2, HO-1 and downregulation of NF-κB expression |
| Qian et al. (2012) [32] | Genistein | Mouse/MCAo | Histological/functional improvement, inhibits ROS production |
| Sakata et al. (2012) [33] | Minocycline | Rat/MCAo with pre-conditioned NSC transplantation, pre-conditined NSC/OGD | Histological/functional improvement, releasing paracrine factors from pre-conditioned NSCs |
| Connell and Saleh (2012) [34] | Apocynin, lipoic acid | Rat/MCAo | Histological improvement |
| Bae et al. (2013) [35] | Carnosine | Rat/MCAo | Histological/functional improvement |
| Sponsor | Condition | Drug | Start year | Completion year | Outcome (If available) |
|---|---|---|---|---|---|
| AstraZeneca | Cerebral Stroke | NXY-059 | 2003 | 2005 | Ineffective for the treatment of acute ischemic stroke within 6 h after the onset of symptoms [36]. |
| AstraZeneca | Cerebral Stroke | NXY-059 | 2003 | 2006 | |
| Mitsubishi Tanabe Pharma Corporation | Cerebral infarction | Edaravone, Sodium Ozagrel | 2004 | 2006 | Edaravone was at least as effective as ozagrel for the treatment of acute noncardioembolic ischemic stroke [37]. |
| Combination Therapy for Acute Ischemic Stroke Study Group | Stroke | Edaravone combined with argatroban | 2004 | 2008 | No favorable effects of edaravone when added to the baseline treatment with argatroban [38]. |
| Mitsubishi Tanabe Pharma Corporation | Acute ischemic stroke | MCI-186 | 2009 | 2010 | Not available |
| Otsuka Beijing Research Institute | Cerebral infarction | Cilostazol, Probucol | 2009 | 2010 | Not available |
| University of Science Malaysia | Cerebrovascular disorders | Palm vitamin E (tocotrienol) | 2008 | 2012 | Not available |
| University of Nottingham | Stroke | Transdermal glyceryl trinitrate patch (combined with prestroke antihypertensives) | 2001 | Ongoing | - |
| Asan Medical Center | Brain ischemia, Intracranial hemorrhages | Cilostazol, Probucol, Aspirin | 2009 | Ongoing | - |
| Brigham and Women’s Hospital | Stroke | Quercetin | 2009 | Ongoing | - |
| Takeda Global Research & Development Center, Inc. | Cardiovascular disease | Febuxostat, Allopurinol | 2010 | Ongoing | - |
| Angel Chamorro | Acute ischemic stroke | Uric acid | 2011 | Ongoing | - |
| Chandan K Sen | Transient ischemic stroke | Vitamin E tocotrienol (TCT) pills, Low dose Aspirin | 2012 | Ongoing | - |
| Nycomed: A Takeda company | Post-stroke cognitive impairment | Actovegin | 2012 | Ongoing | - |
| Zhejiang Hospital | Stroke | Aspirin, Warfarin, Atrvastatin, Edaravone (combined with autologous hematopoiesis stem cell transplantation) | 2012 | Ongoing | - |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shinozuka, K.; Staples, M.; Borlongan, C.V. Melatonin-Based Therapeutics for Neuroprotection in Stroke. Int. J. Mol. Sci. 2013, 14, 8924-8947. https://doi.org/10.3390/ijms14058924
Shinozuka K, Staples M, Borlongan CV. Melatonin-Based Therapeutics for Neuroprotection in Stroke. International Journal of Molecular Sciences. 2013; 14(5):8924-8947. https://doi.org/10.3390/ijms14058924
Chicago/Turabian StyleShinozuka, Kazutaka, Meaghan Staples, and Cesar V. Borlongan. 2013. "Melatonin-Based Therapeutics for Neuroprotection in Stroke" International Journal of Molecular Sciences 14, no. 5: 8924-8947. https://doi.org/10.3390/ijms14058924




