Growth and Potential Damage of Human Bone-Derived Cells on Fresh and Aged Fullerene C60 Films
Abstract
:1. Introduction
2. Results and Discussion
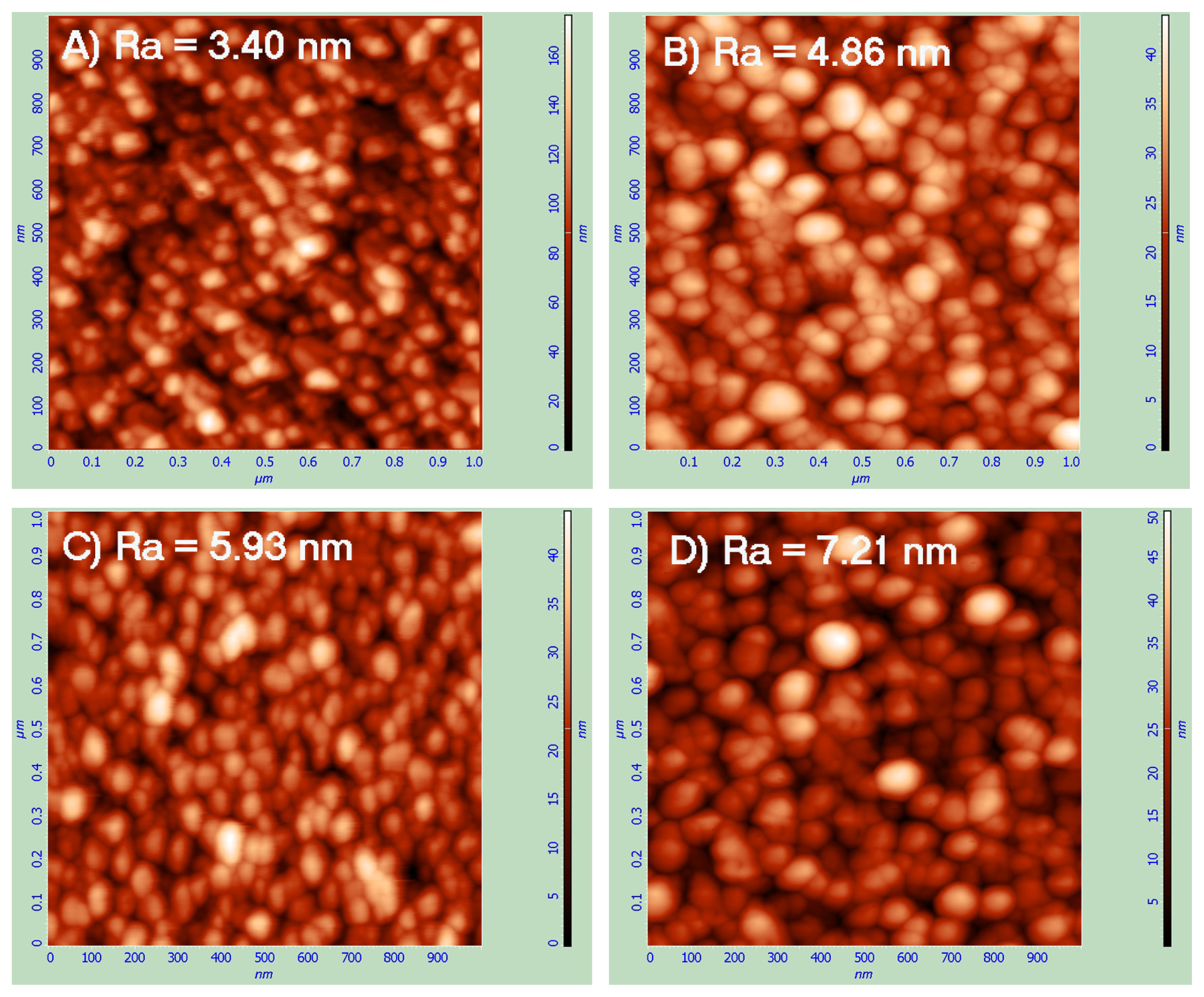
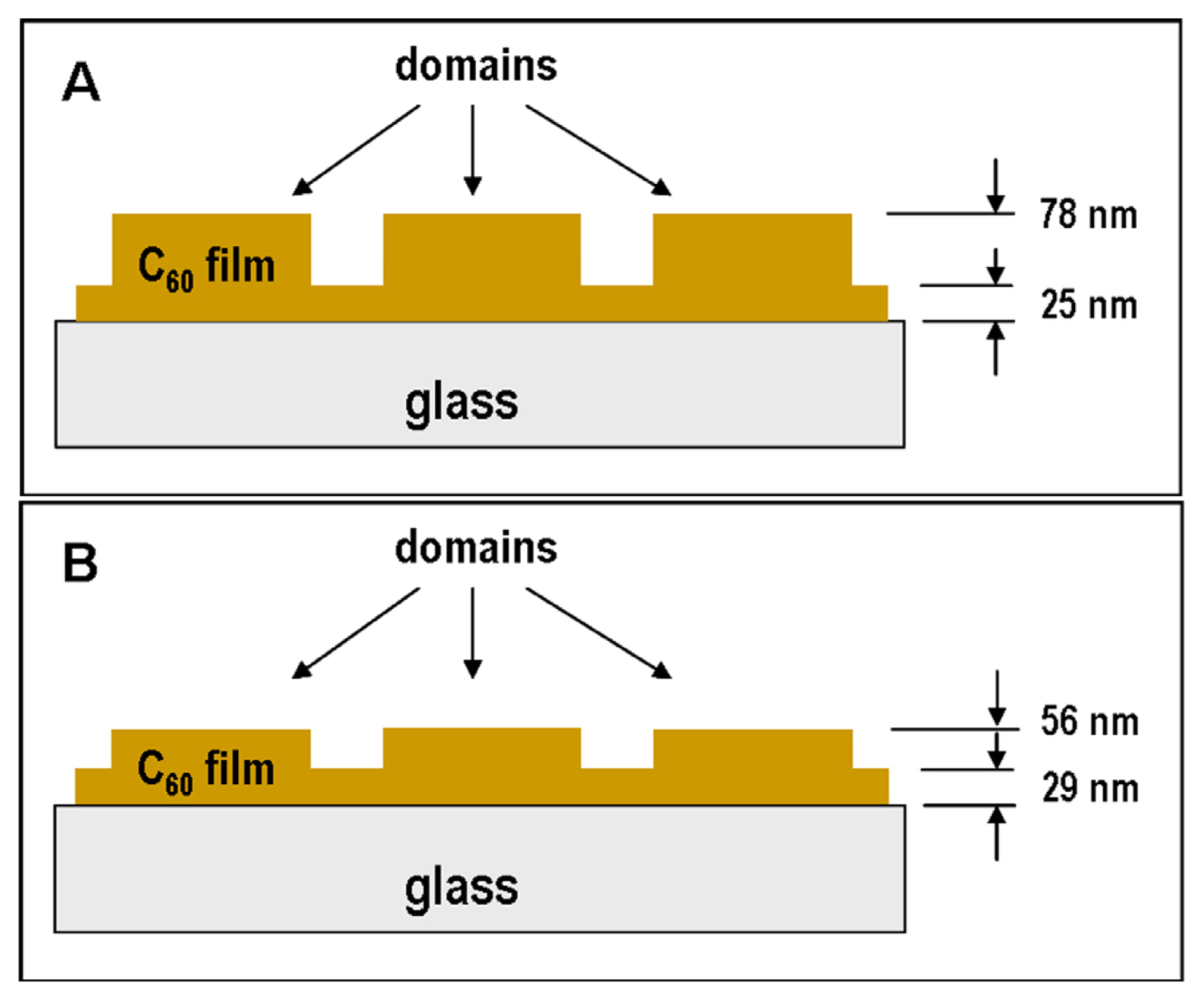
2.1. Atomic Force Microscopy (AFM)
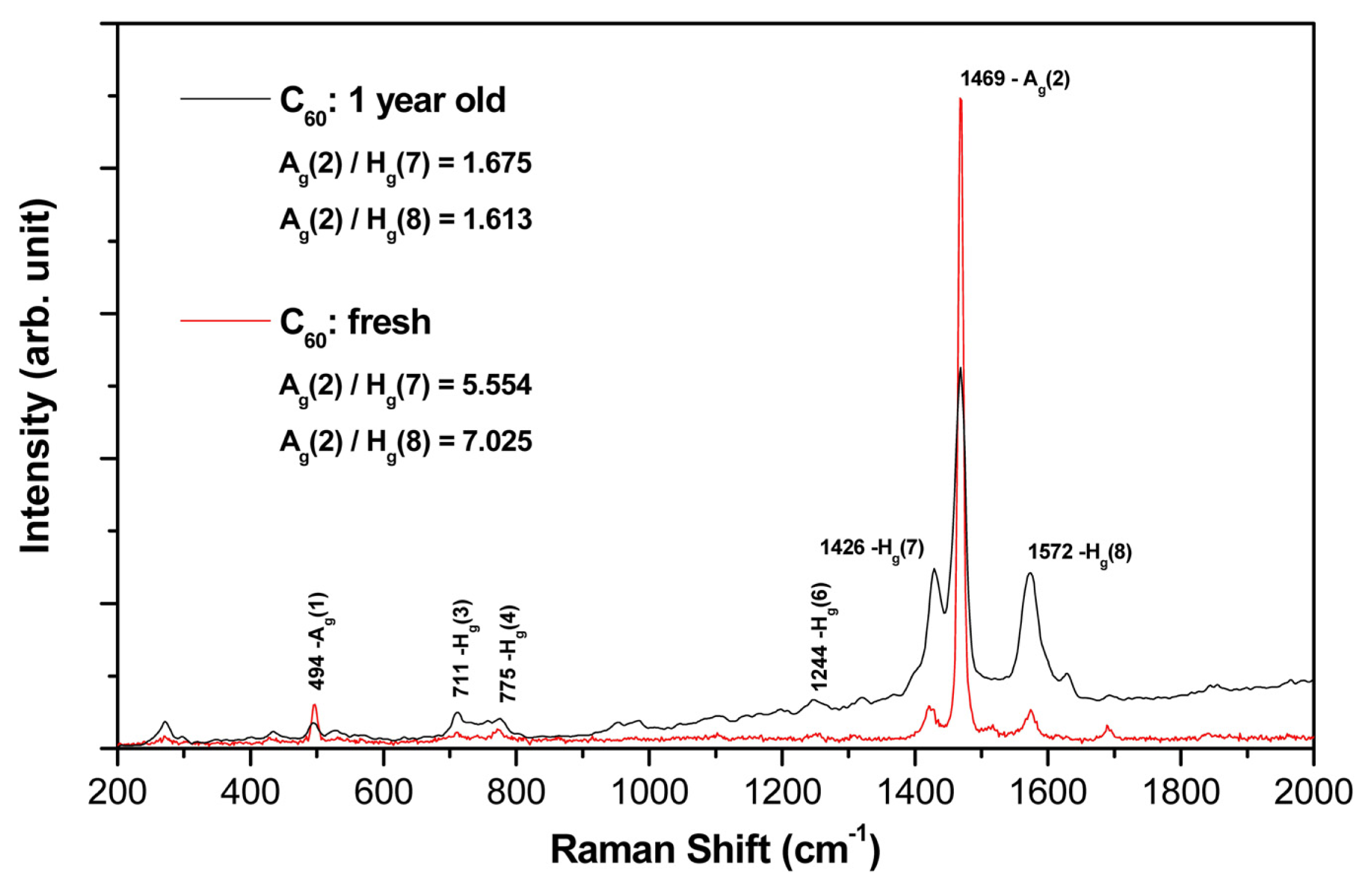
2.2. Raman Spectroscopy
- (a)
- Both films (aged and fresh) exhibit typical features of the fullerene films with dominant Hg(7), Ag(2) and Hg(8) peaks.
- (b)
- In the aged samples, however, a main Ag(2) peak (pentagonal pinch mode) dropped down dramatically (see the area peak ratios above) and showed a slight red-shift asymmetry (seen in the detailed examination of the spectrum).
- (c)
- Interestingly, there is also a clear difference in the symmetric (Ag) and asymmetric (Hg) mode changes of the aged sample (in comparison with the fresh one); all symmetric mode peaks decreased in intensity, though the asymmetric modes increased.
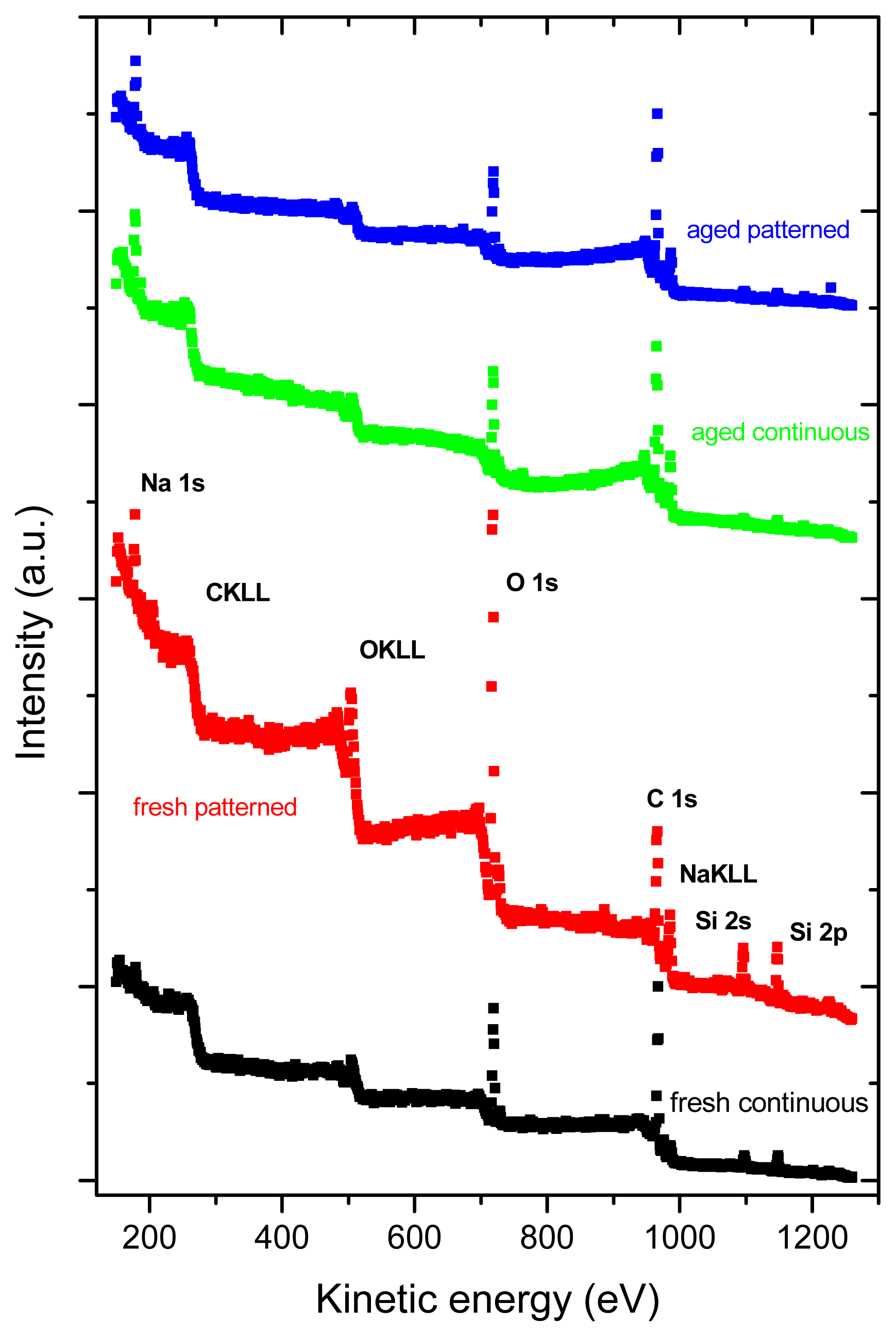
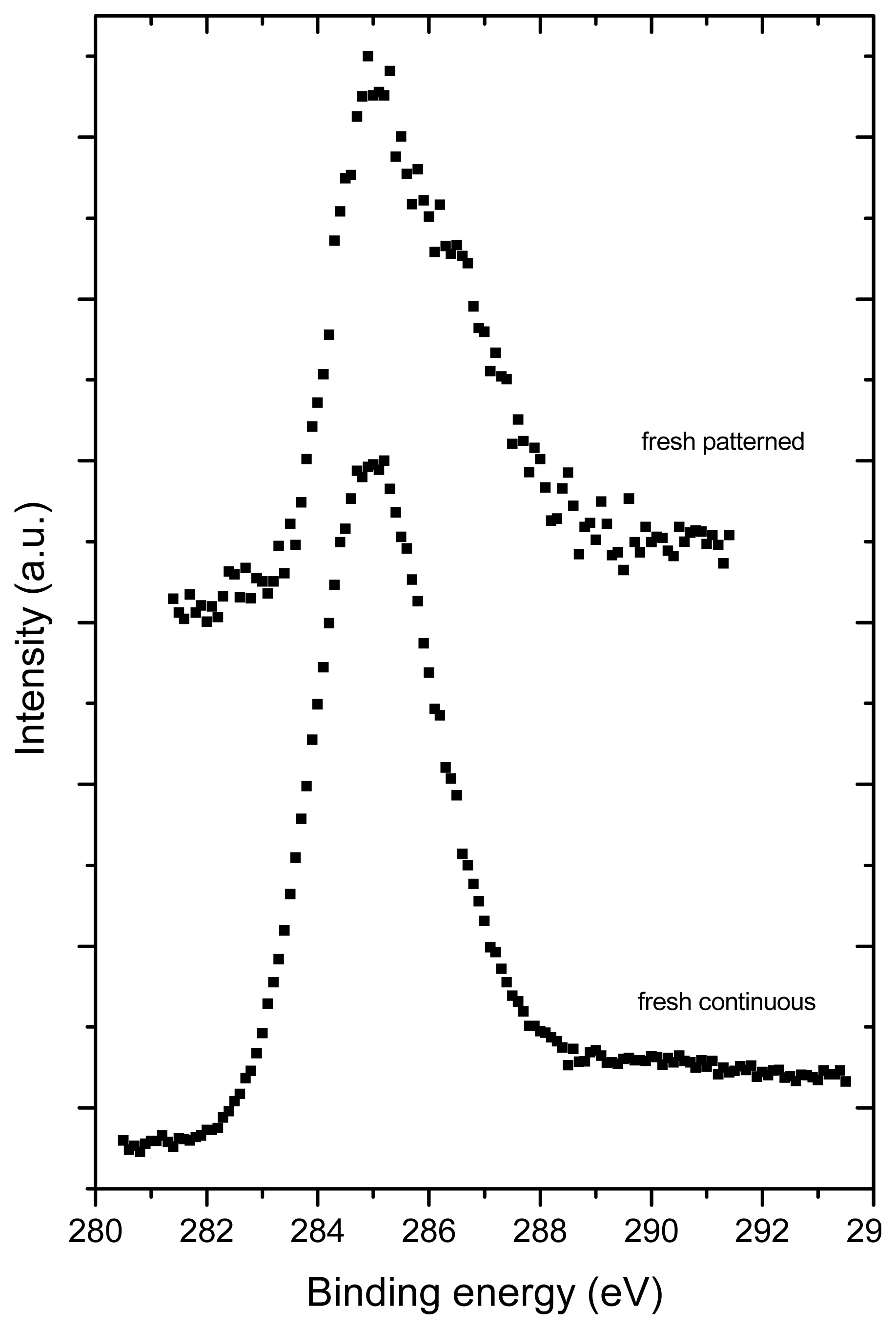
2.3. X-ray Photoelectron Spectroscopy (XPS)
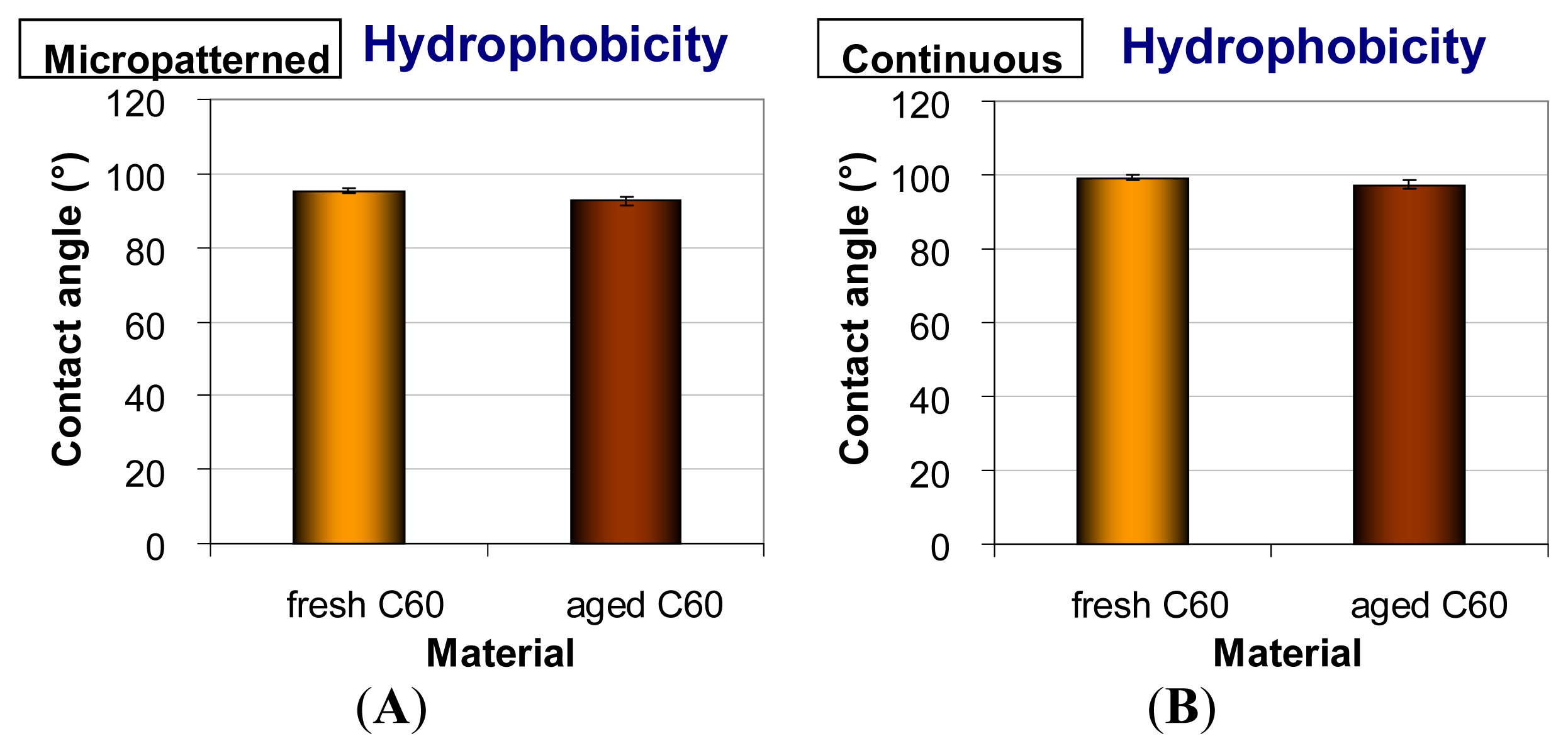
2.4. Hydrophobicity of Fullerene C60 Layers
2.5. Initial Adhesion, Proliferation and Morphology of Cells on Fullerene C60 Layers
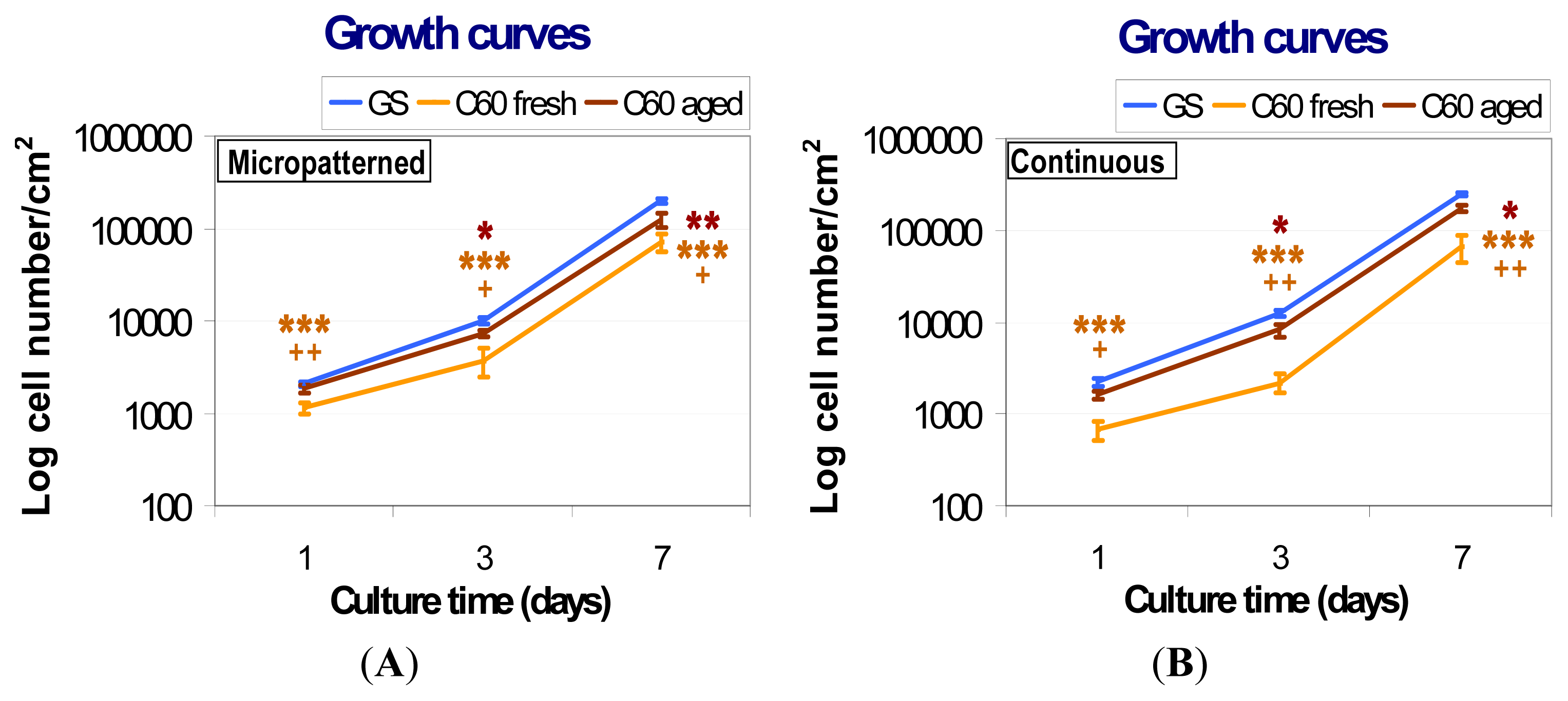
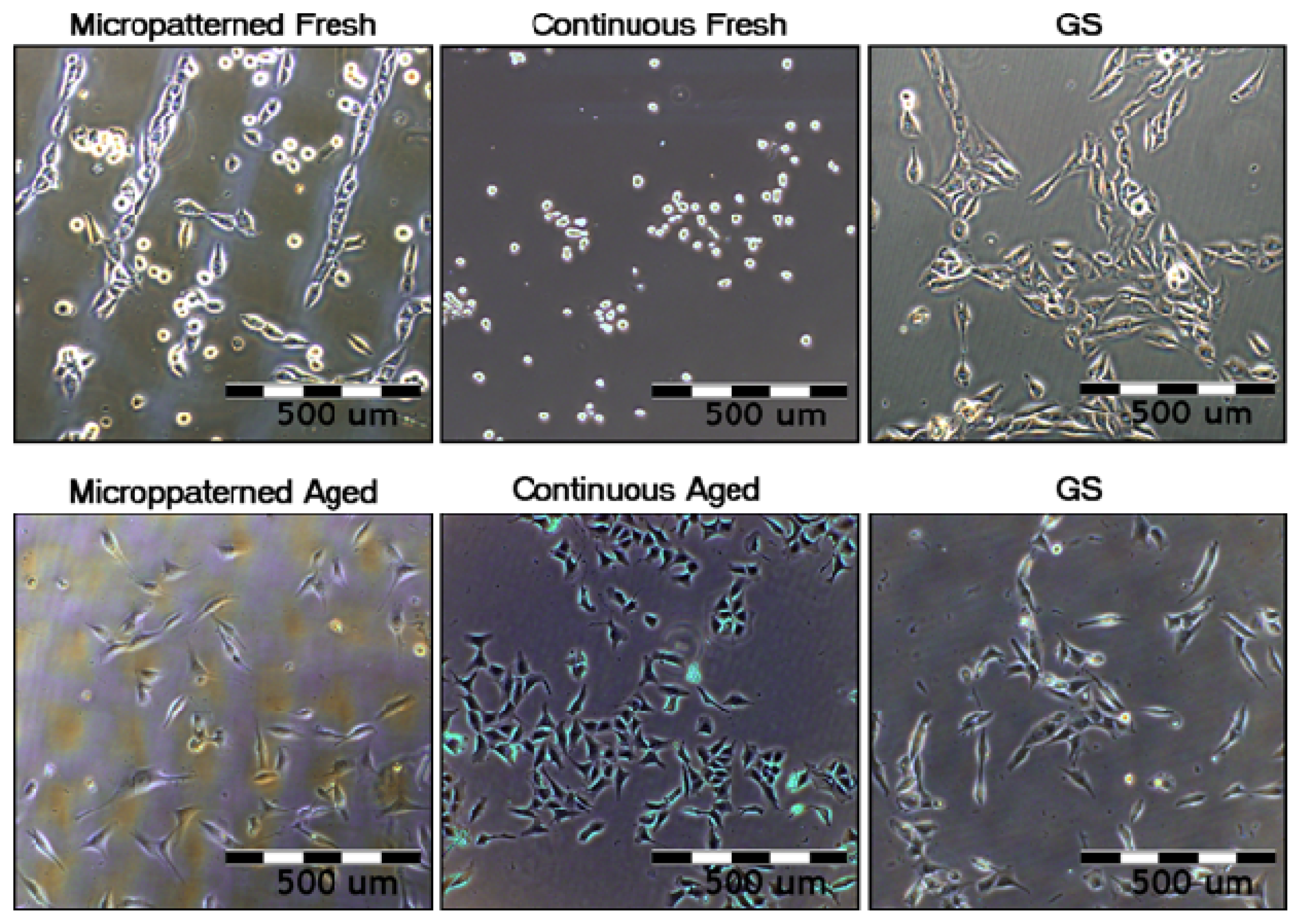
2.5.1. Comparison of Cell Behavior on Fresh and Aged Fullerene Films
2.5.2. Comparison of Cell Behavior on Micropatterned and Continuous Fullerene Films
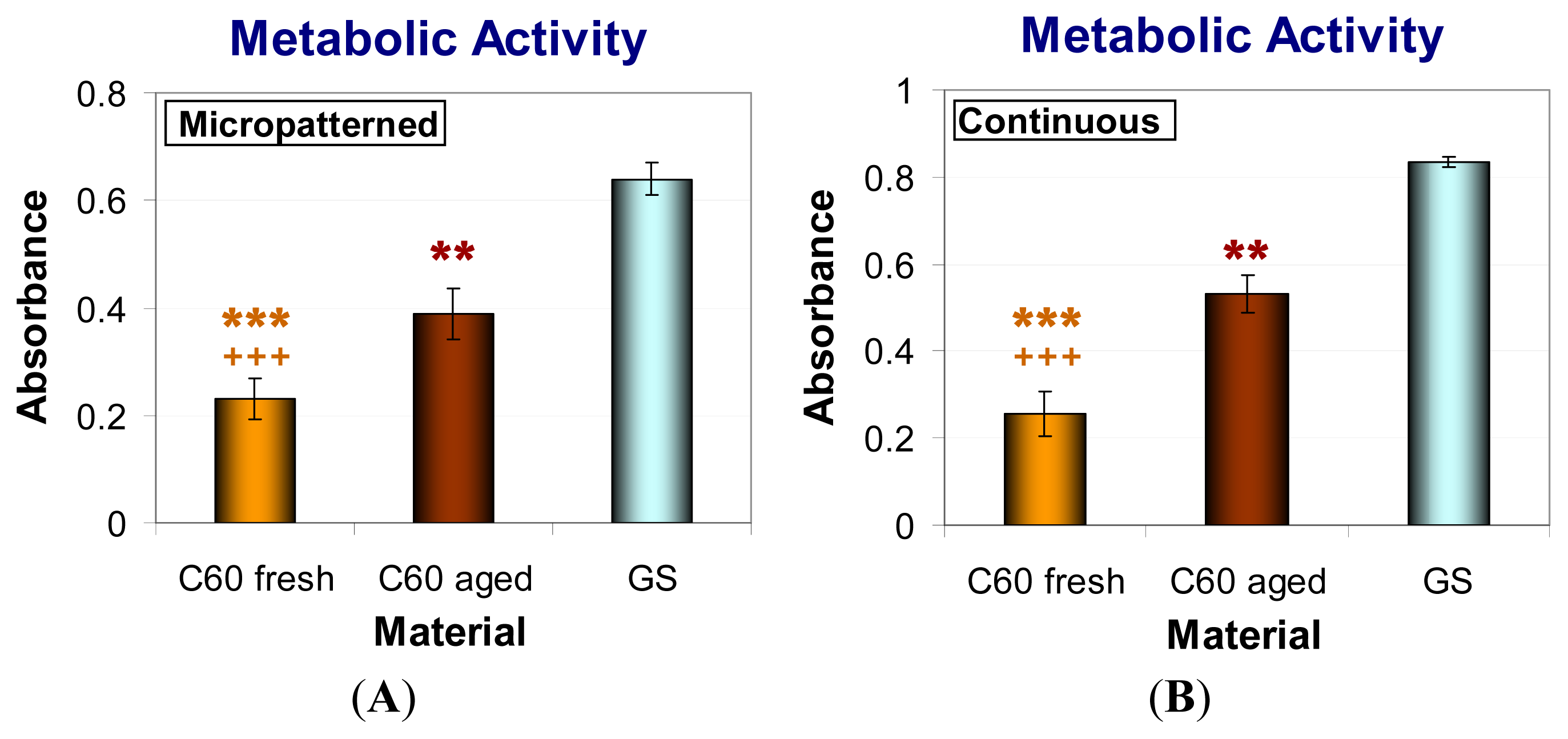
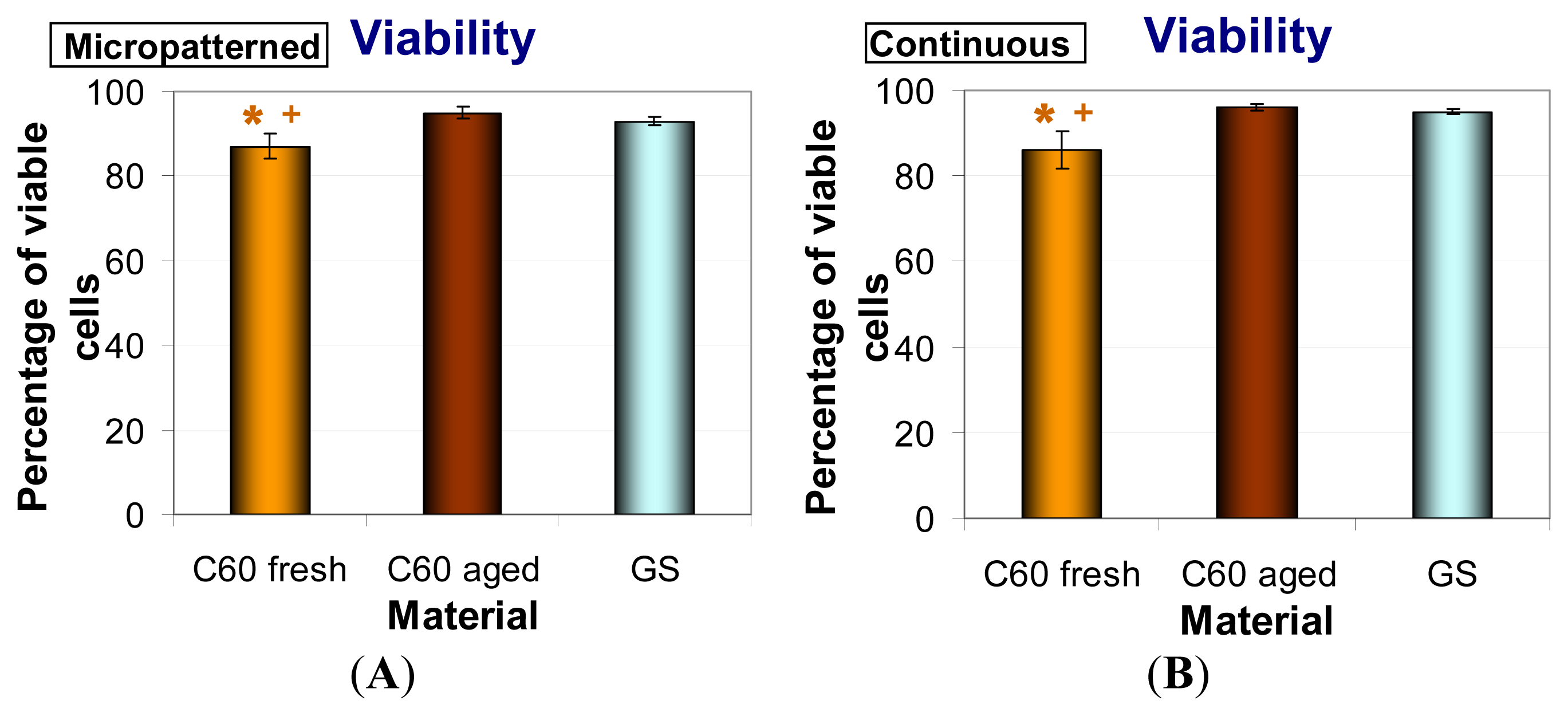
2.6. Metabolic Activity and Viability of Cells on Fullerene C60 Layers
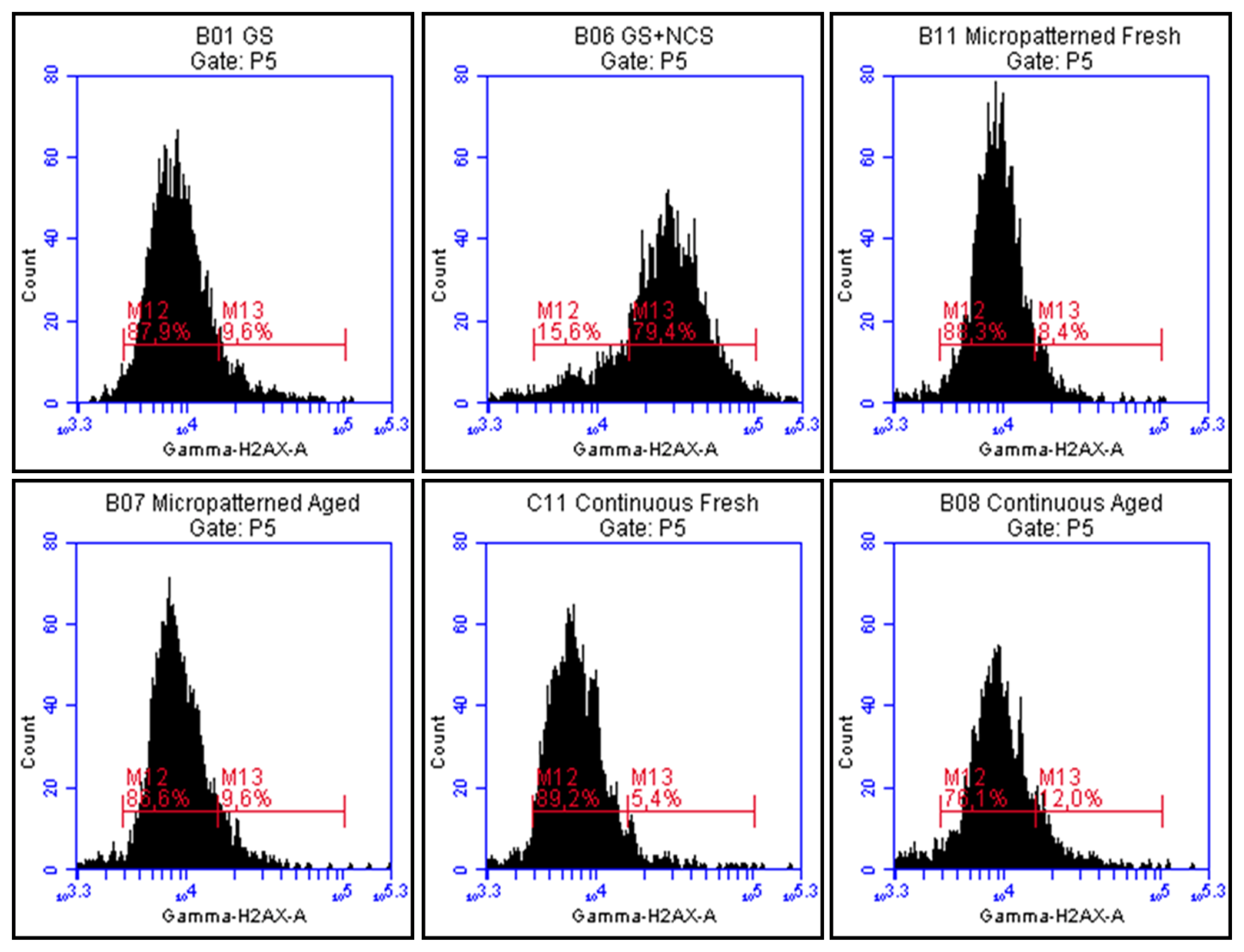
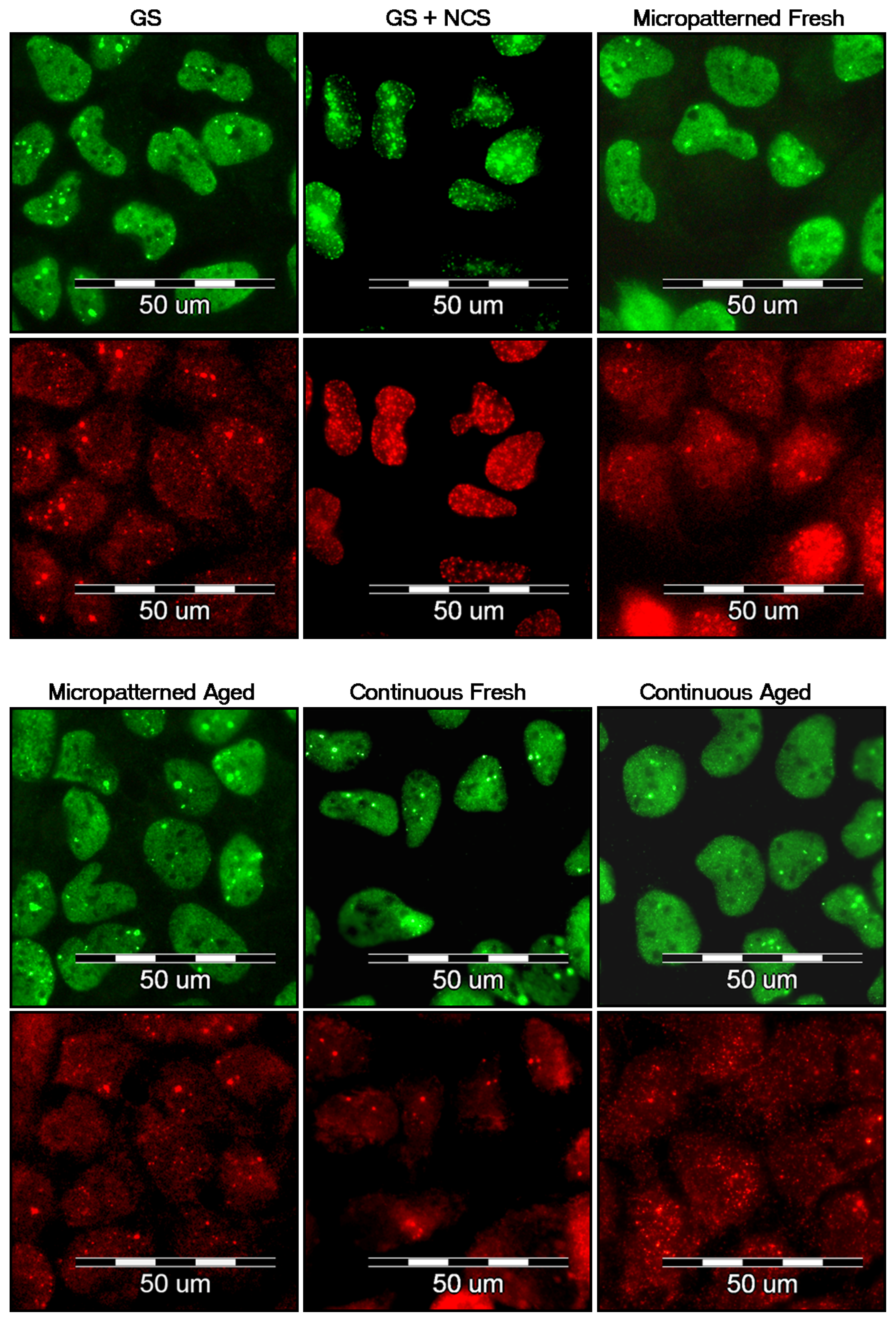
2.7. DNA Damage Response
3. Experimental Section
3.1. Material Deposition and Storage Condition
3.2. Atomic Force Microscopy (AFM)
3.3. Raman Spectroscopy
3.4. X-ray Photoelectron Microscopy (XPS)
3.5. Measurement of Wettability
3.6. Cells and Culture Conditions
3.7. Evaluation of Cell Morphology, Initial Adhesion and Proliferation (Growth Curves)
3.8. Evaluation of Cell Metabolic Activity
3.9. Evaluation of Membrane Damage and Cell Viability
3.10. Evaluation of DNA Damage Response
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Kroto, H.W.; Heath, J.R.; Obrien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar]
- Aitken, R.J.; Chaudhry, M.Q.; Boxall, A.B.A.; Hull, M. Manufacture and use of nanomaterials: Current status in the UK and global trends. Occup. Med 2006, 56, 300–306. [Google Scholar]
- Kato, S.; Taira, H.; Aoshima, H.; Saitoh, Y.; Miwa, N. Clinical evaluation of fullerene-C60 dissolved in squalane for anti-wrinkle cosmetics. J. Nanosci. Nanotechnol 2010, 10, 6769–6774. [Google Scholar]
- Kato, S.; Aoshima, H.; Saitoh, Y.; Miwa, N. Fullerene-C60/liposome complex: Defensive effects against UVA-induced damages in skin structure, nucleus and collagen type I/IV fibrils, and the permeability into human skin tissue. J. Photochem. Photobiol. B Biol 2010, 98, 99–105. [Google Scholar]
- Foley, S.; Crowley, C.; Smaihi, M.; Bonfils, C.; Erlanger, B.F.; Seta, P.; Larroque, C. Cellular localisation of a water-soluble fullerene derivative. Biochem. Biophys. Res. Commun 2002, 294, 116–119. [Google Scholar]
- Venkatesan, N.; Yoshimitsu, J.; Ito, Y.; Shibata, N.; Takada, K. Liquid filled nanoparticles as a drug delivery tool for protein therapeutics. Biomaterials 2005, 26, 7154–7163. [Google Scholar]
- Isobe, H.; Nakanishi, W.; Tomita, N.; Jinno, S.; Okayama, H.; Nakamura, E. Nonviral gene delivery by tetraamino fullerene. Mol. Pharm 2006, 3, 124–134. [Google Scholar]
- Dugan, L.L.; Gabrielsen, J.K.; Yu, S.P.; Lin, T.S.; Choi, D.W. Buckminsterfullerenol free radical scavengers reduce excitotoxic and apoptotic death of cultured cortical neurons. Neurobiol. Dis 1996, 3, 129–135. [Google Scholar]
- Dugan, L.L.; Lovett, E.G.; Quick, K.L.; Lotharius, J.; Lin, T.T.; O’Malley, K.L. Fullerene-based antioxidants and neurodegenerative disorders. Parkinsonism Related Disord 2001, 7, 243–246. [Google Scholar]
- Ryan, J.J.; Bateman, H.R.; Stover, A.; Gomez, G.; Norton, S.K.; Zhao, W.; Schwartz, L.B.; Lenk, R.; Kepley, C.L. Fullerene nanomaterials inhibit the allergic response. J. Immunol 2007, 179, 665–672. [Google Scholar]
- Tabata, Y.; Murakami, Y.; Ikada, Y. Photodynamic effect of polyethylene glycol-modified fullerene on tumor. Jpn. J. Cancer Res 1997, 88, 1108–1116. [Google Scholar]
- Tegos, G.P.; Demidova, T.N.; Arcila-Lopez, D.; Lee, H.; Wharton, T.; Gali, H.; Hamblin, M.R. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem. Biol 2005, 12, 1127–1135. [Google Scholar]
- Käsermann, F.; Kempf, C. Photodynamic inactivation of enveloped viruses by buckminsterfullerene. Antivir. Res 1997, 34, 65–70. [Google Scholar]
- Bacakova, L.; Grausova, L.; Vandrovcova, M.; Vacik, J.; Frazcek, A.; Blazewicz, S.; Kromka, A.; Rezek, B.; Vanecek, M.; Nesladek, M.; et al. Carbon Nanoparticles as Substrates for Cell Adhesion and Growth. In Nanoparticles: New Research; Lombardi, S.L., Ed.; Nova Science Publishers, Inc: Hauppauge, NY, USA, 2008; pp. 39–107. [Google Scholar]
- Spohn, P.; Hirsch, C.; Hasler, F.; Bruinink, A.; Krug, H.F.; Wick, P. C60 fullerene: A powerful antioxidant or a damaging agent? The importance of an in-depth material characterization prior to toxicity assays. Environ. Pollut 2009, 157, 1134–1139. [Google Scholar]
- Kovochich, M.; Espinasse, B.; Auffan, M.; Hotze, E.M.; Wessel, L.; Xia, T.; Nel, A.E.; Wiesner, M.R. Comparative toxicity of C60 aggregates toward mammalian cells: Role of tetrahydrofuran (THF) decomposition. Environ. Sci. Technol 2009, 43, 6378–6384. [Google Scholar]
- Henry, T.B.; Menn, F.-M.; Fleming, J.T.; Wilgus, J.; Compton, R.N.; Sayler, G.S. Attributing effects of aqueous C60 nano-aggregates to tetrahydrofuran decomposition products in larval zebrafish by assessment of gene expression. Environ. Health Perspect 2007, 115, 1059–1065. [Google Scholar]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett 2005, 5, 2578–2585. [Google Scholar]
- Huczko, A.; Lange, H.; Calco, E. Fullerenes: Experimental evidence for a null risk of skin irritation and allergy. Full. Sci. Technol 1999, 7, 935–939. [Google Scholar]
- Nelson, M.A.; Domann, F.E.; Bowden, G.T.; Hooser, S.B.; Fernando, Q.; Carter, D.E. Effects of acute and subchronic exposure of topically applied fullerene extracts on the mouse skin. Toxicol. Ind. Health 1993, 9, 623–630. [Google Scholar]
- Sayes, C.M.; Marchione, A.A.; Reed, K.L.; Warheit, D.B. Comparative pulmonary toxicity assessments of C60 water suspensions in rats: Few differences in fullerene toxicity in vivo in contrast to in vitro profiles. Nano Lett 2007, 7, 2399–2406. [Google Scholar]
- Baker, G.L.; Gupta, A.; Clark, M.L.; Valenzuela, B.R.; Staska, L.M.; Harbo, S.J.; Pierce, J.T.; Dill, J.A. Inhalation toxicity and lung toxicokinetics of C60 fullerene nanoparticles and microparticles. Toxicol. Sci 2008, 101, 122–131. [Google Scholar]
- Yudoh, K.; Shishido, K.; Murayama, H.; Yano, M.; Matsubayashi, K.; Takada, H.; Nakamura, H.; Masuko, K.; Kato, T.; Nishioka, K. Water-soluble C60 fullerene prevents degeneration of articular cartilage in osteoarthritis via down-regulation of chondrocyte catabolic activity and inhibition of cartilage degeneration during disease development. Arthrit. Rheum 2007, 56, 3307–3318. [Google Scholar]
- Yudoh, K.; Karasawa, R.; Masuko, K.; Kato, T. Water-soluble fullerene (C60) inhibits the development of arthritis in the rat model of arthritis. Int. J. Nanomed 2009, 4, 217–225. [Google Scholar]
- Yudoh, K.; Karasawa, R.; Masuko, K.; Kato, T. Water-soluble fullerene (C60) inhibits the osteoclast differentiation and bone destruction in arthritis. Int. J. Nanomed 2009, 4, 233–239. [Google Scholar]
- Kasai, T.; Matsumura, S.; Iizuka, T.; Shiba, K.; Kanamori, T.; Yudasaka, M.; Iijima, S.; Yokoyama, A. Carbon nanohorns accelerate bone regeneration in rat calvarial bone defect. Nanotechnology 2011, 22, 065102. [Google Scholar]
- Bacakova, L.; Grausova, L.; Vacik, J.; Fraczek, A.; Blazewicz, S.; Kromka, A.; Vanecek, M.; Svorcik, V. Improved adhesion and growth of human osteoblast-like MG 63 cells on biomaterials modified with carbon nanoparticles. Diam. Related Mater 2007, 16, 2133–2140. [Google Scholar]
- Grausova, L.; Vacik, J.; Bilkova, P.; Vorlicek, V.; Svorcik, V.; Soukup, D.; Bacakova, M.; Lisa, V.; Bacakova, L. Regionally-selective adhesion and growth of human osteoblast-like MG 63 cells on micropatterned fullerene C60 layers. J. Optoelectron. Adv. Mater 2008, 10, 2071–2076. [Google Scholar]
- Grausova, L.; Vacik, J.; Vorlicek, V.; Svorcik, V.; Slepicka, P.; Bilkova, P.; Vandrovcova, M.; Lisa, V.; Bacakova, L. Fullerene C60 films of continuous and micropatterned morphology as substrates for adhesion and growth of bone cells. Diam. Related Mater 2009, 18, 578–586. [Google Scholar]
- Vandrovcova, M.; Vacik, J.; Svorcik, V.; Slepicka, P.; Kasalkova, N.; Vorlicek, V.; Lavrentiev, V.; Vosecek, V.; Grausova, L.; Lisa, V.; et al. Fullerene C60 and hybrid C60/Ti films as substrates for adhesion and growth of bone cells. Phys. Status Solidi A 2008, 205, 2252–2261. [Google Scholar]
- Vacik, J.; Lavrentiev, V.; Novotna, K.; Bacakova, L.; Lisa, V.; Vorlicek, V.; Fajgar, R. Fullerene (C60)–transitional metal (Ti) composites: Structural and biological properties of the thin films. Diam. Related Mater 2010, 19, 242–246. [Google Scholar]
- Jirka, I.; Vandrovcova, M.; Frank, O.; Tolde, Z.; Plsek, J.; Luxbacher, T.; Bacakova, L.; Stary, V. On the role of Nb-related sites of an oxidized β-TiNb alloy surface in its interaction with osteoblast-like MG-63 cells. Mater. Sci. Eng. C 2013, 33, 1636–1645. [Google Scholar]
- Bacakova, L.; Svorcik, V. Cell Colonization Control by Physical and Chemical Modification of Materials. In Cell Growth Processes: New Research; Kimura, D., Ed.; Nova Science Publishersm, Inc: Hauppauge, NY, USA, 2008; pp. 5–56. [Google Scholar]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv 2011, 29, 739–767. [Google Scholar]
- Bacakova, L.; Grausova, L.; Vacik, J.; Kromka, A.; Biederman, H.; Choukourov, A.; Stary, V. Nanocomposite and Nanostructured Carbon-based Films as Growth Substrates for Bone Cells. In Advances in Diverse Industrial Applications of Nanocomposites; Reddy, B., Ed.; InTech: Vienna, Austria, 2011; pp. 399–435. [Google Scholar]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res 2000, 51, 475–483. [Google Scholar]
- Kim, K.T.; Jang, M.H.; Kim, J.Y.; Kim, S.D. Effect of preparation methods on toxicity of fullerene water suspensions to Japanese medaka embryos. Sci. Total Environ 2010, 408, 5606–5612. [Google Scholar]
- Zhao, X.; Striolo, A.; Cummings, P.T. C60 binds to and deforms nucleotides. Biophys. J 2005, 89, 3856–3862. [Google Scholar]
- Xu, X.; Wang, X.; Li, Y.; Wang, Y.; Yang, L. A large-scale association study for nanoparticle C60 uncovers mechanisms of nanotoxicity disrupting the native conformations of DNA/RNA. Nucleic Acids Res 2012, 40, 7622–7632. [Google Scholar]
- Band, I.M.; Kharitonov Yu, I.; Trzhaskovskaya, M.B. Photoionization cross sections and photoelectron angular distributions for X-ray line energies in the range 0.132–4.509 keV targets: 1 ≤ Z ≤ 100. At. Data Nuclear Data Tables 1979, 23, 443–505. [Google Scholar]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. IX. Data for 41 elemental solids over the 50 eV to 30 keV range. Surf. Interface Anal 2011, 43, 689–713. [Google Scholar]
- Jiricek, P. Measurement of the transmission function of the hemispherical energy analyzer of ADES 400 electron spectrometer. Czechoslov. J. Phys 1994, 44, 261–267. [Google Scholar]

| Sample | C | O | Si | Na | F |
|---|---|---|---|---|---|
| (at.%) | (at.%) | (at.%) | (at.%) | (at.%) | |
| Fresh, continuous | 57.2 | 29.3 | 11.3 | 2.3 | - |
| Fresh, micropatterned | 43.8 | 36.1 | 16.4 | 3.6 | - |
| Aged, continuous | 72.2 | 16.4 | 3.8 | 7.6 | - |
| Aged, micropatterned | 73.7 | 15.6 | 4.9 | 5.8 | - |
| Glass substrate | 21.9 | 49.4 | 22.4 | 4.1 | 2.3 |
| Sample | C (at.%) | O (at.%) |
|---|---|---|
| Fresh, continuous | 91 | 9.0 |
| Fresh, micropatterned | 80.0 | 20.0 |
| Aged, continuous | 93.5 | 6.5 |
| Aged, micropatterned | 96.2 | 3.8 |
| Fullerene powder | 95.2 | 4.8 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kopova, I.; Bacakova, L.; Lavrentiev, V.; Vacik, J. Growth and Potential Damage of Human Bone-Derived Cells on Fresh and Aged Fullerene C60 Films. Int. J. Mol. Sci. 2013, 14, 9182-9204. https://doi.org/10.3390/ijms14059182
Kopova I, Bacakova L, Lavrentiev V, Vacik J. Growth and Potential Damage of Human Bone-Derived Cells on Fresh and Aged Fullerene C60 Films. International Journal of Molecular Sciences. 2013; 14(5):9182-9204. https://doi.org/10.3390/ijms14059182
Chicago/Turabian StyleKopova, Ivana, Lucie Bacakova, Vasily Lavrentiev, and Jiri Vacik. 2013. "Growth and Potential Damage of Human Bone-Derived Cells on Fresh and Aged Fullerene C60 Films" International Journal of Molecular Sciences 14, no. 5: 9182-9204. https://doi.org/10.3390/ijms14059182




