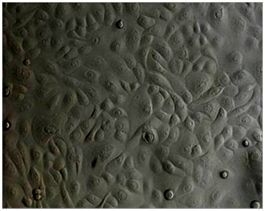
Isolation and Enhancement of a Homogenous in Vitro Human Hertwig’s Epithelial Root Sheath Cell Population
Abstract
:1. Introduction
2. Results
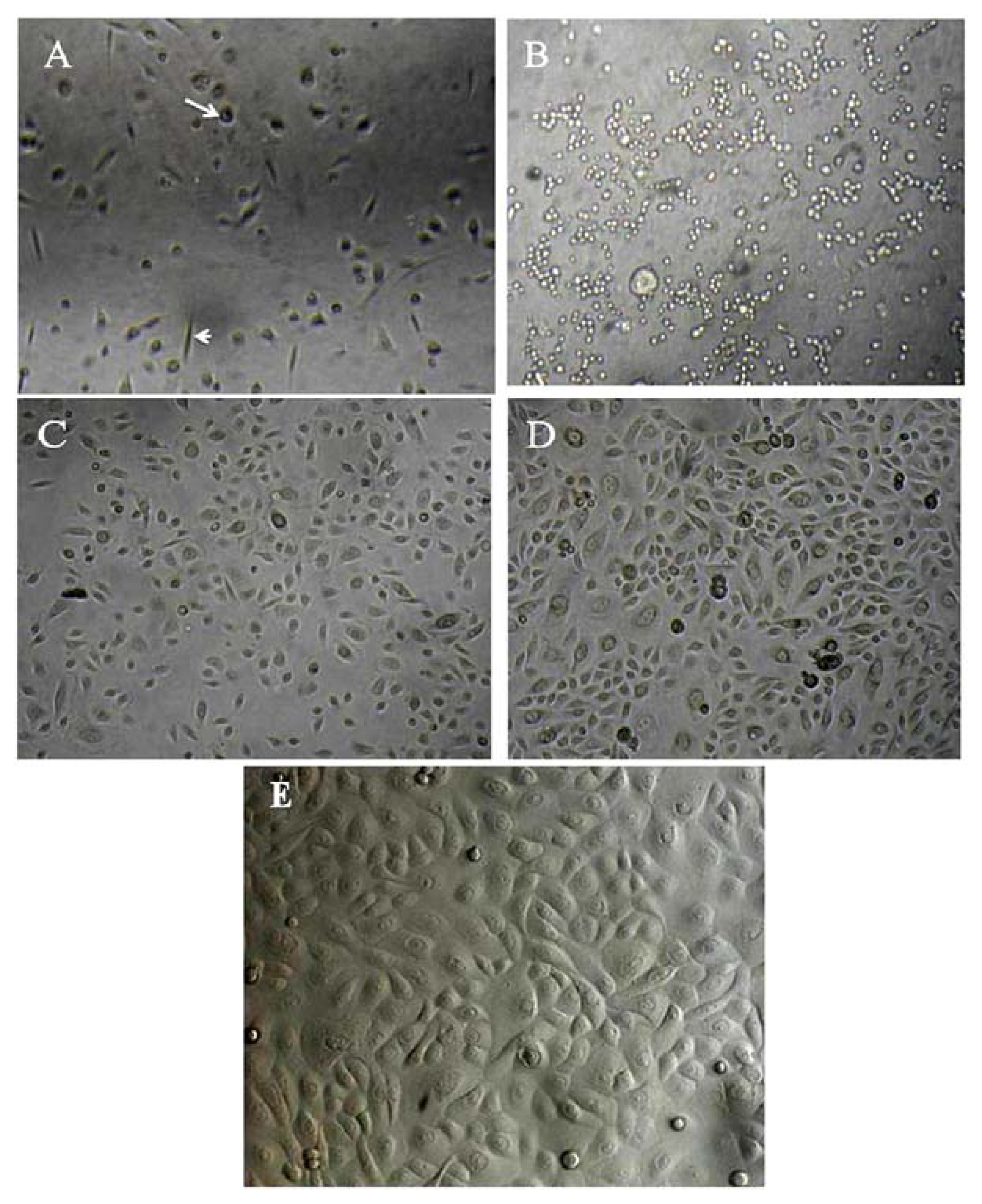
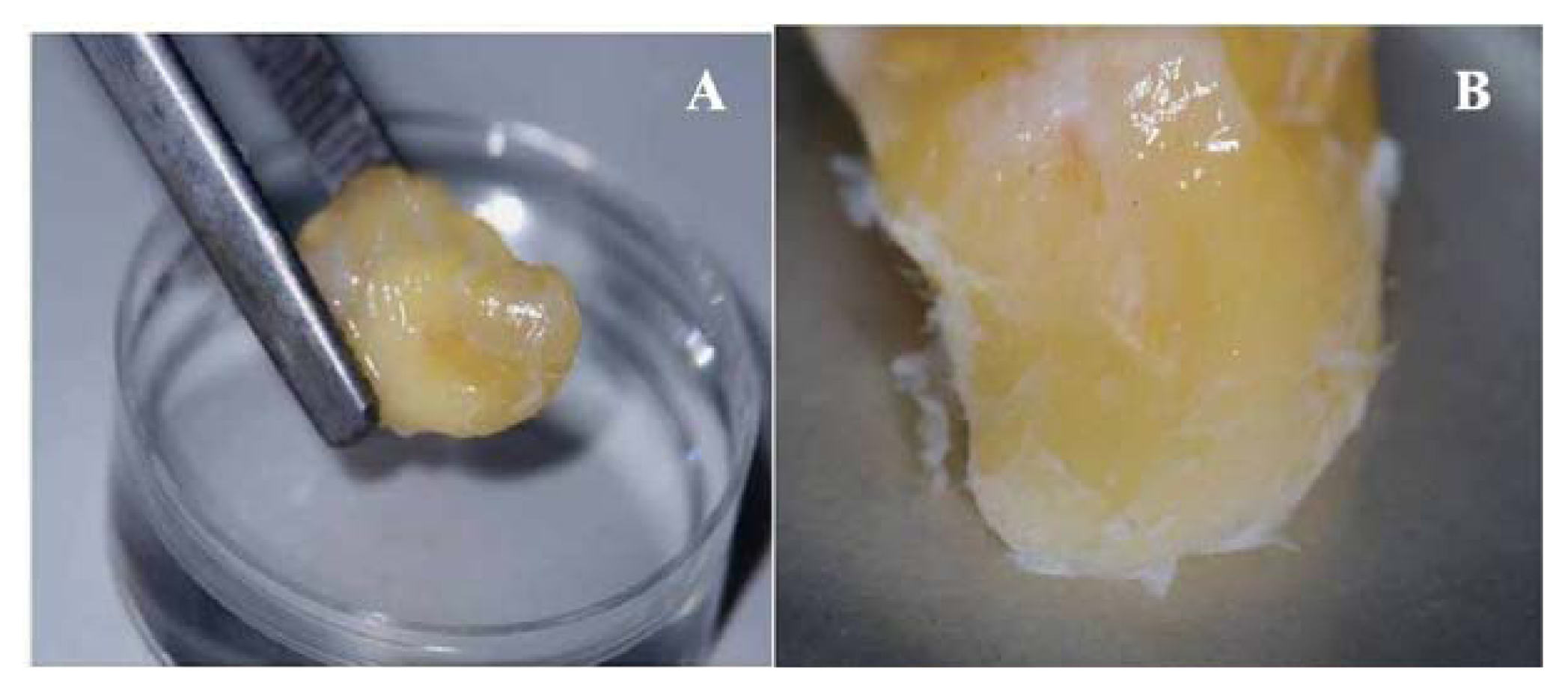
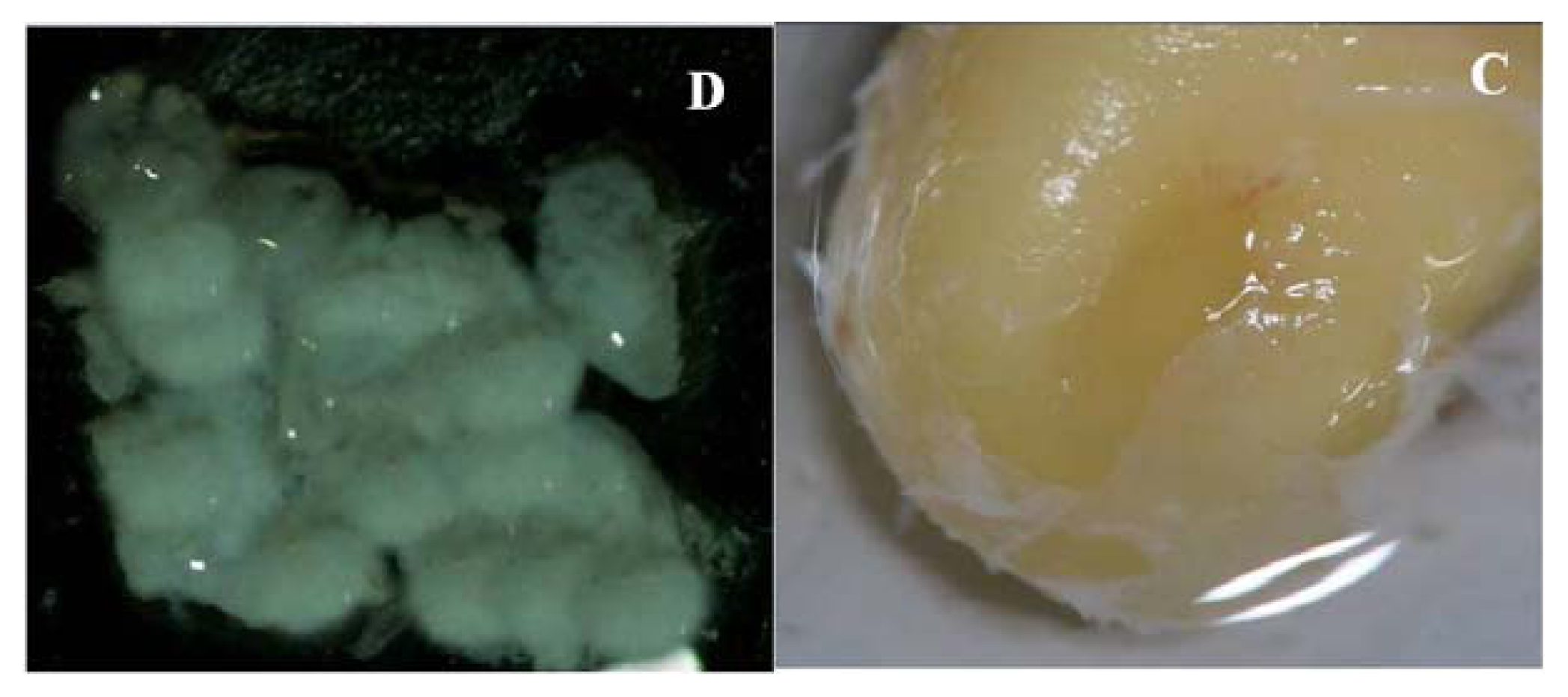
2.1. Primary Isolation of HERS Cells
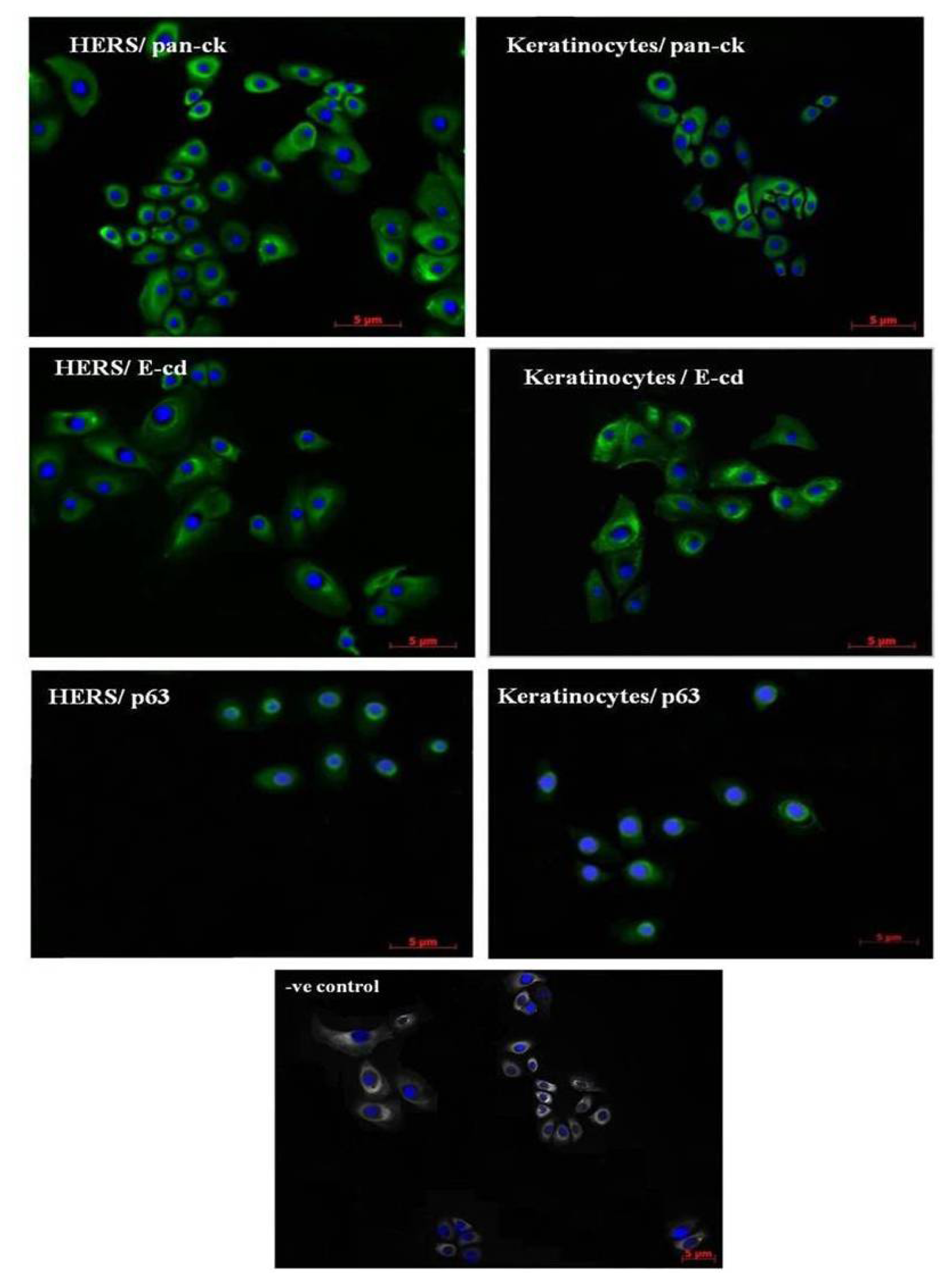
2.2. Immunofluorescence Staining of HERS Cells
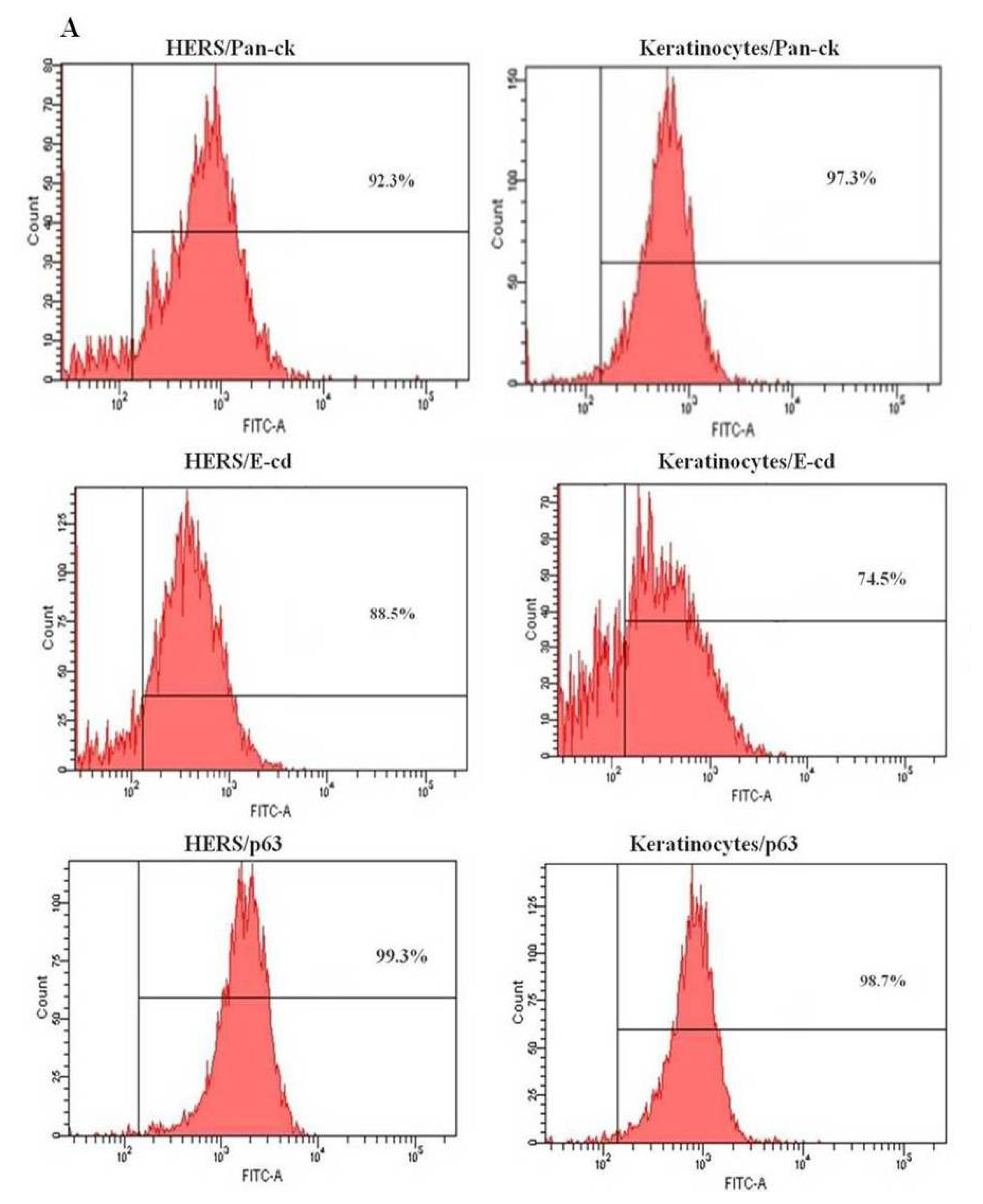
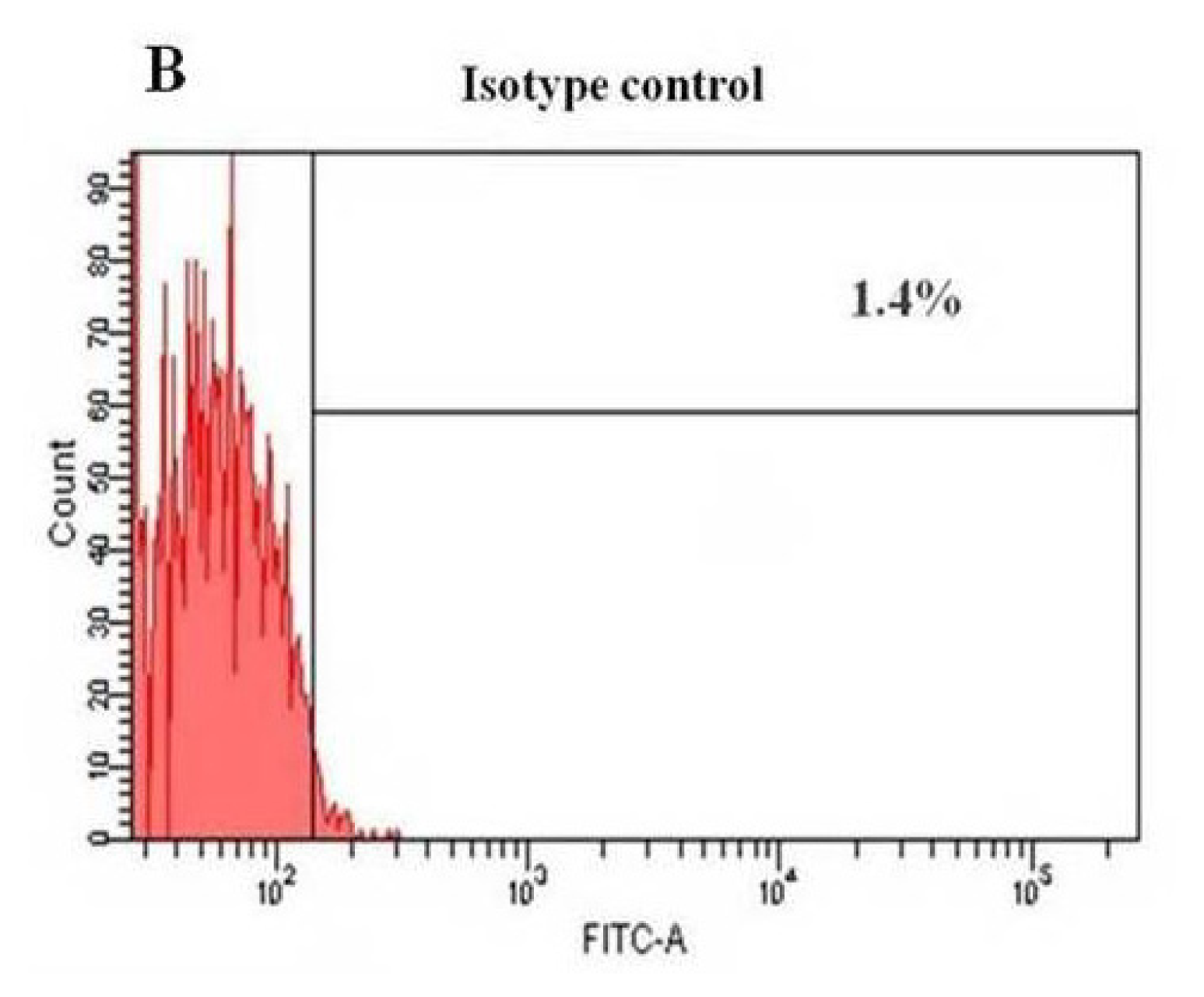
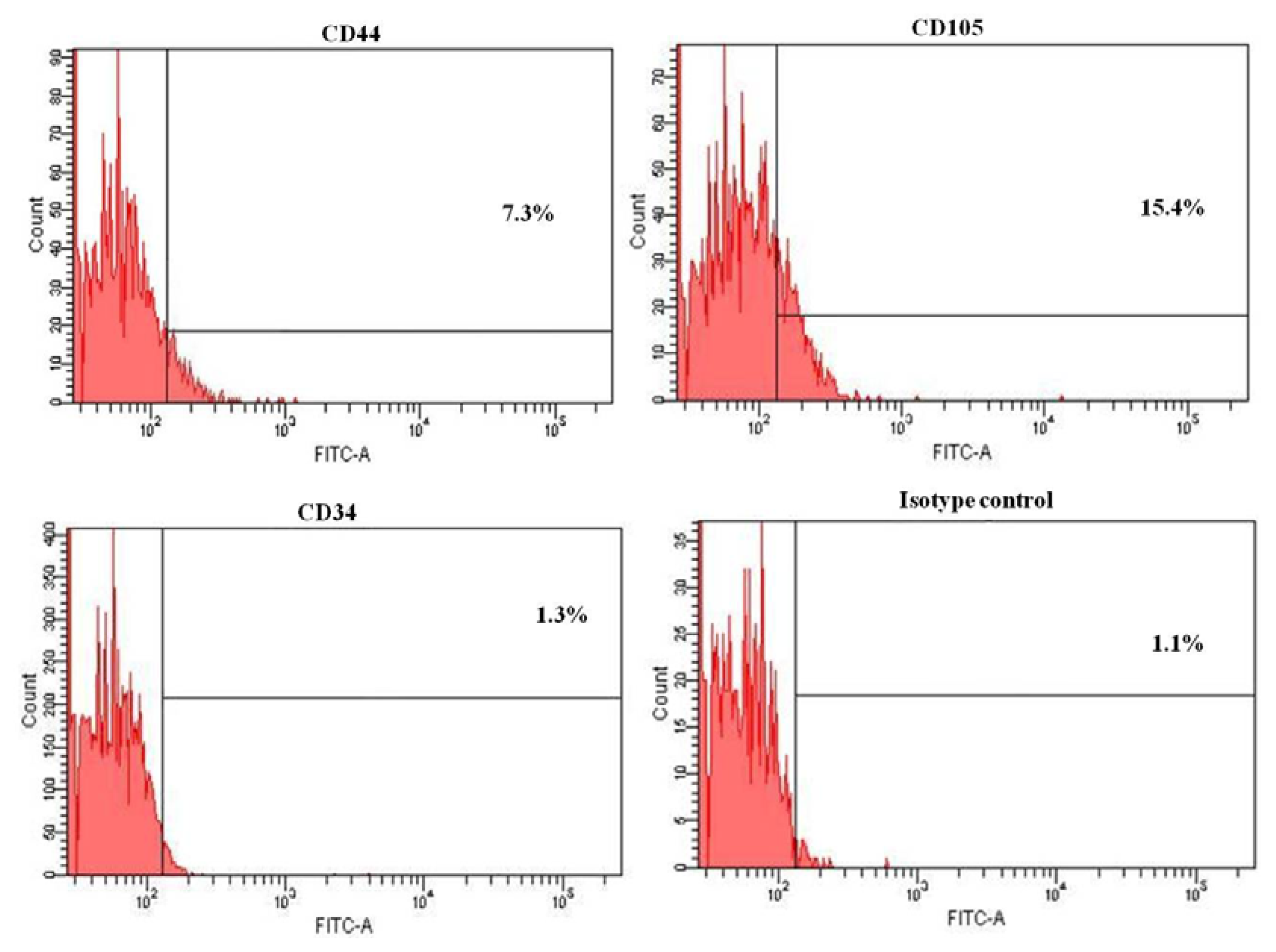
2.3. Flow Cytometry Analysis of HERS Cells
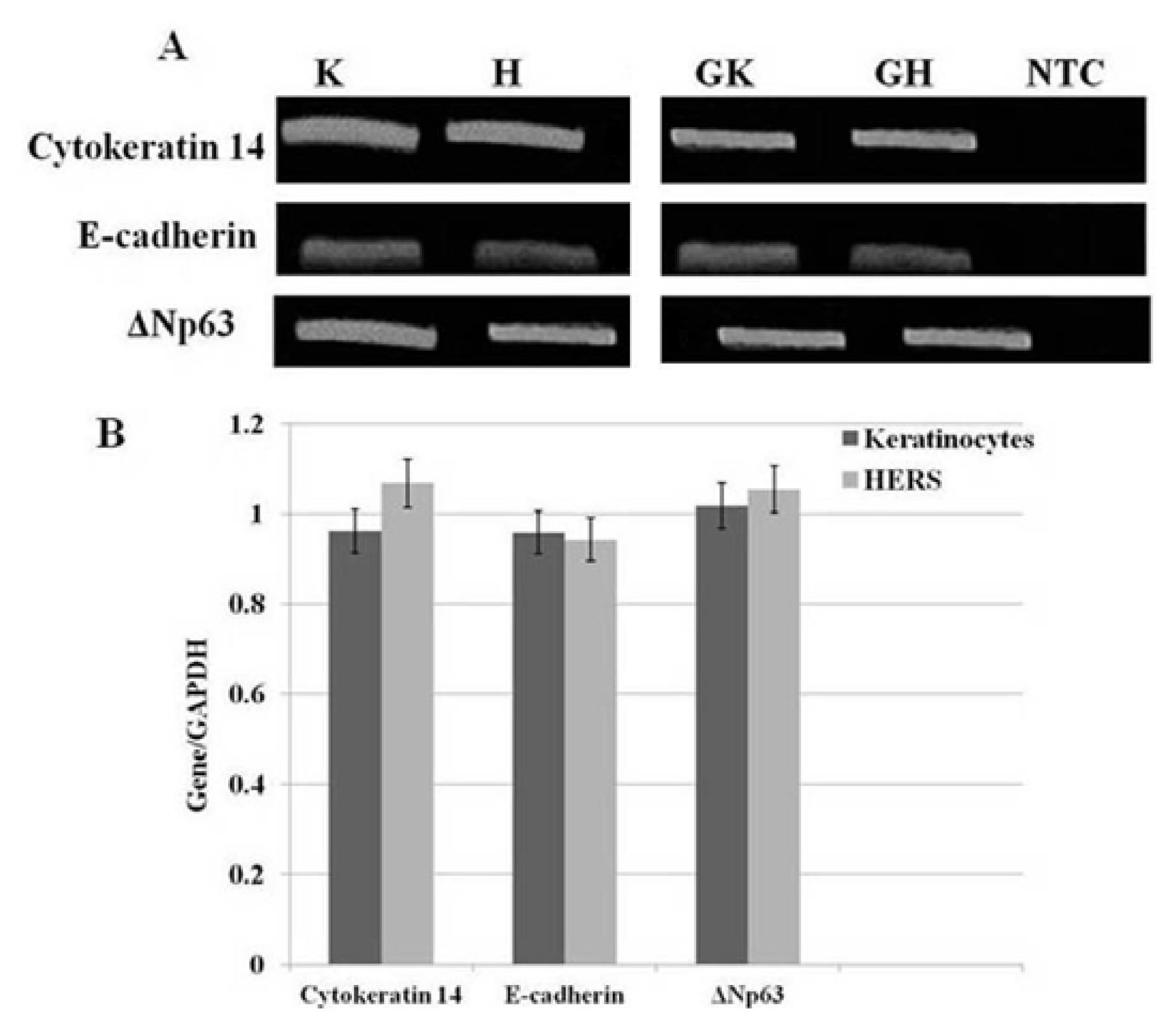
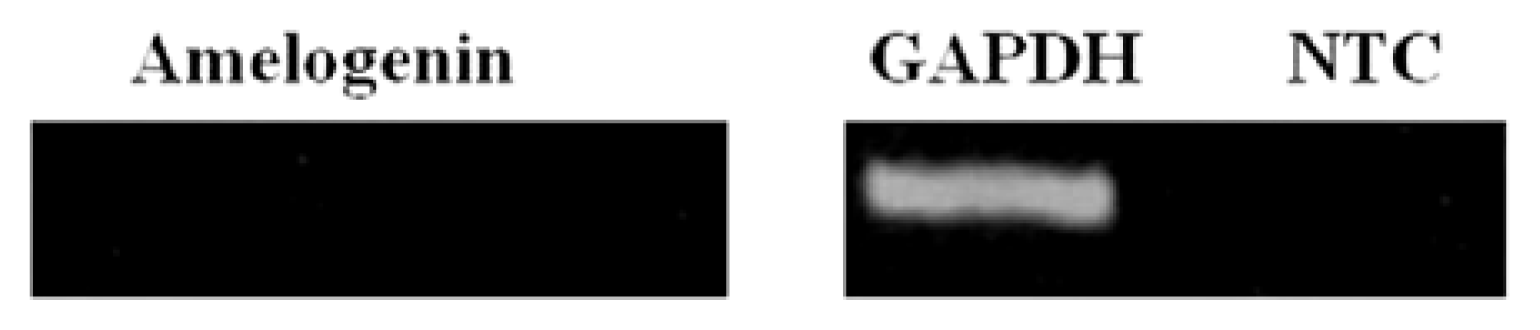
2.4. sqRT-PCR
3. Discussion
4. Experimental Section
4.1. Primary Isolation and Culture of Human HERS Cells
4.2. Indirect Immunofluorescence Staining
4.3. Flow Cytometry Analysis
4.4. Semi-Quantitative Reverse Transcription-Polymerase Chain Reaction (sqRT-PCR)
5. Conclusions
Acknowledgements
Conflict of Interest
References
- Fujiwara, N.; Akimoto, T.; Otsu, K.; Kagiya, T.; Ishizeki, K.; Harada, H. Reduction of Egf signaling decides transition from crown to root in the development of mouse molars. J. Exp. Zool. B. Mol. Dev. Evol. 2009, 486–494. [Google Scholar]
- Wentz, F.M.; Weinmann, J.P.; Schour, I. The prevalence, distribution, and morphologic changes of the epithelial remnants in the molar region of the rat. J. Dent. Res 1950, 29, 637–646. [Google Scholar]
- Spouge, J.D. A new look at the rests of Malassez. A review of their embryological origin, anatomy, and possible role in periodontal health and disease. J. Periodontol 1980, 51, 437–444. [Google Scholar]
- Sonoyama, W.; Seo, B.M.; Yamaza, T.; Shi, S. Human Hertwig’s epithelial root sheath cells play crucial roles in cementum formation. J. Dent. Res 2007, 86, 594–599. [Google Scholar]
- Nam, H.; Lee, G. Identification of novel epithelial stem cell-like cells in human deciduous dental pulp. Biochem. Biophys. Res. Commun 2009, 386, 135–139. [Google Scholar]
- Jung, H.S.; Lee, D.S.; Lee, J.H.; Park, S.J.; Lee, G.; Seo, B.M.; Ko, J.S.; Park, J.C. Directing the differentiation of human dental follicle cells into cementoblasts and/or osteoblasts by a combination of HERS and pulp cells. J. Mol. Histol 2011, 42, 227–235. [Google Scholar]
- Nam, H.; Kim, J.; Park, J.; Park, J.C.; Kim, J.W.; Seo, B.M.; Lee, J.C.; Lee, G. Expression profile of the stem cell markers in human Hertwig’s epithelial root sheath/Epithelial rests of Malassez cells. Mol. Cells 2011, 31, 355–360. [Google Scholar]
- Zeichner-David, M.; Oishi, K.; Su, Z.; Zakartchenko, V.; Chen, L.S.; Arzate, H.; Bringas, P., Jr. Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev. Dyn. 2003, 228, 651–663. [Google Scholar]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar]
- Arzate, H. Preliminary characterization of epithelial root sheath cells in vitro. Bol. Estud. Med. Biol 1994, 42, 27–30. [Google Scholar]
- Akimoto, T.; Fujiwara, N.; Kagiya, T.; Otsu, K.; Ishizeki, K.; Harada, H. Establishment of Hertwig’s epithelial root sheath cell line from cells involved in epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun 2011, 404, 308–312. [Google Scholar]
- Luan, X.; Ito, Y.; Diekwisch, T.G. Evolution and development of Hertwig’s epithelial root sheath. Dev. Dyn 2006, 235, 1167–1180. [Google Scholar]
- Thomas, H.F. Root formation. Int. J. Dev. Biol 1995, 39, 231–137. [Google Scholar]
- De Paiva, C.S.; Chen, Z.; Corrales, R.M.; Pflugfelder, S.C.; Li, D.Q. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem. Cells 2005, 23, 63–73. [Google Scholar]
- Parikh, N.; Nagarajan, P.; Sei-ichi, M.; Sinha, S.; Garrett-Sinha, L.A. Isolation and characterization of an immortalized oral keratinocyte cell line of mouse origin. Arch. Oral. Biol 2008, 53, 1091–1100. [Google Scholar]
- Parsa, R.; Yang, A.; McKeon, F.; Green, H. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J. Invest. Dermatol 1999, 113, 1099–1105. [Google Scholar]
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; de Luca, M. P63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161. [Google Scholar]
- Romano, R.A.; Birkaya, B.; Sinha, S. Defining the regulatory elements in the proximal promoter of DeltaNp63 in keratinocytes: Potential roles for Sp1/Sp3, NF-Y, and p63. J. Invest. Dermatol 2006, 126, 1469–1479. [Google Scholar]
- Takagi, R.; Yamato, M.; Murakami, D.; Sugiyama, H.; Okano, T. Low calcium culture condition induces mesenchymal cell-like phenotype in normal human epidermal keratinocytes. Biochem. Biophys. Res. Commun 2011, 412, 226–231. [Google Scholar]
- Garlick, J.A.; Fenjves, E.S. Keratinocyte gene transfer and gene therapy. Crit. Rev. Oral. Biol. Med 1996, 7, 204–221. [Google Scholar]
- Presland, R.B.; Dale, B.A. Epithelial structural proteins of the skin and oral cavity: Function in health and disease. Crit. Rev. Oral. Biol. Med 2000, 11, 383–408. [Google Scholar]
- Glade, C.P.; Seegers, B.A.; Meulen, E.F.; van Hooijdonk, C.A.; van Erp, P.E.; van de Kerkhof, P.C. Multiparameter flow cytometric characterization of epidermal cell suspensions prepared from normal and hyperproliferative human skin using an optimized thermolysin-trypsin protocol. Arch. Dermatol. Res 1996, 288, 203–210. [Google Scholar]
- Melican, D.; Butler, R.; Hawkins, N.; Chen, L.H.; Hayden, E.; Destrempes, M.; Williams, J.; Lewis, T.; Behboodi, E.; Ziomek, C.; et al. Effect of serum concentration, method of trypsinization and fusion/activation utilizing transfected fetal cells to generate transgenic dairy goats by somatic cell nuclear transfer. Theriogenology 2005, 63, 1549–1563. [Google Scholar]
- Sutradhar, B.C.; Park, J.; Hong, G.; Choi, S.H.; Kim, G. Effects of trypsinization on viability of equine chondrocytes in cell culture. Pak. Vet. J 2010, 30, 232–238. [Google Scholar]
- Lähdesmäki, H.; Shmulevich, L.; Dunmire, V.; Yli-Harja, O.; Zhang, W. In silico microdissection of microarray data from heterogeneous cell populations. BMC Bioinform 2005, 14, 6, :54.. [Google Scholar]
- Zhao, Y.; Simon, R. Gene expression deconvolution in clinical samples. Genome Med 2010, 2, 93. [Google Scholar]
- Kuhn, A.; Thu, D.; Waldvogel, H.J.; Faull, R.L.; Luthi-Carter, R. Population-specific expression analysis (PSEA) reveals molecular changes in diseased brain. Nat. Methods 2011, 8, 945–947. [Google Scholar]
- Yang, A.; Schweitzer, R.; Sun, D.; Kaghad, M.; Walker, N.; Bronson, R.T.; Tabin, C.; Sharpe, A.; Caput, D.; Crum, C.; et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999, 398, 714–718. [Google Scholar]
- Barbareschi, M.; Pecciarini, L.; Cangi, M.G.; Macri, E.; Rizzo, A.; Viale, G.; Doglioni, C. p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am. J. Surg. Pathol 2001, 25, 1054–1060. [Google Scholar]
- Yang, A.; Kaghad, M.; Wang, Y.; Gillett, E.; Fleming, M.D.; Dötsch, V.; Andrews, N.C.; Caput, D.; McKeon, F. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 1998, 2, 305–316. [Google Scholar]
- Inai, T.; Kukita, T.; Ohsaki, Y.; Nagata, K.; Kukita, A.; Kurisu, K. Immunohistochemical demonstration of amelogenin penetration toward the dental pulp in the early stages of ameloblast development in rat molar tooth germs. Anat. Rec 1991, 229, 259–270. [Google Scholar]
- Kukita, A.; Harada, H.; Kukita, T.; Inai, T.; Matsuhashi, S.; Kurisu, K. Primary and secondary culture of rat ameloblasts in serum-free medium. Calcif. Tissue. Int 1992, 51, 393–398. [Google Scholar]
- Tabata, M.J.; Matsumura, T.; Fujii, T.; Abe, M.; Kurisu, K. Fibronectin accelerates the growth and differentiation of ameloblast lineage cells in vitro. J. Histochem. Cytochem 2003, 51, 1673–1679. [Google Scholar]
- Luo, W.; Slavkin, H.C.; Snead, M.L. Cells from Hertwig’s epithelial root sheath do not transcribe amelogenin. J. Periodontal. Res 1991, 26, 42–47. [Google Scholar]
- Diekwisch, T.G. The developmental biology of cementum. Int. J. Dev. Biol 2001, 45, 695–706. [Google Scholar]
| Gene | Sequence |
|---|---|
| Cytokeratin14 | 5′-GTCCTTCTGCAGATTGACAATGA-3′ (forward) 5′-TTCACCAAGACAGAGGAGCTGAA-3′ (reverse) |
| E-cadherin | 5′-GAAGATTGCACCGGTCGACAAA-3′ (forward) 5′-CCACCAAAGTCACGCTGAATAC-3′ (reverse) |
| ΔNp63 | 5′-CAGACTCAATTTAGTGAG-3′ (forward) 5′-AGCTCATGGTTGGGGCAC-3′ (reverse) |
| Amelogenin | 5′-GGAGCAGCTTTTGCCATGCCT-3′ (forward) 5′-GTGGCCTTATGCTCTGGTAC-3′ (reverse) |
| GAPDH | 5′-GAGTCAACGGATTTGGTCGT-3′ (forward) 5′-GACAAGCTTCCCGTTCTCAG-3′ (reverse) |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Farea, M.; Halim, A.S.; Abdullah, N.A.; Lim, C.K.; Mokhtar, K.I.; Berahim, Z.; Mokhtar, K.; Rani, A.Q.; Husein, A. Isolation and Enhancement of a Homogenous in Vitro Human Hertwig’s Epithelial Root Sheath Cell Population. Int. J. Mol. Sci. 2013, 14, 11157-11170. https://doi.org/10.3390/ijms140611157
Farea M, Halim AS, Abdullah NA, Lim CK, Mokhtar KI, Berahim Z, Mokhtar K, Rani AQ, Husein A. Isolation and Enhancement of a Homogenous in Vitro Human Hertwig’s Epithelial Root Sheath Cell Population. International Journal of Molecular Sciences. 2013; 14(6):11157-11170. https://doi.org/10.3390/ijms140611157
Chicago/Turabian StyleFarea, Manal, Ahmad Sukari Halim, Nurul Asma Abdullah, Chin Keong Lim, Khairani Idah Mokhtar, Zurairah Berahim, Kasmawati Mokhtar, Abdul Qawee Rani, and Adam Husein. 2013. "Isolation and Enhancement of a Homogenous in Vitro Human Hertwig’s Epithelial Root Sheath Cell Population" International Journal of Molecular Sciences 14, no. 6: 11157-11170. https://doi.org/10.3390/ijms140611157