Angiotensin II Regulates microRNA-132/-212 in Hypertensive Rats and Humans
Abstract
:1. Introduction
2. Results
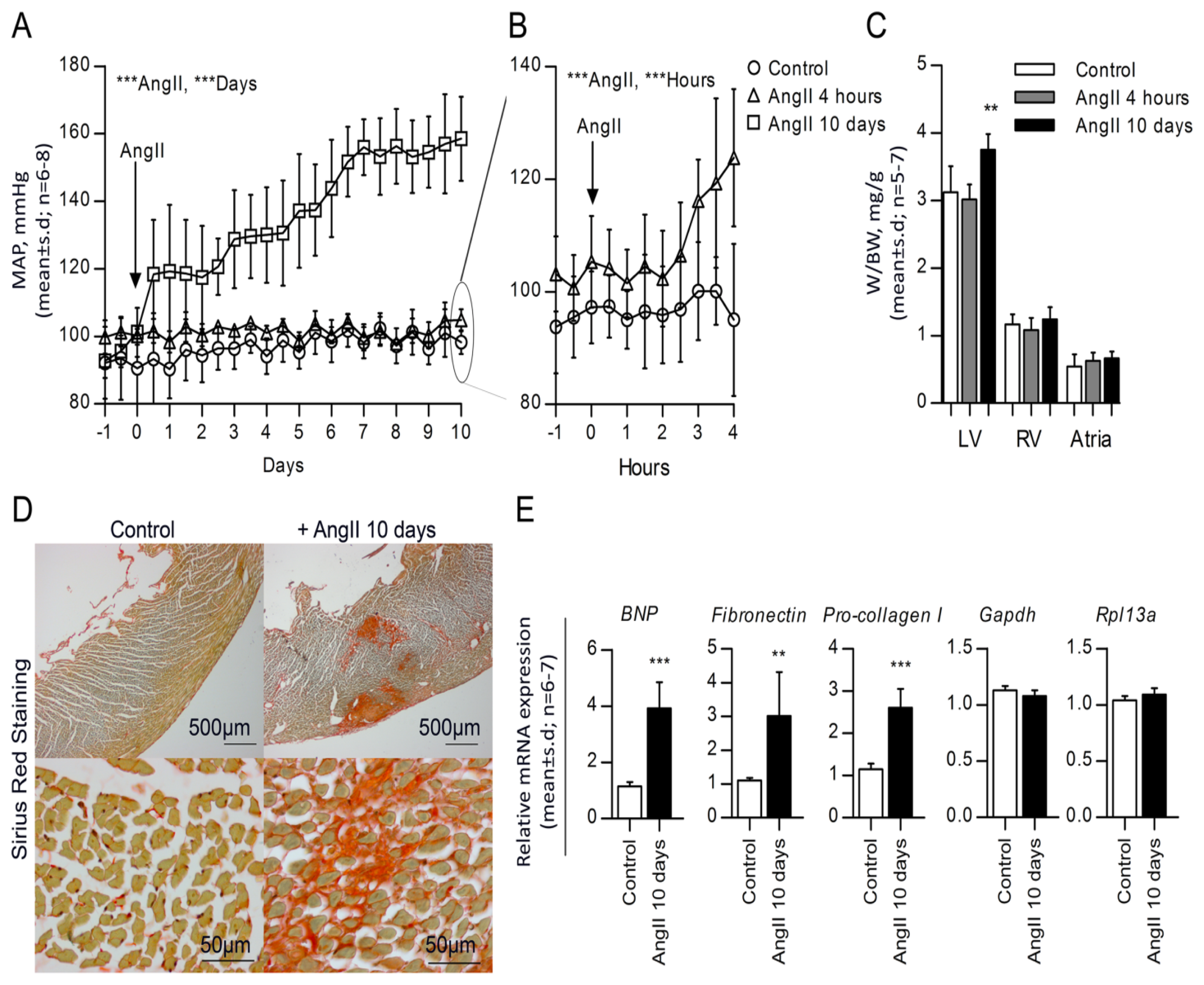
2.1. High Blood Pressure, Cardiac Hypertrophy and Fibrosis Are Sustained in the Rat Model
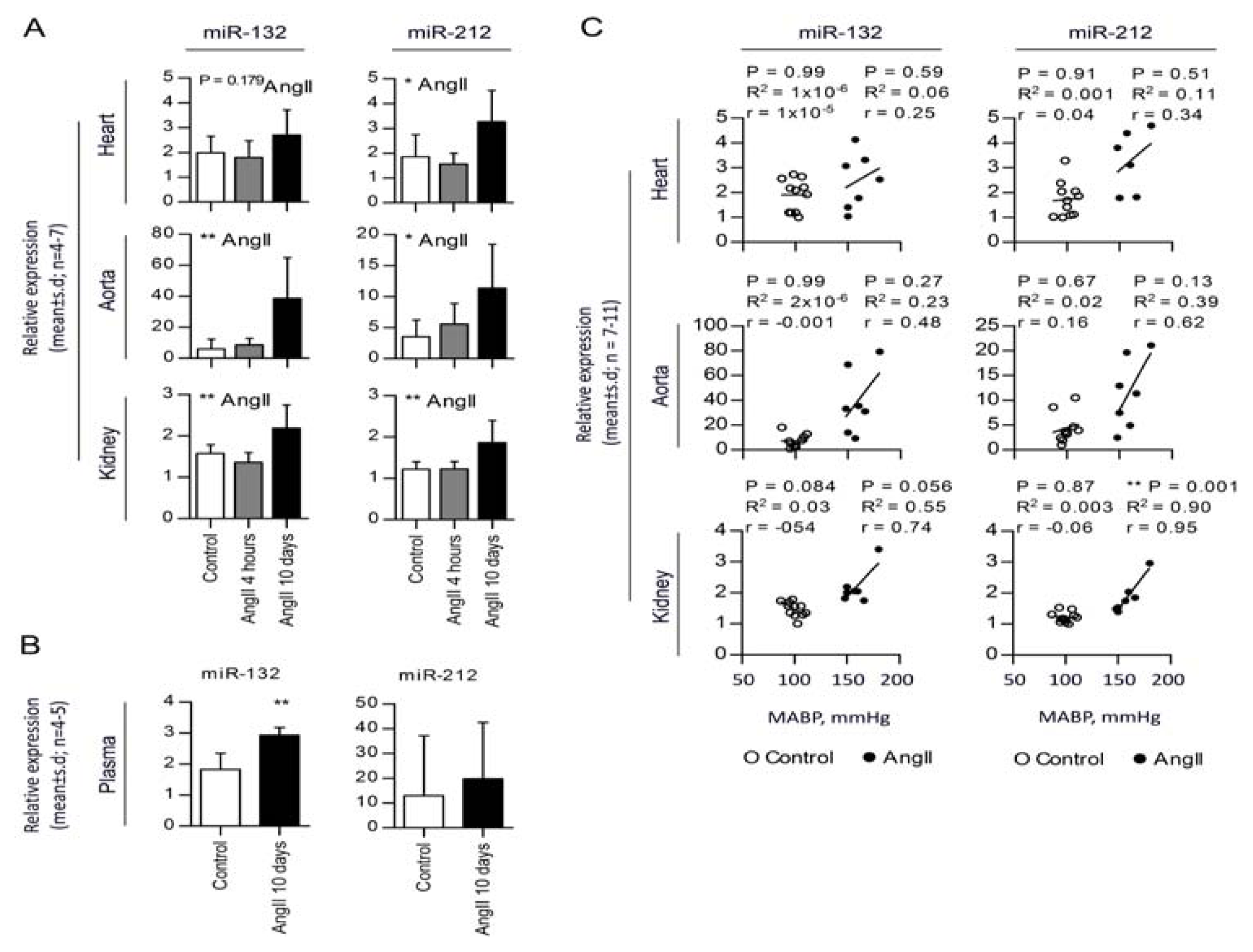
2.2. Chronic AngII-Mediated Hypertension in Rats Increases miR-132/-212 Cluster Expression in Blood Pressure Regulating Organs: Heart, Aorta and Kidney
2.3. miR-132 and -212 Regulation in Response to AngII in Mice
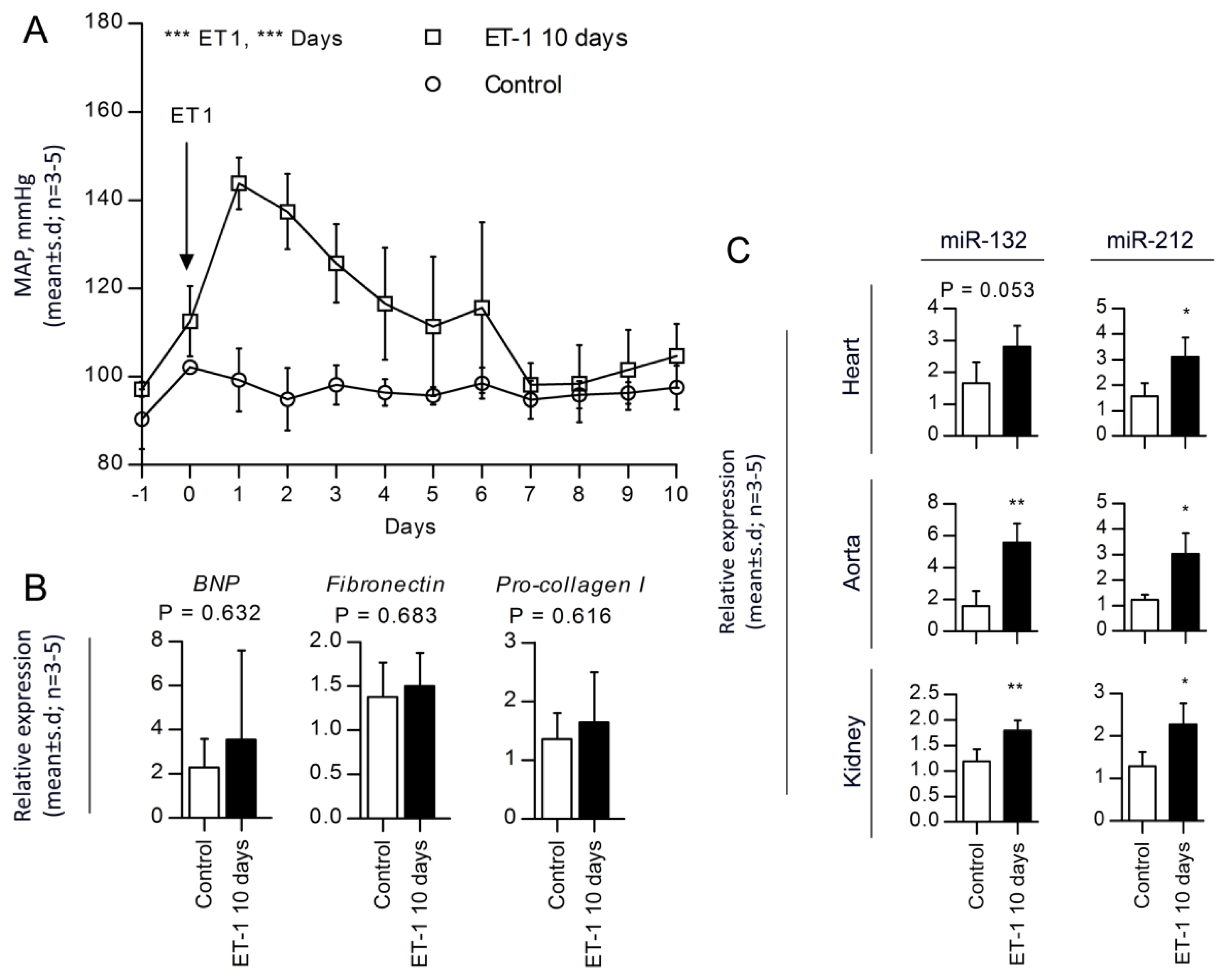
2.4. miR-132 and -212 Regulations in Response to ET-1, Vasopressor-Induced Hypertension
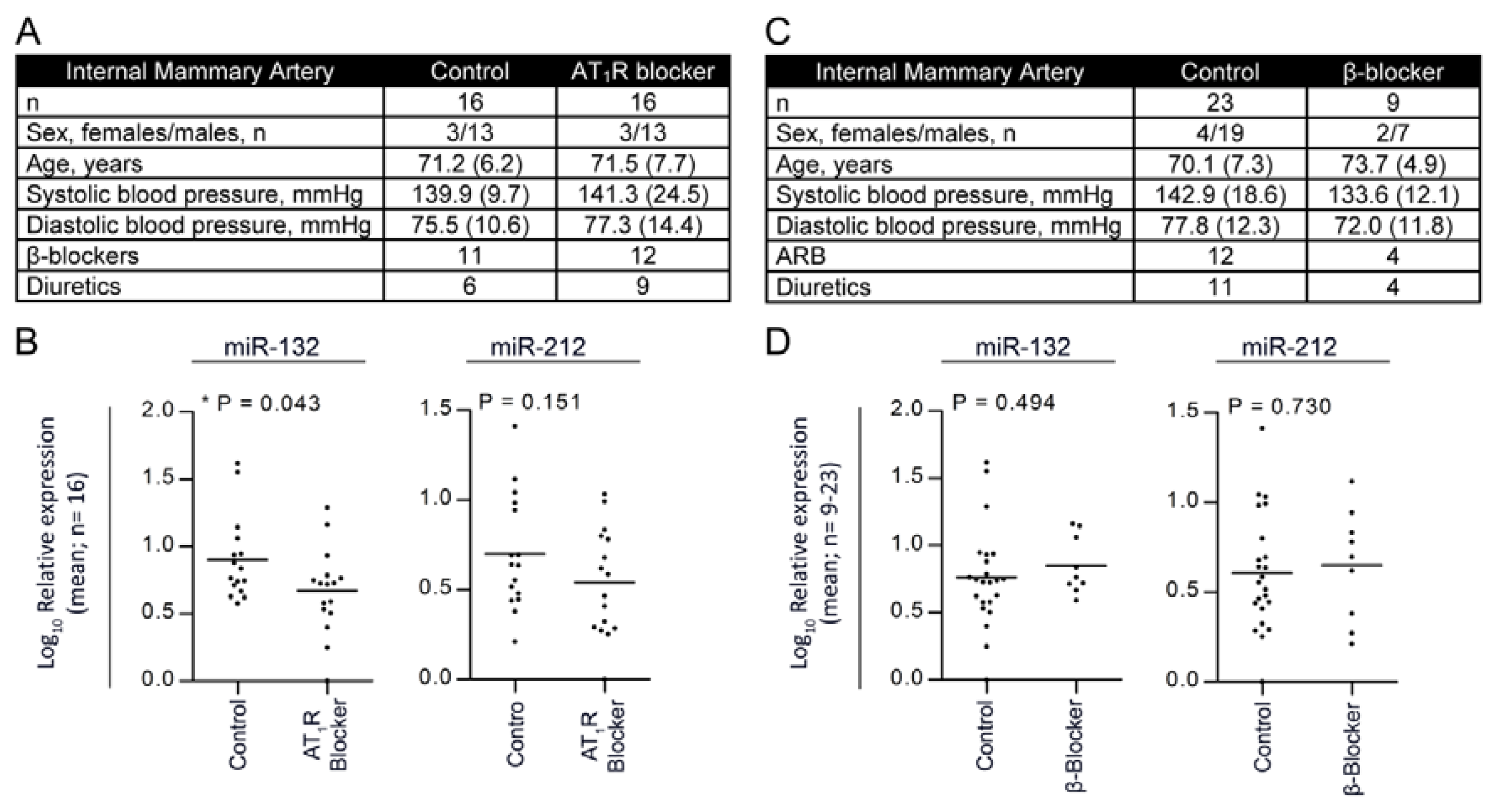
2.5. Treatment with Angiotensin II Receptor Blocker Attenuates the Expression of the miR-132/-212 Cluster in Human Hypertension
3. Discussion
4. Experimental Section
4.1. Animal Care
4.2. Angiotensin II (AngII) Model
4.3. Endothelin 1 (ET-1) Model
4.4. Mice AngII Model
4.5. Patients, Internal Mammary Artery Study
4.6. Sirius Red Staining
4.7. Microarray and Data Processing
4.8. mRNA and miRNA Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Information
ijms-14-11190-s001.pdfAcknowledgments
Conflict of Interest
References
- Coffman, T.M. Under pressure: The search for the essential mechanisms of hypertension. Nat. Med 2011, 17, 1402–1409. [Google Scholar]
- Batkai, S.; Thum, T. MicroRNAs in hypertension: Mechanisms and therapeutic targets. Curr. Hypertens. Rep 2012, 14, 79–87. [Google Scholar]
- Crowley, S.D.; Gurley, S.B.; Coffman, T.M. AT(1) receptors and control of blood pressure: The kidney and more. Trends Cardiovasc. Med 2007, 17, 30–34. [Google Scholar]
- Bader, M.; Ganten, D. Update on tissue renin-angiotensin systems. J. Mol. Med. (Berl. ) 2008, 86, 615–621. [Google Scholar]
- Hansen, J.L.; Aplin, M.; Hansen, J.T.; Christensen, G.L.; Bonde, M.M.; Schneider, M.; Haunso, S.; Schiffer, H.H.; Burstein, E.S.; Weiner, D.M.; et al. The human angiotensin AT(1) receptor supports G protein-independent extracellular signal-regulated kinase 1/2 activation and cellular proliferation. Eur. J. Pharmacol 2008, 590, 255–263. [Google Scholar]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar]
- Vo, N.; Klein, M.E.; Varlamova, O.; Keller, D.M.; Yamamoto, T.; Goodman, R.H.; Impey, S. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 16426–16431. [Google Scholar]
- Neyses, L.; Nouskas, J.; Luyken, J.; Fronhoffs, S.; Oberdorf, S.; Pfeifer, U.; Williams, R.S.; Sukhatme, V.P.; Vetter, H. Induction of immediate-early genes by angiotensin II and endothelin-1 in adult rat cardiomyocytes. J. Hypertens 1993, 11, 927–934. [Google Scholar]
- Christensen, G.L.; Kelstrup, C.D.; Lyngso, C.; Sarwar, U.; Bogebo, R.; Sheikh, S.P.; Gammeltoft, S.; Olsen, J.V.; Hansen, J.L. Quantitative phosphoproteomics dissection of seven-transmembrane receptor signaling using full and biased agonists. Mol. Cell Proteomics 2010, 9, 1540–1553. [Google Scholar]
- Olson, E.N. Gene regulatory networks in the evolution and development of the heart. Science 2006, 313, 1922–1927. [Google Scholar]
- Van Rooij, E.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Hill, J.; Olson, E.N. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 2007, 316, 575–579. [Google Scholar]
- Care, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med 2007, 13, 613–618. [Google Scholar]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar]
- Van Rooij, E.; Marshall, W.S.; Olson, E.N. Toward microRNA-based therapeutics for heart disease: the sense in antisense. Circ. Res 2008, 103, 919–928. [Google Scholar]
- Martin, M.M.; Lee, E.J.; Buckenberger, J.A.; Schmittgen, T.D.; Elton, T.S. MicroRNA-155 regulates human angiotensin II type 1 receptor expression in fibroblasts. J. Biol. Chem 2006, 281, 18277–18284. [Google Scholar]
- Zhu, N.; Zhang, D.; Chen, S.; Liu, X.; Lin, L.; Huang, X.; Guo, Z.; Liu, J.; Wang, Y.; Yuan, W.; et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis 2011, 215, 286–293. [Google Scholar]
- Van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar]
- Jeppesen, P.L.; Christensen, G.L.; Schneider, M.; Nossent, A.Y.; Jensen, H.B.; Andersen, D.C.; Eskildsen, T.; Gammeltoft, S.; Hansen, J.L.; Sheikh, S.P. Angiotensin II type 1 receptor signalling regulates microRNA differentially in cardiac fibroblasts and myocytes. Br. J. Pharmacol 2011, 164, 394–404. [Google Scholar]
- Nossent, A.Y.; Eskildsen, T.V.; Andersen, L.B.; Bie, P.; Brønnum, H.; Schneider, M.; Andersen, D.C.; Welten, S.M.J.; Jeppesen, P.L.; Hamming, J.F.; et al. The 14q32 MicroRNA-487b targets the anti-apoptotic insulin receptor substrate 1 in hypertension induced remodeling of the aorta. Ann. Surg. 2013, in press. [Google Scholar]
- Tognini, P.; Pizzorusso, T. MicroRNA212/132 family: Molecular transducer of neuronal function and plasticity. Int. J. Biochem. Cell Biol 2012, 44, 6–10. [Google Scholar]
- Mortensen, L.H.; Pawloski, C.M.; Kanagy, N.L.; Fink, G.D. Chronic hypertension produced by infusion of endothelin in rats. Hypertension 1990, 15, 729–733. [Google Scholar]
- Anand, S.; Majeti, B.K.; Acevedo, L.M.; Murphy, E.A.; Mukthavaram, R.; Scheppke, L.; Huang, M.; Shields, D.J.; Lindquist, J.N.; Lapinski, P.E.; et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat. Med 2010, 16, 909–914. [Google Scholar]
- Nossent, A.Y.; Hansen, J.L.; Doggen, C.; Quax, P.H.; Sheikh, S.P.; Rosendaal, F.R. SNPs in microRNA binding sites in 3′-UTRs of RAAS genes influence arterial blood pressure and risk of myocardial infarction. Am. J. Hypertens 2011, 24, 999–1006. [Google Scholar]
- Katare, R.; Riu, F.; Mitchell, K.; Gubernator, M.; Campagnolo, P.; Cui, Y.; Fortunato, O.; Avolio, E.; Cesselli, D.; Beltrami, A.P.; et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ. Res 2011, 109, 894–906. [Google Scholar]
- Nudelman, A.S.; DiRocco, D.P.; Lambert, T.J.; Garelick, M.G.; Le, J.; Nathanson, N.M.; Storm, D.R. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus 2010, 20, 492–498. [Google Scholar]
- Strum, J.C.; Johnson, J.H.; Ward, J.; Xie, H.; Feild, J.; Hester, A.; Alford, A.; Waters, K.M. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol. Endocrinol 2009, 23, 1876–1884. [Google Scholar]
- Jin, W.; Reddy, M.A.; Chen, Z.; Putta, S.; Lanting, L.; Kato, M.; Park, J.T.; Chandra, M.; Wang, C.; Tangirala, R.; et al. Small RNA sequencing reveals microRNAs that modulate angiotensin II effects in vascular smooth muscle cells. J. Biol. Chem 2012, 287, 15672–15683. [Google Scholar]
- Cassis, L.A.; Huang, J.; Gong, M.C.; Daugherty, A. Role of metabolism and receptor responsiveness in the attenuated responses to Angiotensin II in mice compared to rats. Regul. Pept 2004, 117, 107–116. [Google Scholar]
- Rautureau, Y.; Schiffrin, E.L. Endothelin in hypertension: An update. Curr. Opin. Nephrol. Hypertens 2012, 21, 128–136. [Google Scholar]
- Gray, G.A.; Webb, D.J. Molecular Biology and Pharmacology of the Endothelins; Springer & R.G. Landes, Co.: New York, NY, USA, 1995; pp. 116–145. [Google Scholar]
- Roberts, R.E. The extracellular signal-regulated kinase (ERK) pathway: A potential therapeutic target in hypertension. J. Exp. Pharmacol 2012, 4, 77–83. [Google Scholar]
- Zaman, M.A.; Oparil, S.; Calhoun, D.A. Drugs targeting the renin-angiotensin-aldosterone system. Nat. Rev. Drug Discov 2002, 1, 621–636. [Google Scholar]
- Bruner, C.A.; Mangiapane, M.L.; Fink, G.D. Subfornical organ. Does it protect against angiotensin II-induced hypertension in the rat? Circ. Res 1985, 56, 462–466. [Google Scholar]
- Mortensen, L.H.; Fink, G.D. Captopril prevents chronic hypertension produced by infusion of endothelin-1 in rats. Hypertension 1992, 19, 676–680. [Google Scholar]
- Cangemi, C.; Skov, V.; Poulsen, M.K.; Funder, J.; Twal, W.O.; Gall, M.A.; Hjortdal, V.; Jespersen, M.L.; Kruse, T.A.; Aagard, J.; et al. Fibulin-1 is a marker for arterial extracellular matrix alterations in type 2 diabetes. Clin. Chem 2011, 57, 1556–1565. [Google Scholar]
- Andersen, D.C.; Jensen, C.H.; Schneider, M.; Nossent, A.Y.; Eskildsen, T.; Hansen, J.L.; Teisner, B.; Sheikh, S.P. MicroRNA-15a fine-tunes the level of Delta-like 1 homolog (DLK1) in proliferating 3T3-L1 preadipocytes. Exp. Cell Res 2010, 316, 1681–1691. [Google Scholar]
- Andersen, D.C.; Andersen, P.; Schneider, M.; Jensen, H.B.; Sheikh, S.P. Murine “cardiospheres” are not a source of stem cells with cardiomyogenic potential. Stem Cells 2009, 27, 1571–1581. [Google Scholar]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002, 3, RESEARCH0034. [Google Scholar]
- Hellemans, J.; Mortier, G.; de Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 2007, 8, R19. [Google Scholar]

| Microarray: miRNAs regulated by AngII | ||
|---|---|---|
| Name of miRNA | Log fold change | p-value |
| ***p < 0.001 | ||
| 21 | 0.7016 | 1.89 × 10−5 |
| 132 | 0.1261 | 5.90 × 10−5 |
| 105 | 0.1883 | 9.28 × 10−5 |
| 155 | 0.1425 | 0.00012 |
| 221 | 0.3414 | 0.00052 |
| 223 | 0.4459 | 0.00085 |
| 208b | 0.5560 | 0.00089 |
| **p < 0.01 | ||
| 222 | 0.1681 | 0.0022 |
| 147b | 0.1151 | 0.0032 |
| 26b | −0.1153 | 0.0034 |
| 15b | 0.2580 | 0.0057 |
| 613 | −0.1047 | 0.0065 |
| 31* | 0.1054 | 0.0075 |
| 520b | 0.0938 | 0.0082 |
| 30c-1* | −0.1409 | 0.0084 |
| 18b | 0.1334 | 0.0092 |
| *p < 0.05 | ||
| 301a | 0.1537 | 0.010 |
| 143 | 0.1242 | 0.011 |
| 434-5p | 0.1692 | 0.012 |
| 484 | 0.0855 | 0.014 |
| 155 | 0.1135 | 0.014 |
| 379 | 0.0813 | 0.015 |
| 29c | −0.2193 | 0.017 |
| 936 | 0.1682 | 0.018 |
| 199a-5p | 0.1523 | 0.021 |
| 201 | −0.1204 | 0.021 |
| 101 | −0.1860 | 0.021 |
| 363* | 0.2352 | 0.021 |
| 760 | −0.0944 | 0.022 |
| 944 | 0.1056 | 0.023 |
| 200b* | 0.1079 | 0.024 |
| 30b | −0.0888 | 0.025 |
| 322 | −0.1890 | 0.026 |
| 337-3p | 0.1065 | 0.026 |
| 29c* | −0.1247 | 0.028 |
| 302c* | 0.1453 | 0.030 |
| 193a-3p | 0.1665 | 0.030 |
| 888 | −0.0852 | 0.033 |
| 299-3p | 0.2128 | 0.035 |
| 142-3p | 0.3121 | 0.035 |
| 517* | 0.0746 | 0.041 |
| 31 | 0.3286 | 0.042 |
| 194 | −0.0860 | 0.042 |
| 701 | 0.0671 | 0.042 |
| 545 | −0.0834 | 0.043 |
| 609 | 0.0617 | 0.044 |
| 141* | −0.0928 | 0.045 |
| 211 | 0.0588 | 0.047 |
| 373 | 0.0712 | 0.048 |
| 218-1* | −0.0678 | 0.049 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Eskildsen, T.V.; Jeppesen, P.L.; Schneider, M.; Nossent, A.Y.; Sandberg, M.B.; Hansen, P.B.L.; Jensen, C.H.; Hansen, M.L.; Marcussen, N.; Rasmussen, L.M.; et al. Angiotensin II Regulates microRNA-132/-212 in Hypertensive Rats and Humans. Int. J. Mol. Sci. 2013, 14, 11190-11207. https://doi.org/10.3390/ijms140611190
Eskildsen TV, Jeppesen PL, Schneider M, Nossent AY, Sandberg MB, Hansen PBL, Jensen CH, Hansen ML, Marcussen N, Rasmussen LM, et al. Angiotensin II Regulates microRNA-132/-212 in Hypertensive Rats and Humans. International Journal of Molecular Sciences. 2013; 14(6):11190-11207. https://doi.org/10.3390/ijms140611190
Chicago/Turabian StyleEskildsen, Tilde V., Pia L. Jeppesen, Mikael Schneider, Anne Y. Nossent, Maria B. Sandberg, Pernille B. L. Hansen, Charlotte H. Jensen, Maria L. Hansen, Niels Marcussen, Lars M. Rasmussen, and et al. 2013. "Angiotensin II Regulates microRNA-132/-212 in Hypertensive Rats and Humans" International Journal of Molecular Sciences 14, no. 6: 11190-11207. https://doi.org/10.3390/ijms140611190





