Myricetin-Mediated Lifespan Extension in Caenorhabditis elegans Is Modulated by DAF-16
Abstract
:1. Introduction
Aim of the study
2. Results and Discussion
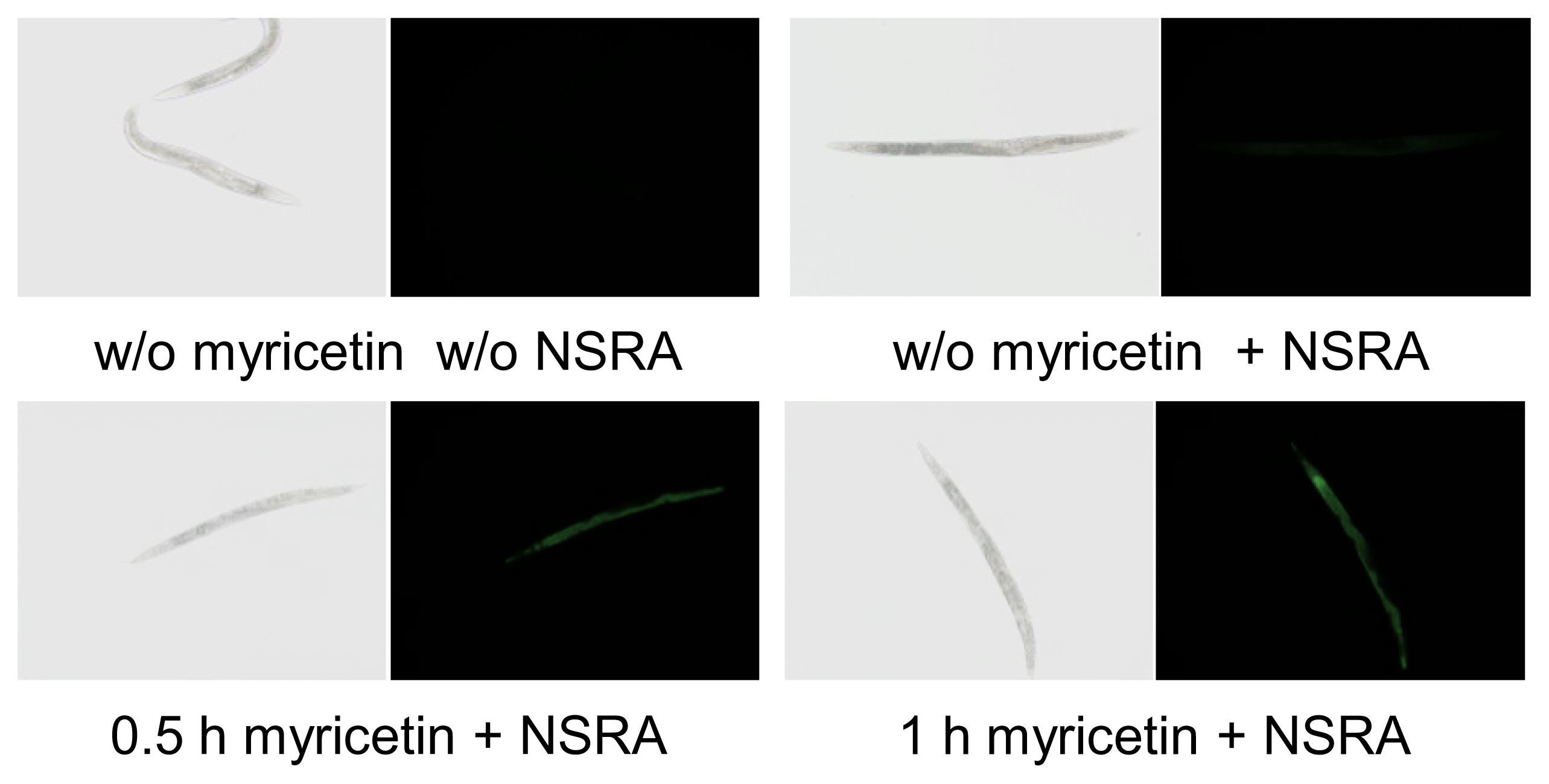
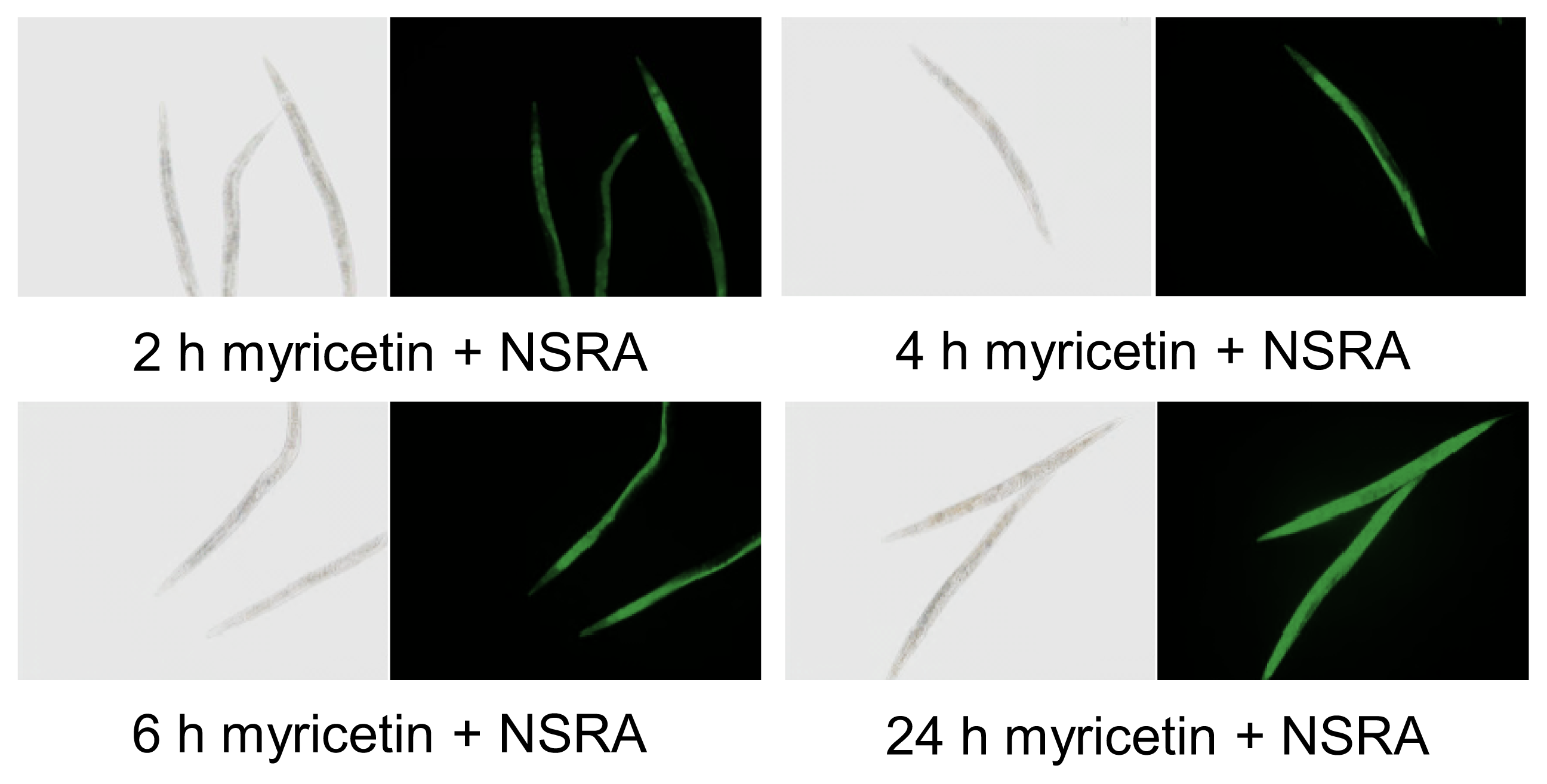
2.1. Uptake of Myricetin by C. elegans
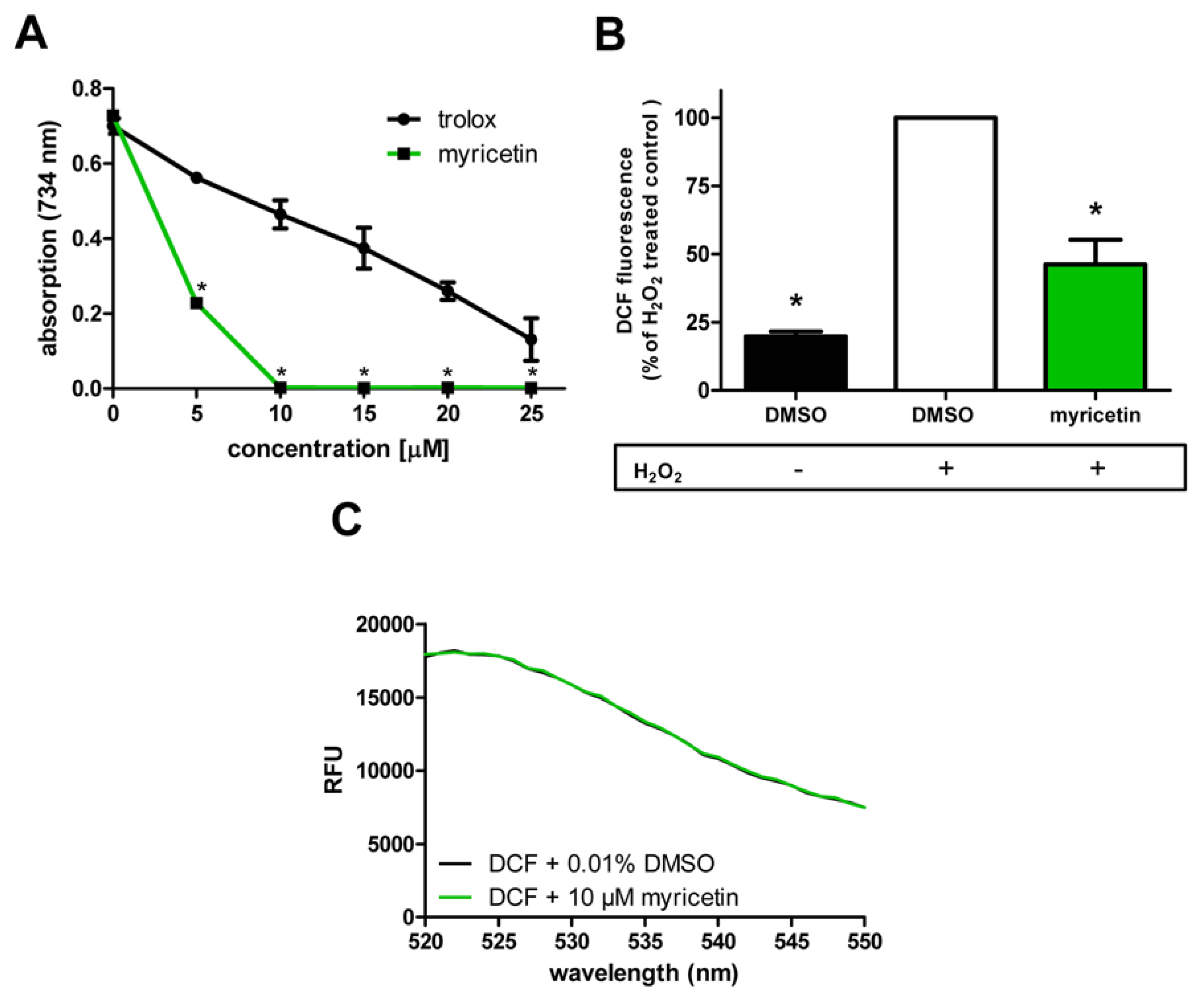
2.2. Anti-Oxidative Effects of Myricetin
2.2.1. Anti-Oxidative Effects of Myricetin in Cell-Free System/in Hct116 Cells
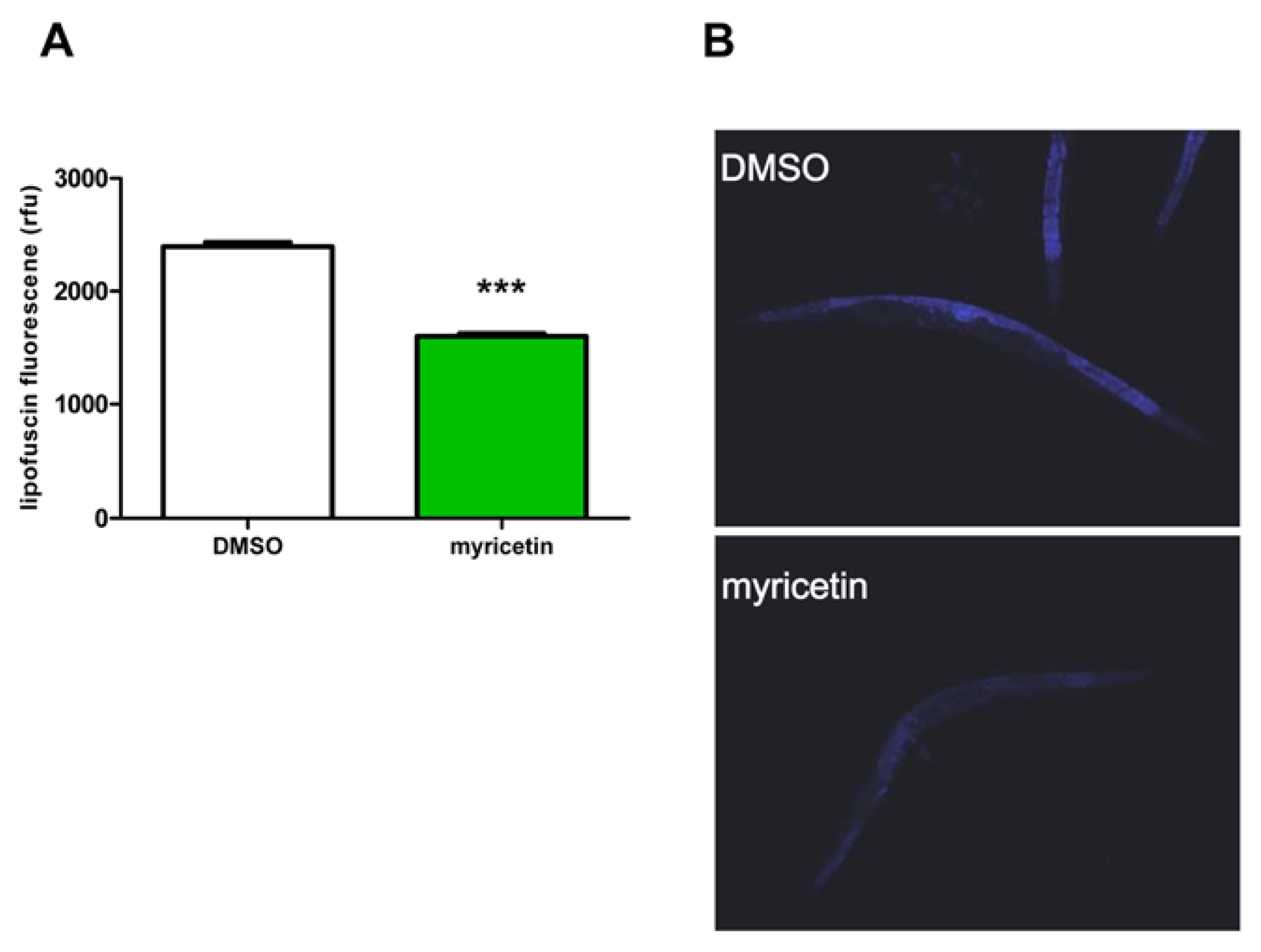
2.2.2. Effect of Myricetin on Lipofuscin Accumulation in C. elegans
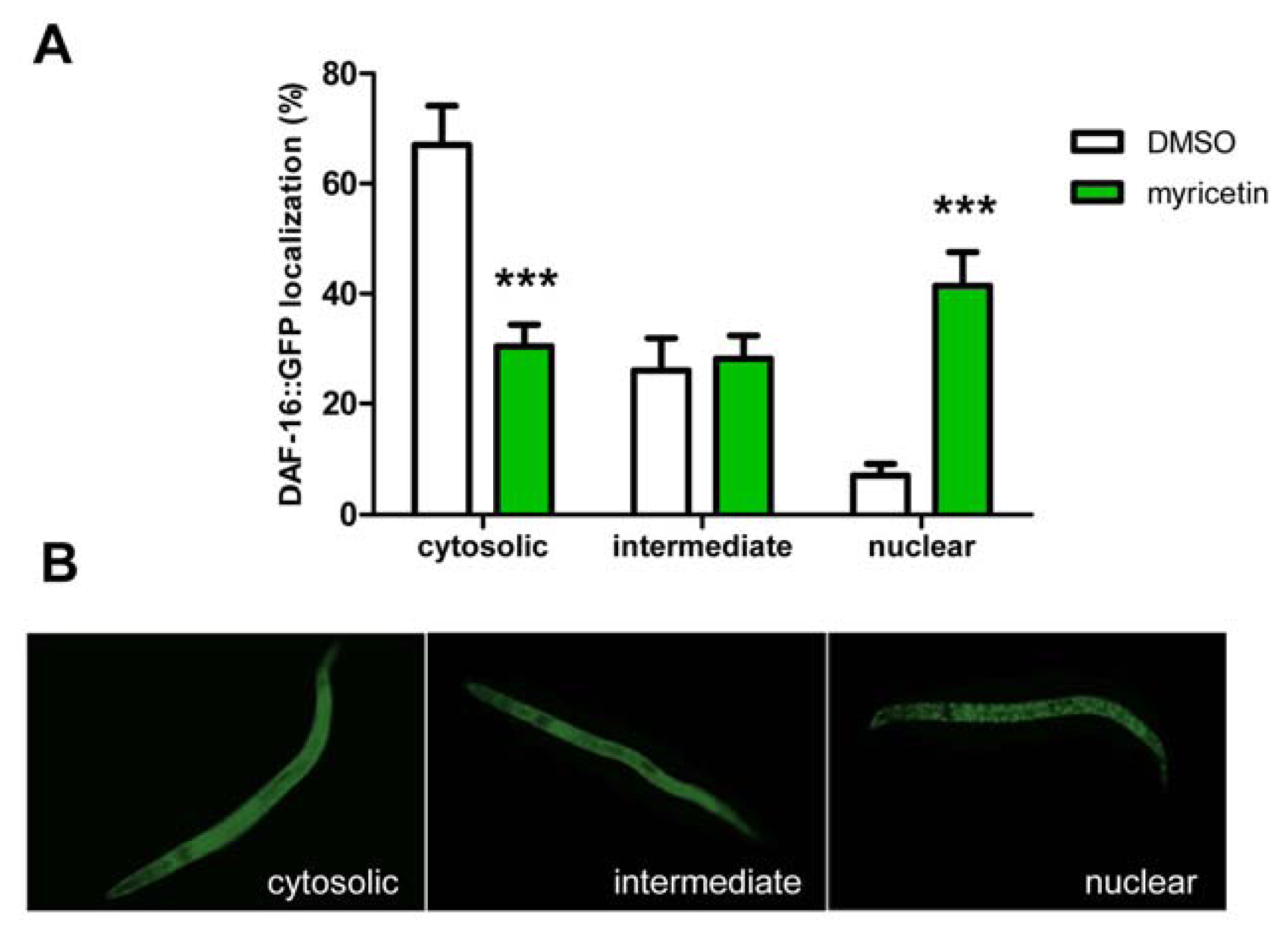
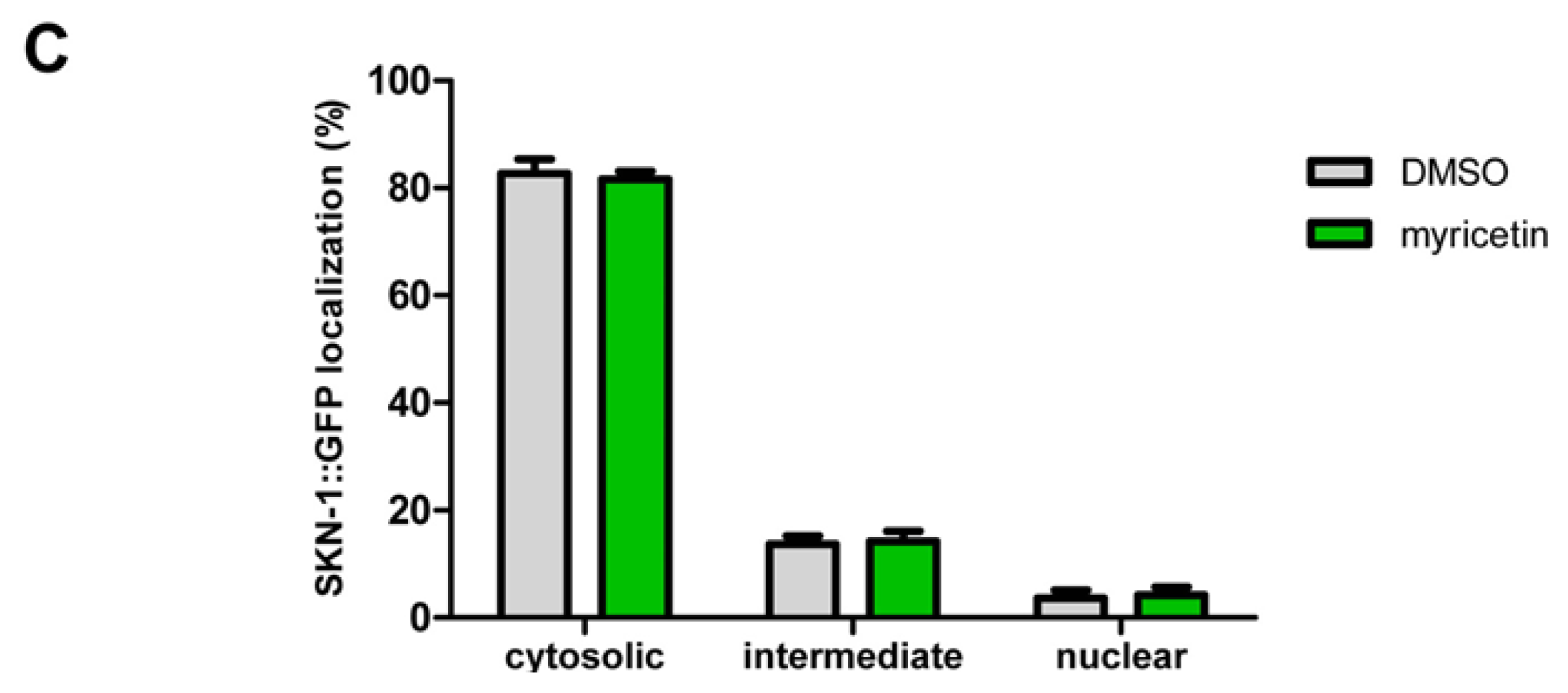
2.2.3. Effect of Myricetin on Translocation of DAF-16 and SKN-1
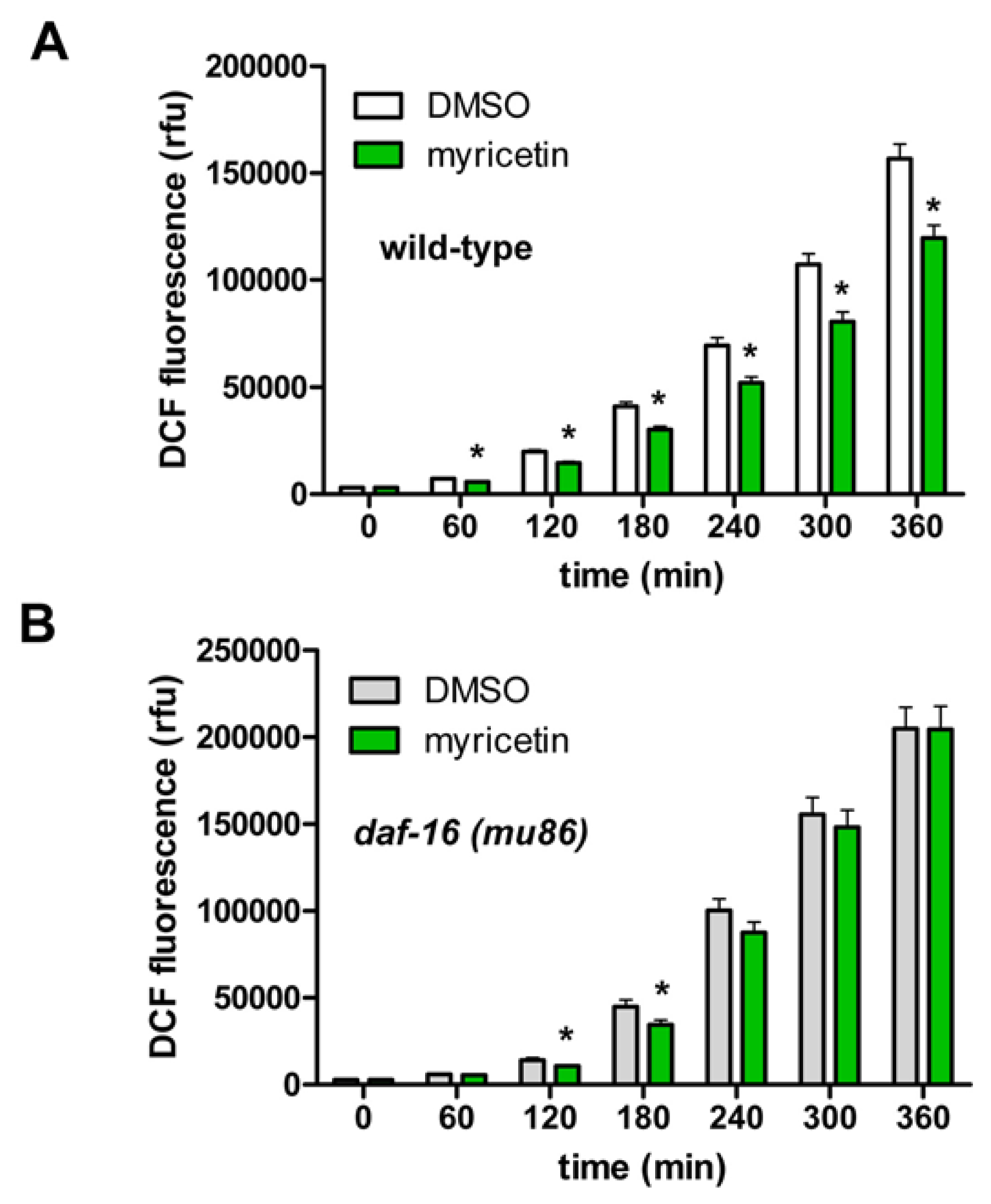
2.2.4. Requirement of DAF-16 for the Anti-oxidative Effects of Myricetin in C. elegans
2.3. DAF-16 Is Required for Myricetin-Induced Prolongation of Lifespan
2.4. Myricetin Has No Effect on Resistance against Thermal Stress
2.5. Effects of Myricetin Are Not Mediated by Caloric Restriction
3. Experimental Section
3.1. C. elegans Strains and Maintenance
3.2. In Vivo Visualization of Myricetin: Fluorescent Staining with 2-Aminoethyl Diphenylborinate
3.3. Determination of Lipofuscin Accumulation
3.4. Intracellular Localization of DAF-16::GFP and SKN-1::GFP
3.5. Lifespan Assays
3.6. Measurement of Intracellular ROS Accumulation in C. elegans
3.7. Cell Culture and Measurement of ROS Accumulation in Hct116 Cells
3.8. Measurement of Fluorescence Emission Spectra
3.9. Trolox Equivalent Anti-Oxidative Capacity (TEAC) Assay
3.10. Thermotolerance Assay
3.11. Pharyngeal Pumping Assay
3.12. Determination of Body Size
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci 1999, 65, 337–359. [Google Scholar]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr 2003, 133, 3248S–3254S. [Google Scholar]
- Wätjen, W.; Chovolou, Y.; Kampkötter, A.; Kahl, R. Anti- and Prooxidative Effects of Flavonoids. In Leading Edge Antioxidants Research; Panglossi, H.V., Ed.; Nova Science Publishers Inc: New York, NY, USA, 2009; pp. 1–16. [Google Scholar]
- Chobot, V.; Hadacek, F. Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin. Redox Rep 2011, 16, 242–247. [Google Scholar]
- Qin, S.; Chen, J.; Tanigawa, S.; Hou, D.X. Microarray and pathway analysis highlight Nrf2/ARE-mediated expression profiling by polyphenolic myricetin. Mol. Nutr. Food Res 2013, 57, 435–446. [Google Scholar]
- Phillips, P.A.; Sangwan, V.; Borja-Cacho, D.; Dudeja, V.; Vickers, S.M.; Saluja, A.K. Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signalling pathway. Cancer Lett 2011, 308, 181–188. [Google Scholar]
- Chen, W.; Li, Y.; Li, J.; Han, Q.; Ye, L.; Li, A. Myricetin affords protection against peroxynitrite-mediated DNA damage and hydroxyl radical formation. Food Chem. Toxicol 2011, 49, 2439–2444. [Google Scholar]
- Kang, N.J.; Jung, S.K.; Lee, K.W.; Lee, H.J. Myricetin is a potent chemopreventive phytochemical in skin carcinogenesis. Ann. N. Y. Acad. Sci 2011, 1229, 124–132. [Google Scholar]
- Duthie, G.; Morrice, P. Antioxidant capacity of flavonoids in hepatic microsomes is not reflected by antioxidant effects in vivo. Oxid. Med. Cell Longev 2012, 2012, 165127. [Google Scholar]
- Tzeng, T.F.; Liou, S.S.; Liu, I.M. Myricetin ameliorates defective post-receptor insulin signalling via β-endorphin signalling in the skeletal muscles of fructose-fed rats. Evid. Based Complement. Alternat. Med 2011, 2011, 150752. [Google Scholar]
- Kampkötter, A.; Timpel, C.; Zurawski, R.F.; Ruhl, S.; Chovolou, Y.; Proksch, P.; Wätjen, W. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp. Biochem. Physiol. B Biochem. Mol Biol 2008, 149, 314–323. [Google Scholar]
- Saul, N.; Pietsch, K.; Menzel, R.; Steinberg, C.E. Quercetin-mediated longevity in Caenorhabditis elegans: Is DAF-16 involved? Mech. Ageing Dev 2008, 129, 611–613. [Google Scholar]
- Zarse, K.; Schmeisser, S.; Birringer, M.; Falk, E.; Schmoll, D.; Ristow, M. Differential effects of resveratrol and SRT1720 on lifespan of adult Caenorhabditis elegans. Horm. Metab. Res 2010, 42, 837–839. [Google Scholar]
- Morselli, E.; Maiuri, M.C.; Markaki, M.; Megalou, E.; Pasparaki, A.; Palikaras, K.; Criollo, A.; Galluzzi, L.; Malik, S.A.; Vitale, I.; et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010, 1. [Google Scholar] [CrossRef]
- Viswanathan, M.; Kim, S.K.; Berdichevsky, A.; Guarente, L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev. Cell 2005, 9, 605–615. [Google Scholar]
- Bartholome, A.; Kampkötter, A.; Tanner, S.; Sies, H.; Klotz, L.O. Epigallocatechin gallate-induced modulation of FoxO signalling in mammalian cells and C. elegans: FoxO stimulation is masked via PI3K/Akt activation by hydrogen peroxide formed in cell culture. Arch. Biochem. Biophys 2010, 501, 58–64. [Google Scholar]
- Abbas, S.; Wink, M. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med 2009, 75, 216–221. [Google Scholar]
- Saul, N.; Pietsch, K.; Menzel, R.; Stürzenbaum, S.R.; Steinberg, C.E. Catechin induced longevity in C. elegans: From key regulator genes to disposable soma. Mech. Ageing Dev 2009, 130, 477–486. [Google Scholar]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–86. [Google Scholar]
- Grünz, G.; Haas, K.; Soukup, S.; Klingenspor, M.; Kulling, S.E.; Daniel, H.; Spanier, B. Structural features and bioavailability of four flavonoids and their implications for lifespan-extending and antioxidant actions in C. elegans. Mech. Ageing Dev 2012, 133, 1–10. [Google Scholar]
- Ong, K.C.; Khoo, H.E. Biological effects of myricetin. Gen. Pharmacol 1997, 29, 121–126. [Google Scholar]
- Wang, Z.H.; Ah Kang, K.; Zhang, R.; Piao, M.J.; Jo, S.H.; Kim, J.S.; Kang, S.S.; Lee, J.S.; Park, D.H.; Hyun, J.W. Myricetin suppresses oxidative stress-induced cell damage via both direct and indirect antioxidant action. Environ. Toxicol. Pharmacol 2010, 29, 12–18. [Google Scholar]
- Kang, K.A.; Wang, Z.H.; Zhang, R.; Piao, M.J.; Kim, K.C.; Kang, S.S.; Kim, Y.W.; Lee, J.; Park, D.; Hyun, J.W. Myricetin protects cells against oxidative stress-induced apoptosis via regulation of PI3K/Akt and MAPK signalling pathways. Int. J. Mol. Sci 2010, 11, 4348–4360. [Google Scholar]
- Jung, T.; Bader, N.; Grune, T. Lipofuscin: Formation, distribution, and metabolic consequences. Ann. N. Y. Acad. Sci 2007, 1119, 97–111. [Google Scholar]
- Hosokawa, H.; Ishii, N.; Ishida, H.; Ichimori, K.; Nakazawa, H.; Suzuki, K. Rapid accumulation of fluorescent material with aging in an oxygen-sensitive mutant mev-1 of Caenorhabditis elegans. Mech. Ageing Dev 1994, 74, 161–170. [Google Scholar]
- Gerstbrein, B.; Stamatas, G.; Kollias, N.; Driscoll, M. In vivospectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell 2005, 4, 127–137. [Google Scholar]
- Honnen, S.J.; Büchter, C.; Schröder, V.; Hoffmann, M.; Kohara, Y.; Kampkötter, A.; Bossinger, O. C. elegans VANG-1 modulates life span via insulin/IGF-1-like signalling. PLoS One 2012, 7, e32183. [Google Scholar]
- Kampkötter, A.; Nkwonkam, C.G.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology 2007, 234, 113–123. [Google Scholar]
- Kampkötter, A.; Gombitang Nkwonkam, C.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch. Toxicol 2007, 81, 849–858. [Google Scholar]
- Pietsch, K.; Saul, N.; Chakrabarti, S.; Stürzenbaum, S.R.; Menzel, R.; Steinberg, C.E. Hormetins, antioxidants and prooxidants: Defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology 2011, 12, 329–347. [Google Scholar]
- Kawasaki, I.; Jeong, M.H.; Oh, B.K.; Shim, Y.H. Apigenin inhibits larval growth of Caenorhabditis elegans through DAF-16 activation. FEBS Lett 2010, 584, 3587–3591. [Google Scholar]
- Havermann, S.; Rohrig, R.; Chovolou, Y.; Humpf, H.U.; Wätjen, W. Molecular effects of baicalein in Hct116 cells and Caenorhabditis elegans: Activation of the Nrf2 signalling pathway and prolongation of lifespan. J. Agric. Food Chem 2013, 61, 2158–2164. [Google Scholar]
- Zhang, L.; Jie, G.; Zhang, J.; Zhao, B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic. Biol. Med 2009, 46, 414–421. [Google Scholar]
- Liao, V.H.C.; Yu, C.W.; Chu, Y.J.; Li, W.H.; Hsieh, Y.C.; Wang, T.T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech. Ageing Dev 2011, 132, 480–487. [Google Scholar]
- Houthoofd, K.; Johnson, T.E.; Vanfleteren, J.R. Dietary restriction in the nematode Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci 2005, 60, 1125–1131. [Google Scholar]
- Piper, M.D.; Bartke, A. Diet and aging. Cell Metab 2008, 8, 99–104. [Google Scholar]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med 1999, 26, 1231–1237. [Google Scholar]
- Gill, M.S.; Olsen, A.; Sampayo, J.N.; Lithgow, G.J. An automated high-throughput assay for survival of the nematode Caenorhabditis elegans. Free Radic. Biol. Med 2003, 35, 558–565. [Google Scholar]
- Kampkötter, A.; Pielarski, T.; Rohrig, R.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. The Ginkgo biloba extract EGb761 reduces stress sensitivity, ROS accumulation and expression of catalase and glutathione S-transferase 4 in Caenorhabditis elegans. Pharmacol. Res 2007, 55, 139–147. [Google Scholar]
- Raizen, D.M.; Lee, R.Y.; Avery, L. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics 1995, 141, 1365–1382. [Google Scholar]




| Strain | Exp. | Treatment | Mean lifespan (days) | SE | n (censored) | % Difference | p value |
|---|---|---|---|---|---|---|---|
| wild-type | 3 | DMSO | 18.7 | 0.9 | 90 (20) | 32.9 | <0.001 |
| wild-type | 3 | myricetin | 24.8 | 0.9 | 90 (18) | ||
| daf-16 (mu86) | 3 | DMSO | 15.1 | 0.5 | 153 (46) | 0.8 | 0.788 |
| daf-16 (mu86) | 3 | myricetin | 15.2 | 0.5 | 150 (46) | ||
| Strain | Exp. | Treatment | Mean lifespan (hours) | SE | n | % Difference | p value |
|---|---|---|---|---|---|---|---|
| wild-type | 3 | DMSO | 3.94 | 0.09 | 48 | 1.8 | 0.473 |
| wild-type | 3 | myricetin | 4.01 | 0.12 | 47 | ||
| daf-16 (mu86) | 4 | DMSO | 3.84 | 0.12 | 64 | 2.1 | 0.403 |
| daf-16 (mu86) | 4 | myricetin | 3.76 | 0.09 | 64 | ||
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Büchter, C.; Ackermann, D.; Havermann, S.; Honnen, S.; Chovolou, Y.; Fritz, G.; Kampkötter, A.; Wätjen, W. Myricetin-Mediated Lifespan Extension in Caenorhabditis elegans Is Modulated by DAF-16. Int. J. Mol. Sci. 2013, 14, 11895-11914. https://doi.org/10.3390/ijms140611895
Büchter C, Ackermann D, Havermann S, Honnen S, Chovolou Y, Fritz G, Kampkötter A, Wätjen W. Myricetin-Mediated Lifespan Extension in Caenorhabditis elegans Is Modulated by DAF-16. International Journal of Molecular Sciences. 2013; 14(6):11895-11914. https://doi.org/10.3390/ijms140611895
Chicago/Turabian StyleBüchter, Christian, Daniela Ackermann, Susannah Havermann, Sebastian Honnen, Yvonni Chovolou, Gerhard Fritz, Andreas Kampkötter, and Wim Wätjen. 2013. "Myricetin-Mediated Lifespan Extension in Caenorhabditis elegans Is Modulated by DAF-16" International Journal of Molecular Sciences 14, no. 6: 11895-11914. https://doi.org/10.3390/ijms140611895




