A Novel Perspective and Approach to Intestinal Octreotide Absorption: Sinomenine-Mediated Reversible Tight Junction Opening and Its Molecular Mechanism
Abstract
:1. Introduction
2. Results and Discussion
2.1. Everted Gut Sacs Studies in Rats
2.2. Intravenous (i.v.) and Oral Pharmacokinetic Studies in Rats
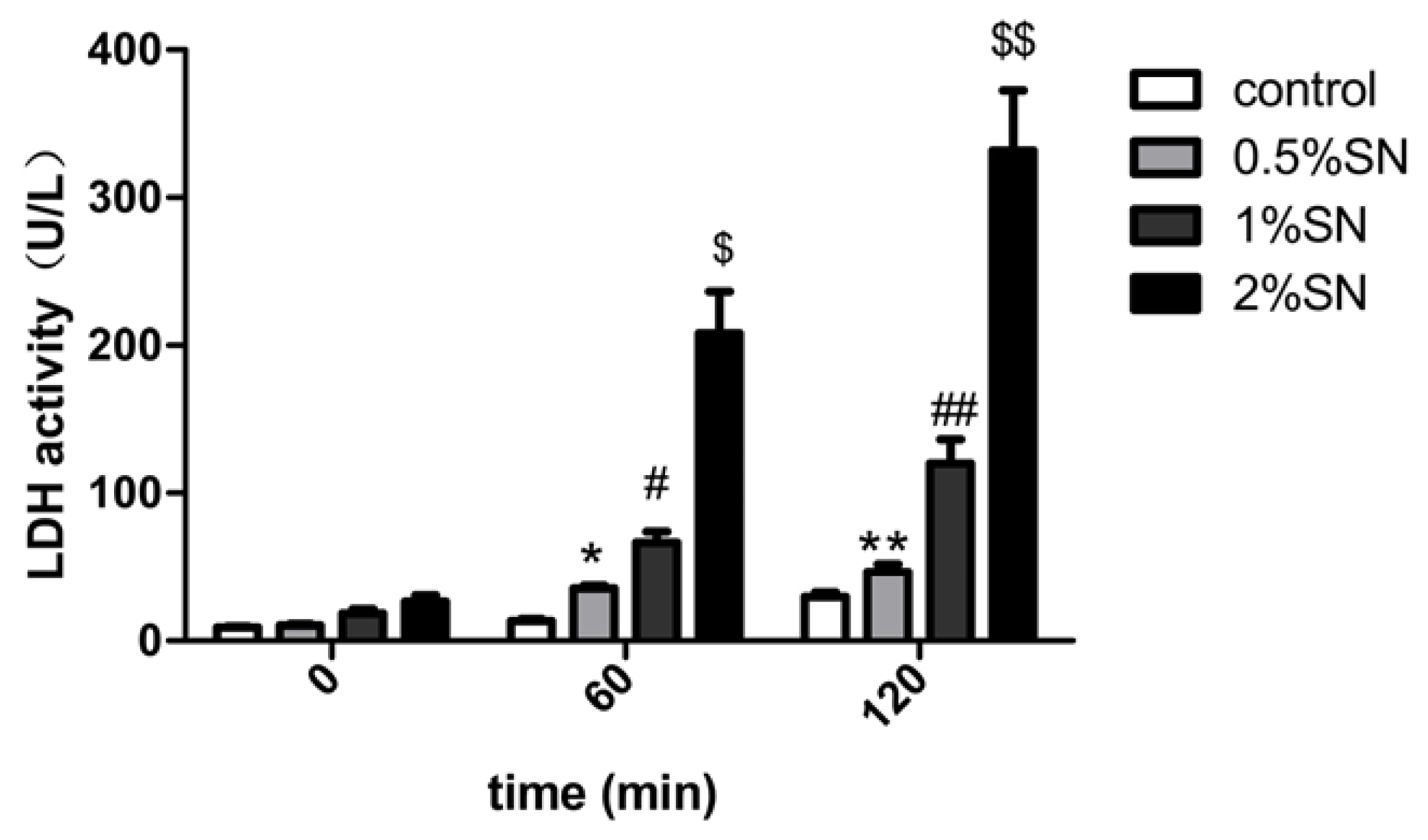
2.3. The Cytotoxic Effect of SN on Caco-2 Cell Monolayers
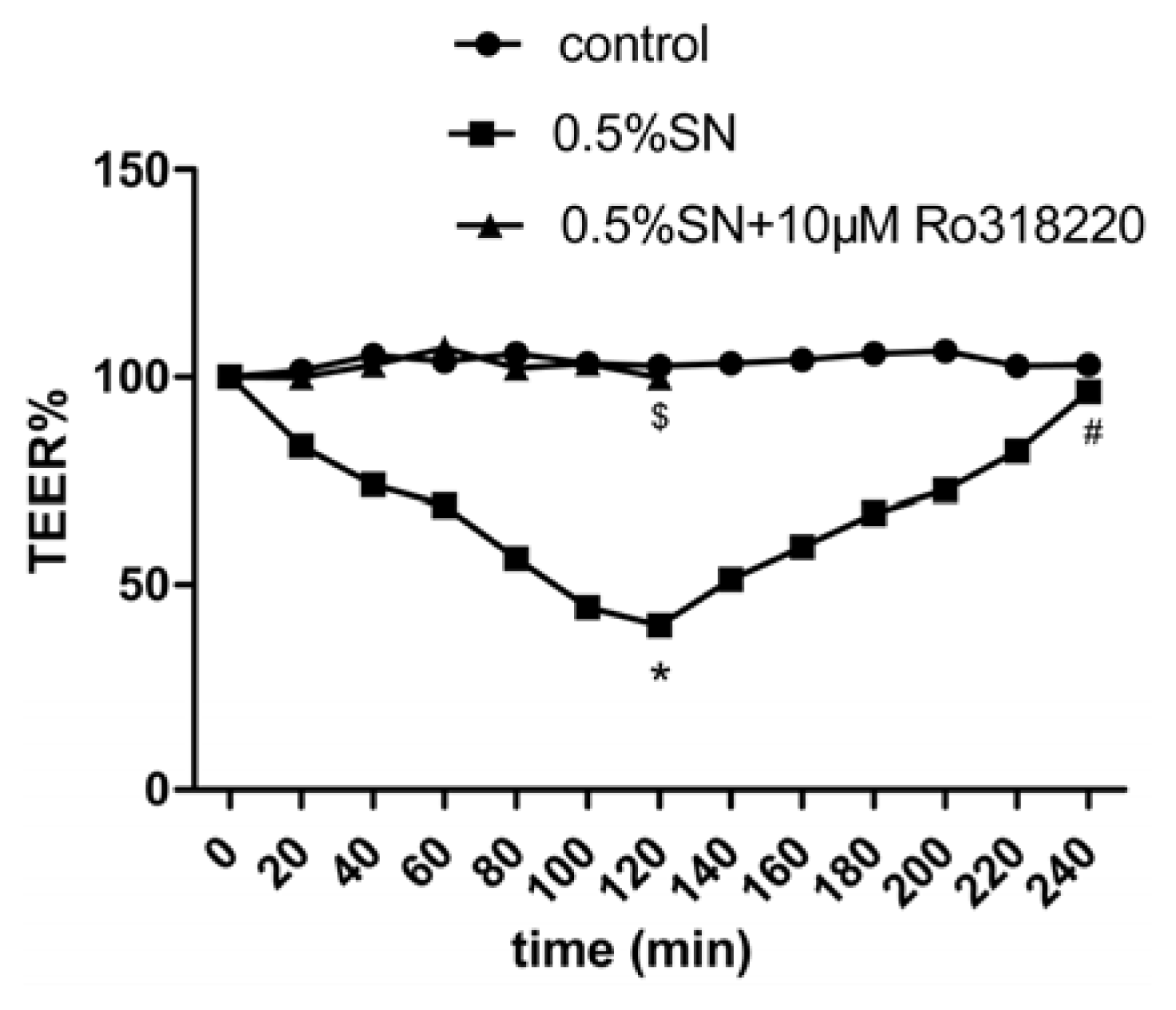
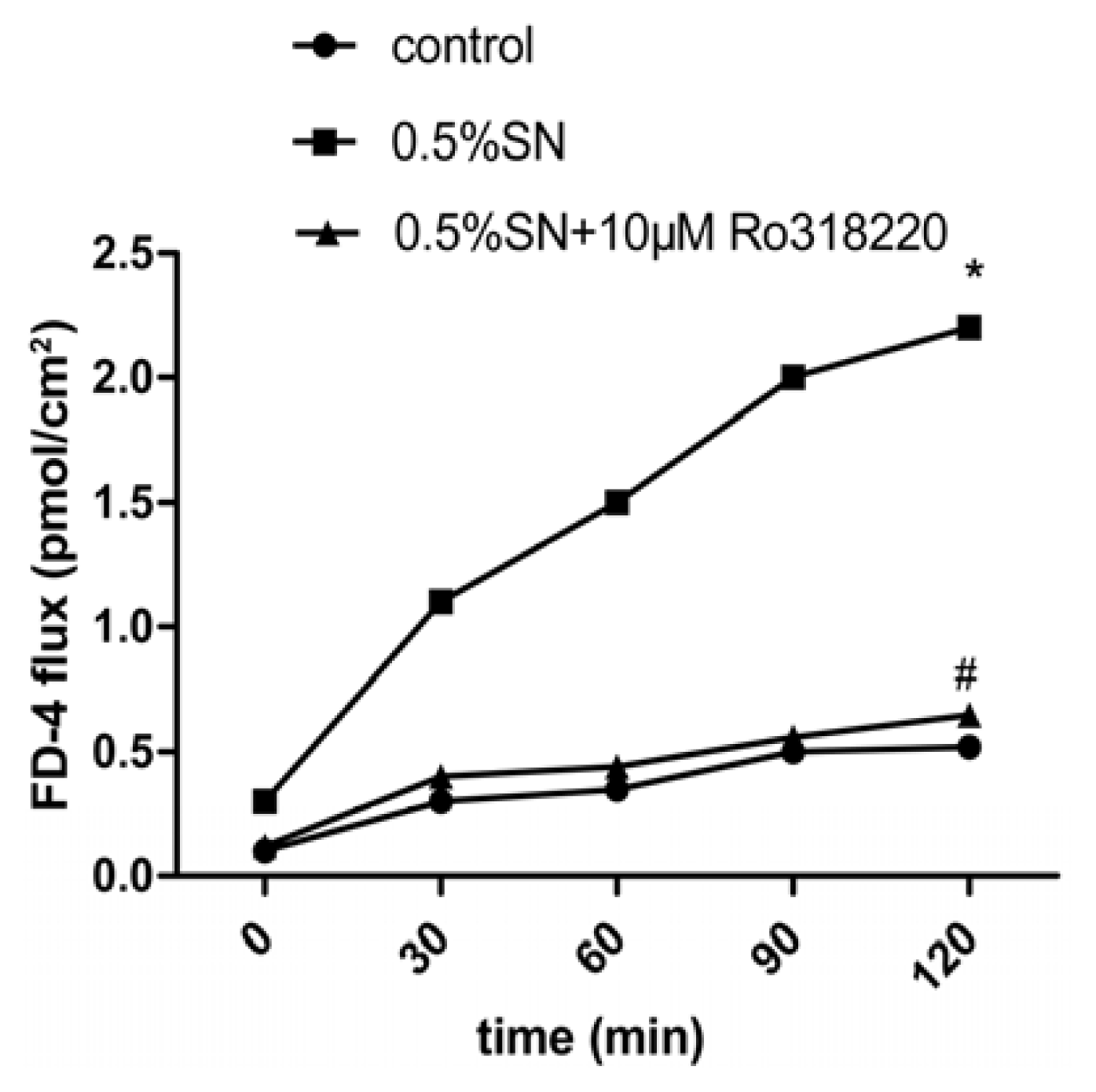
2.4. Effect of SN on the TEER and FD-4 Permeability of Caco-2 Cell Monolayers
2.5. Effect of Sinomenine on the Transport of OCT across Caco-2 Cell Monolayers
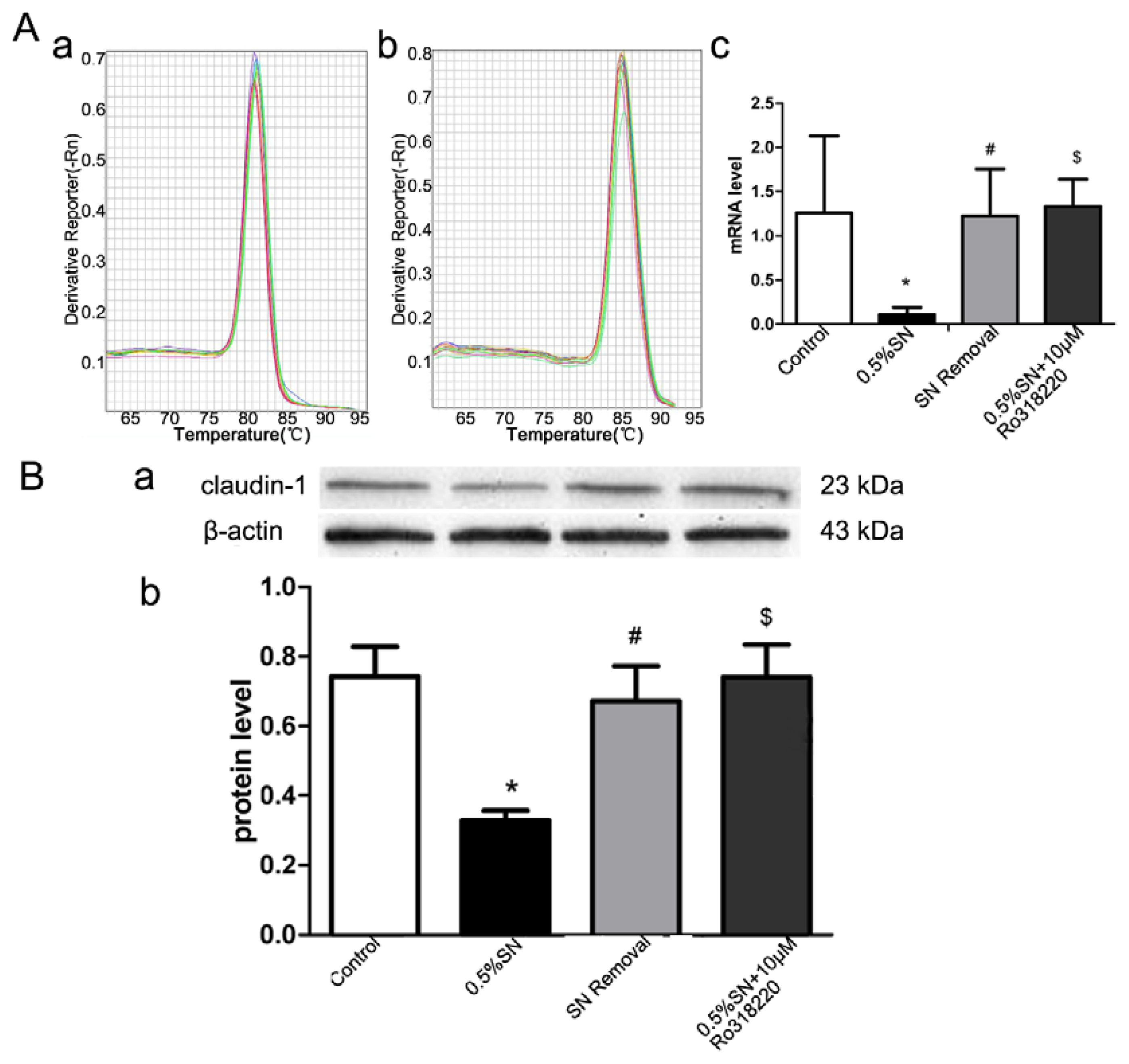
2.6. Gene and Protein Levels for the Expression of Claudin-1
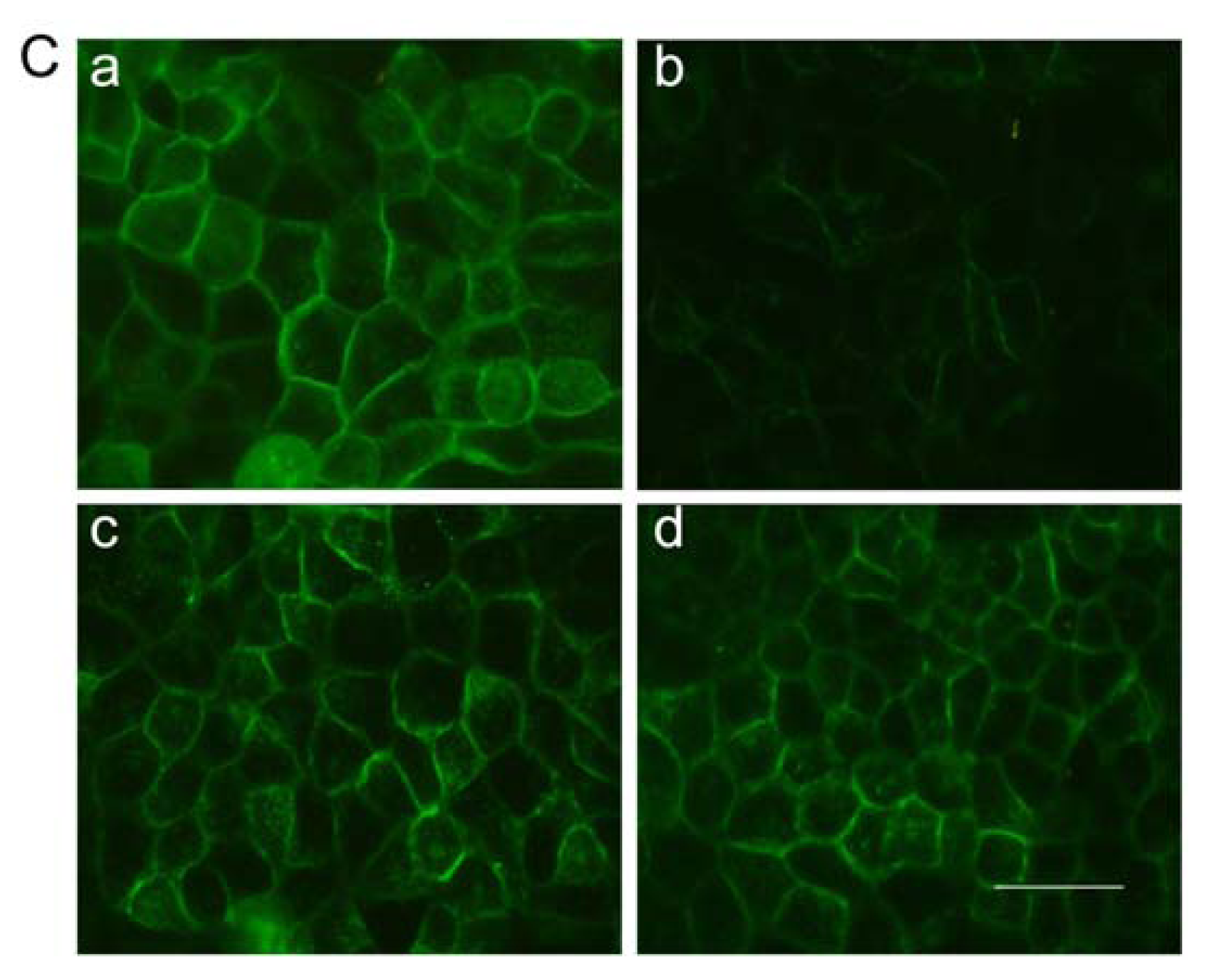
2.7. Immunofluorescent Staining for Claudin-1
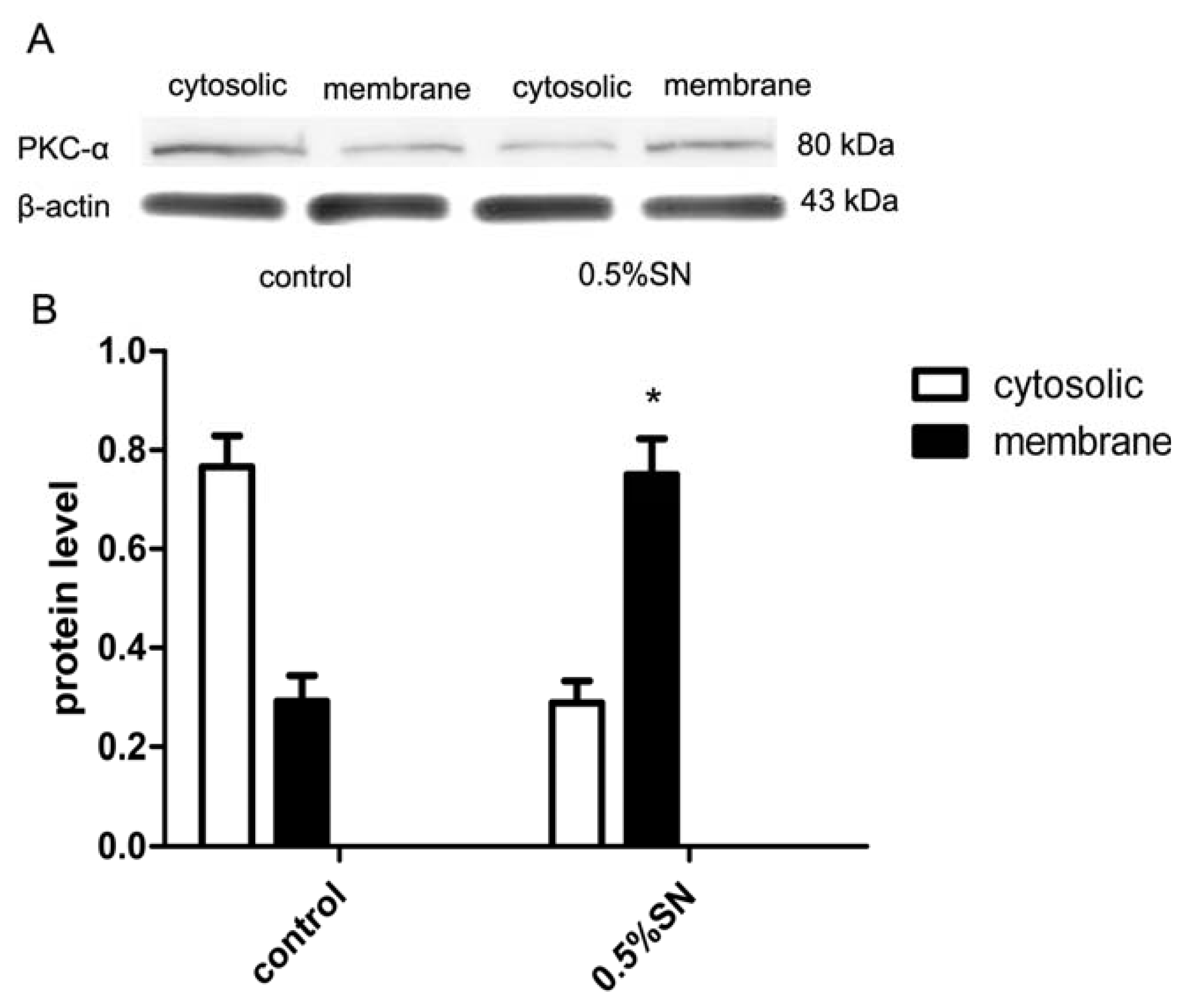
2.8. Mechanism of SN-Mediated Reversible TJ Opening—Shift of PKC-α
2.9. Discussion
3. Experimental Section
3.1. Materials
3.2. Animals Ethics
3.3. Methods
3.3.1. Everted Gut Sacs in Rats
3.3.2. Pharmacokinetics of OCT after i.v. and Oral Administration
3.3.3. Cell Culture
3.3.4. Lactate Dehydrogenase Assay
3.3.5. Transepithelial Electrical Resistance Measurement and FD-4 Permeability
3.3.6. OCT Transport on the Caco-2 Cell Monolayers
3.3.7. Real-Time PCR Analysis
3.3.8. Western Blotting
3.3.9. Immunofluorescent Staining
3.3.10. Statistical Analysis
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Adelman, D.T.; Burgess, A.; Davies, P.R. Evaluation of long-acting somatostatin analog injection devices by nurses: A quantitative study. Med. Devices Auckl 2012, 5, 103–109. [Google Scholar]
- Le Quan, S.K.; Arnoux, J.B.; Mamoune, A.; Saint-Martin, C.; Bellanne-Chantelot, C.; Valayannopoulos, V.; Brassier, A.; Kayirangwa, H.; Barbier, V.; Broissand, C.; et al. Successful treatment of congenital hyperinsulinism with long-acting release octreotide. Eur. J. Endocrinol 2012, 166, 333–339. [Google Scholar]
- Biron, E.; Chatterjee, J.; Ovadia, O.; Langenegger, D.; Brueggen, J.; Hoyer, D.; Schmid, H.A.; Jelinek, R.; Gilon, C.; Hoffman, A.; Kessler, H. Improving oral bioavailability of peptides by multiple N-methylation: Somatostatin analogues. Angew. Chem. Int. Ed. Engl 2008, 47, 2595–2599. [Google Scholar]
- Maggio, E.T.; Grasso, P. Oral delivery of octreotide acetate in Intravail® improves uptake, half-life, and bioavailability over subcutaneous administration in male Swiss webster mice. Regul Pept 2011, 167, 233–238. [Google Scholar]
- Garcia-Pagan, J.C.; Reverter, E.; Abraldes, J.G.; Bosch, J. Acute variceal bleeding. Semin. Respir. Crit. Care Med 2012, 33, 46–54. [Google Scholar]
- Rosenthal, R.; Heydt, M.S.; Amasheh, M.; Stein, C.; Fromm, M.; Amasheh, S. Analysis of absorption enhancers in epithelial cell models. Ann. N. Y. Acad. Sci 2012, 1258, 86–92. [Google Scholar]
- Lu, Z.; Chen, W.; Viljoen, A.; Hamman, J.H. Effect of sinomenine on the in vitro intestinal epithelial transport of selected compounds. Phytother. Res 2010, 24, 211–218. [Google Scholar]
- Chen, Z.M.; Liu, B.Q.; Boreham, J.; Wu, Y.P.; Chen, J.S.; Peto, R. Smoking and liver cancer in China: Case-control comparison of 36,000 liver cancer deaths vs. 17,000 cirrhosis deaths. Int. J. Cancer 2003, 107, 106–112. [Google Scholar]
- Dorkoosh, F.A.; Verhoef, J.C.; Verheijden, J.H.; Rafiee-Tehrani, M.; Borchard, G.; Junginger, H.E. Peroral absorption of octreotide in pigs formulated in delivery systems on the basis of superporous hydrogel polymers. Pharm. Res 2002, 19, 1532–1536. [Google Scholar]
- Tuvia, S.; Atsmon, J.; Teichman, S.L.; Katz, S.; Salama, P.; Pelled, D.; Landau, I.; Karmeli, I.; Bidlingmaier, M.; Strasburger, C.J.; et al. Oral octreotide absorption in human subjects: Comparable pharmacokinetics to parenteral octreotide and effective growth hormone suppression. J. Clin. Endocrinol. Metab 2012, 97, 2362–2369. [Google Scholar]
- Dorkoosh, F.A.; Broekhuizen, C.A.; Borchard, G.; Rafiee-Tehrani, M.; Verhoef, J.C.; Junginger, H.E. Transport of octreotide and evaluation of mechanism of opening the paracellular tight junctions using superporous hydrogel polymers in Caco-2 cell monolayers. J. Pharm. Sci 2004, 93, 743–752. [Google Scholar]
- Chan, K.; Liu, Z.Q.; Jiang, Z.H.; Zhou, H.; Wong, YF.; Xu, H.X.; Liu, L. The effects of sinomenine on intestinal absorption of paeoniflorin by the everted rat gut sac model. J. Ethnopharmacol 2006, 103, 425–432. [Google Scholar]
- Liu, Z.Q.; Jiang, Z.H.; Liu, L.; Hu, M. Mechanisms responsible for poor oral bioavailability of paeoniflorin: Role of intestinal disposition and interactions with sinomenine. Pharm. Res 2006, 23, 2768–2780. [Google Scholar]
- Liu, Z.Q.; Zhou, H.; Liu, L.; Jiang, Z.H.; Wong, Y.F.; Xie, Y.; Cai, X.; Xu, H.X.; Chan, K. Influence of co-administrated sinomenine on pharmacokinetic fate of paeoniflorin in unrestrained conscious rats. J. Ethnopharmacol 2005, 99, 61–67. [Google Scholar]
- Zhao, X.X.; Peng, C.; Zhang, H.; Qin, L.P. Sinomenium acutum: A review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharm. Biol 2012, 50, 1053–1061. [Google Scholar]
- Cano-Cebrian, M.J.; Zornoza, T.; Granero, L.; Polache, A. Intestinal absorption enhancement via the paracellular route by fatty acids, chitosans and others: A target for drug delivery. Curr. Drug Deliv 2005, 2, 9–22. [Google Scholar]
- Liang, X.L.; Zhao, L.J.; Liao, Z.G.; Zhao, G.W.; Zhang, J.; Chao, Y.C.; Yang, M.; Yin, R.L. Transport properties of puerarin and effect of Radix Angelicae Dahuricae extract on the transport of puerarin in Caco-2 cell model. J. Ethnopharmacol 2012, 144, 677–682. [Google Scholar]
- Konishi, T.; Satsu, H.; Hatsugai, Y.; Aizawa, K.; Inakuma, T.; Nagata, S.; Sakuda, S.H.; Nagasawa, H.; Shimizu, M. Inhibitory effect of a bitter melon extract on the P-glycoprotein activity in intestinal Caco-2 cells. Br. J. Pharmacol 2004, 143, 379–387. [Google Scholar]
- Doi, N.; Tomita, M.; Hayashi, M. Absorption enhancement effect of acylcarnitines through changes in tight junction protein in Caco-2 cell monolayers. Drug Metab. Pharmacokinet 2011, 26, 162–170. [Google Scholar]
- Aungst, B.J. Intestinal permeation enhancers. J. Pharm. Sci 2000, 89, 429–442. [Google Scholar]
- Yeh, T.H.; Hsu, L.W.; Tseng, M.T.; Lee, P.L.; Sonjae, K.; Ho, Y.C.; Sung, H.W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar]
- Chiba, H.; Osanai, M.; Murata, M.; Kojima, T.; Sawada, N. Transmembrane proteins of tight junctions. Biochim. Biophys. Acta 2008, 1778, 588–600. [Google Scholar]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol 2002, 156, 1099–1111. [Google Scholar]
- Gonzalez-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 2008, 1778, 729–756. [Google Scholar]
- Pinton, P.; Nougayrede, J.P.; Del, R.J.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol 2009, 237, 41–48. [Google Scholar] [Green Version]
- Matter, K.; Balda, M.S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol 2003, 4, 225–236. [Google Scholar]
- Smith, J.M.; Dornish, M.; Wood, E.J. Involvement of protein kinase C in chitosan glutamate-mediated tight junction disruption. Biomaterials 2005, 26, 3269–3276. [Google Scholar]
- Morreale, A.; Mallon, B.; Beale, G.; Watson, J.; Rumsby, M. Ro31-8220 inhibits protein kinase C to block the phorbol ester-stimulated release of choline- and ethanolamine-metabolites from C6 glioma cells: p70 S6 kinase and MAPKAP kinase-1beta do not function downstream of PKC in activating PLD. FEBS Lett 1997, 417, 38–42. [Google Scholar]
- Feng, X.; Becker, K.P.; Stribling, S.D.; Peters, K.G.; Hannun, Y.A. Regulation of receptor-mediated protein kinase C membrane trafficking by autophosphorylation. J. Biol. Chem 2000, 275, 17024–17034. [Google Scholar]
- Barthe, L.; Woodley, J.; Houin, G. Gastrointestinal absorption of drugs: methods and studies. Fundam Clin. Pharmacol 1999, 13, 154–168. [Google Scholar]
- Eltink, C.; Lee, J.; Schaddelee, M.; Zhang, W.; Kerbusch, V.; Meijer, J.; van Marle, S.; Grunenberg, N.; Kowalski, D.; Drogendijk, T.; et al. Single dose pharmacokinetics and absolute bioavailability of mirabegron, a beta(3)-adrenoceptor agonist for treatment of overactive bladder. Int. J. Clin. Pharmacol. Ther 2012, 50, 838–850. [Google Scholar]
- Madara, J.L.; Stafford, J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J. Clin. Invest 1989, 83, 724–727. [Google Scholar]
- Thanou, M.M.; Kotze, A.F.; Scharringhausen, T.; Luessen, H.L.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J. Control. Release 2000, 64, 15–25. [Google Scholar]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc 2006, 1, 581–585. [Google Scholar]
- Kakinuma, S.; Tanaka, Y.; Chinzei, R.; Watanabe, M.; Shimizu-Saito, K.; Hara, Y.; Teramoto, K.; Arii, S.; Sato, C.; Takase, K.; et al. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells 2003, 21, 217–227. [Google Scholar]

| Group | Tmax (min) | Cmax (ng/mL) | AUC (ng/mL/min) | F% | ER |
|---|---|---|---|---|---|
| OCT p.o. | 30 | 44.33 ± 6.66 | 4,023.33 ± 417.14 | 1.63 ± 0.31 | 1 |
| OCT + 0.5% SN p.o. | 30 | 255.00 ± 8.72 * | 21,268.33 ± 1,136.48 # | 8.67 ± 1.73 $ | 5.3 |
| OCT i.v. | 10 | 307.00 ± 6.00 | 25,135.83 ± 4,548.65 |
| TEER reading | 1 | 2 | 3 | 4 | 5 | 7 | TEER (Ω·cm2) |
|---|---|---|---|---|---|---|---|
| Blankgroup | 104 | 107 | 101 | 105 | 106 | 102 | 104.17 ± 2.32 |
| cells | 760 | 710 | 830 | 760 | 772 | 790 | 746.11 ± 46.57 |
| Group | Papp (×10−6 cm/s) | R (Papptest/Pappcontrol) |
|---|---|---|
| Control (10 μM OCT) | 2.67 ± 0.015 | 1 |
| 0.5% SN + 10 μM OCT | 5.89 ± 0.038 * | 2.2 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Y.; Duan, Z.; Tian, Y.; Liu, Z.; Wang, Q. A Novel Perspective and Approach to Intestinal Octreotide Absorption: Sinomenine-Mediated Reversible Tight Junction Opening and Its Molecular Mechanism. Int. J. Mol. Sci. 2013, 14, 12873-12892. https://doi.org/10.3390/ijms140612873
Li Y, Duan Z, Tian Y, Liu Z, Wang Q. A Novel Perspective and Approach to Intestinal Octreotide Absorption: Sinomenine-Mediated Reversible Tight Junction Opening and Its Molecular Mechanism. International Journal of Molecular Sciences. 2013; 14(6):12873-12892. https://doi.org/10.3390/ijms140612873
Chicago/Turabian StyleLi, Yuling, Zhijun Duan, Yan Tian, Zhen Liu, and Qiuming Wang. 2013. "A Novel Perspective and Approach to Intestinal Octreotide Absorption: Sinomenine-Mediated Reversible Tight Junction Opening and Its Molecular Mechanism" International Journal of Molecular Sciences 14, no. 6: 12873-12892. https://doi.org/10.3390/ijms140612873




