Anti-Tumor Effects of Mfn2 in Gastric Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
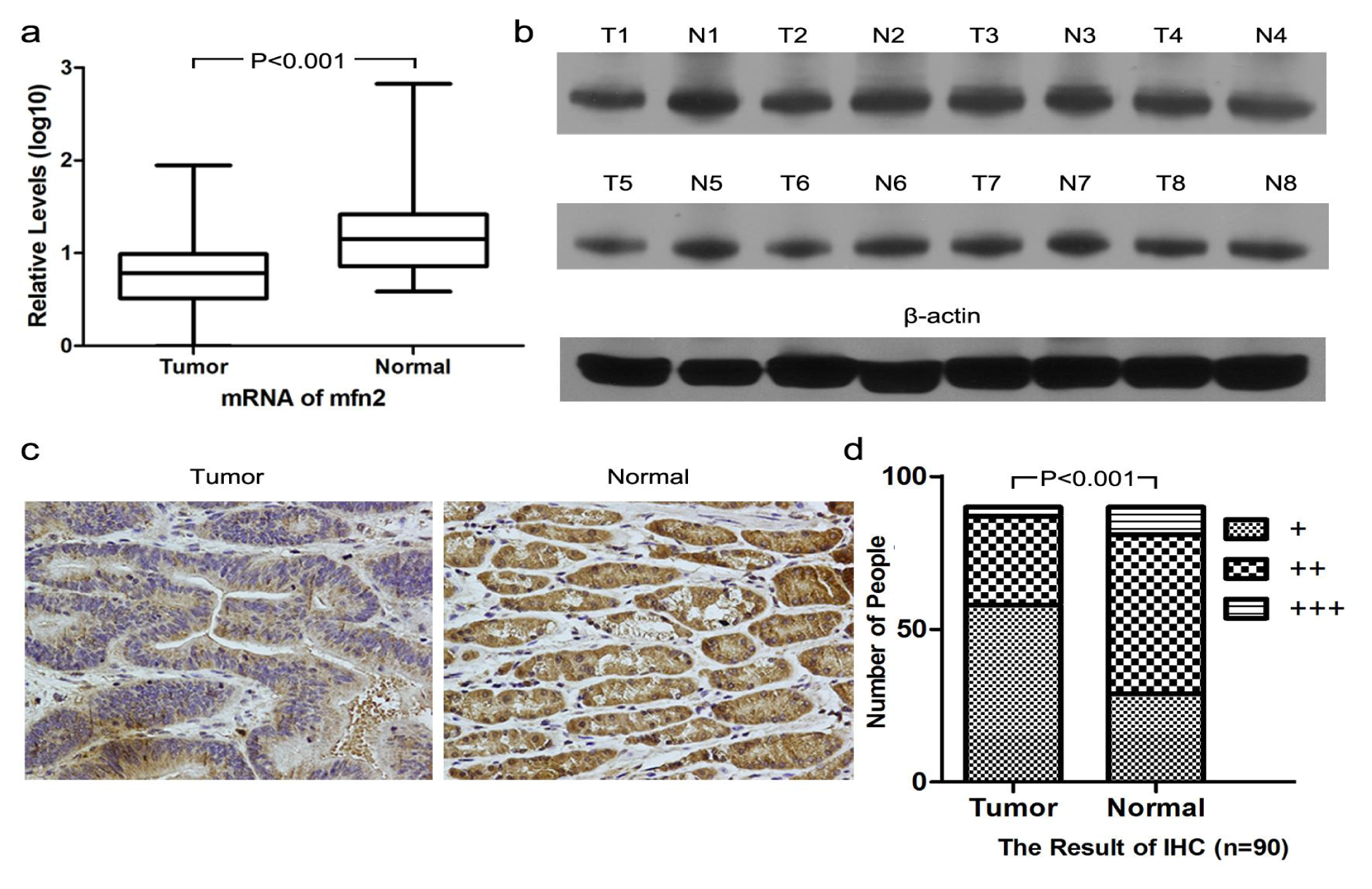
2.1.1. Higher Mfn2 Expression in Normal Mucosal Tissue than in Tumor Tissue
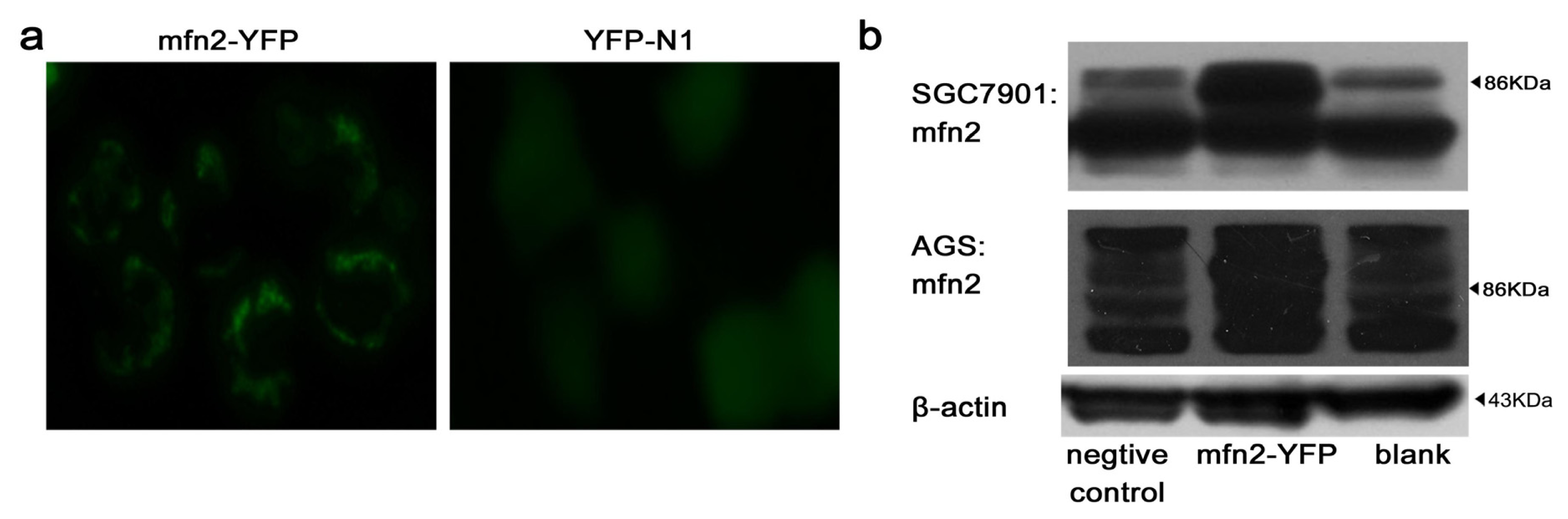
2.1.2. Efficiency of Transfection
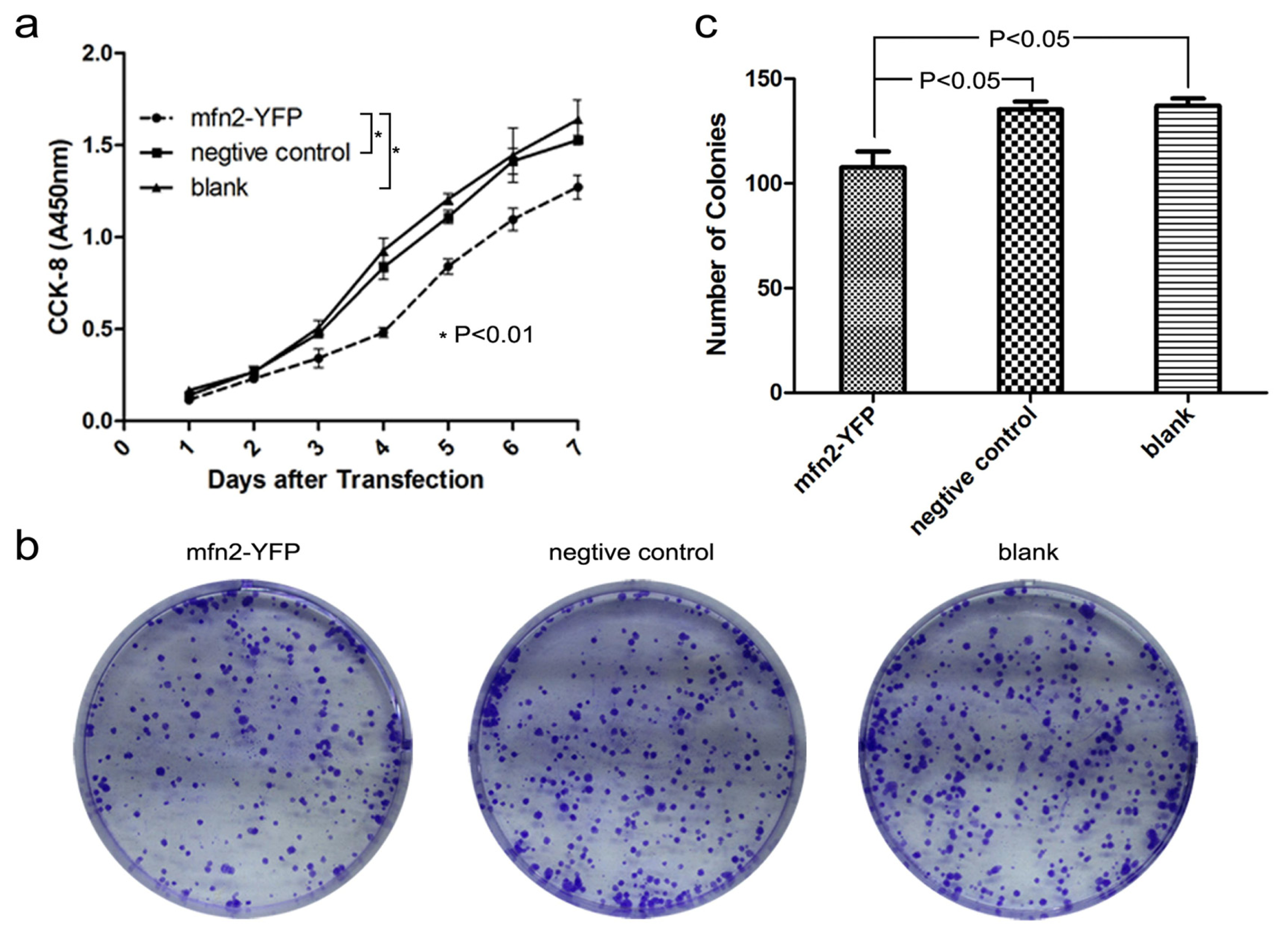
2.1.3. Mfn2 Significantly Suppressed Gastric Cancer Cell Proliferation
2.1.4. Mfn2 Halted the Cell Cycle in the G0/G1 Phase
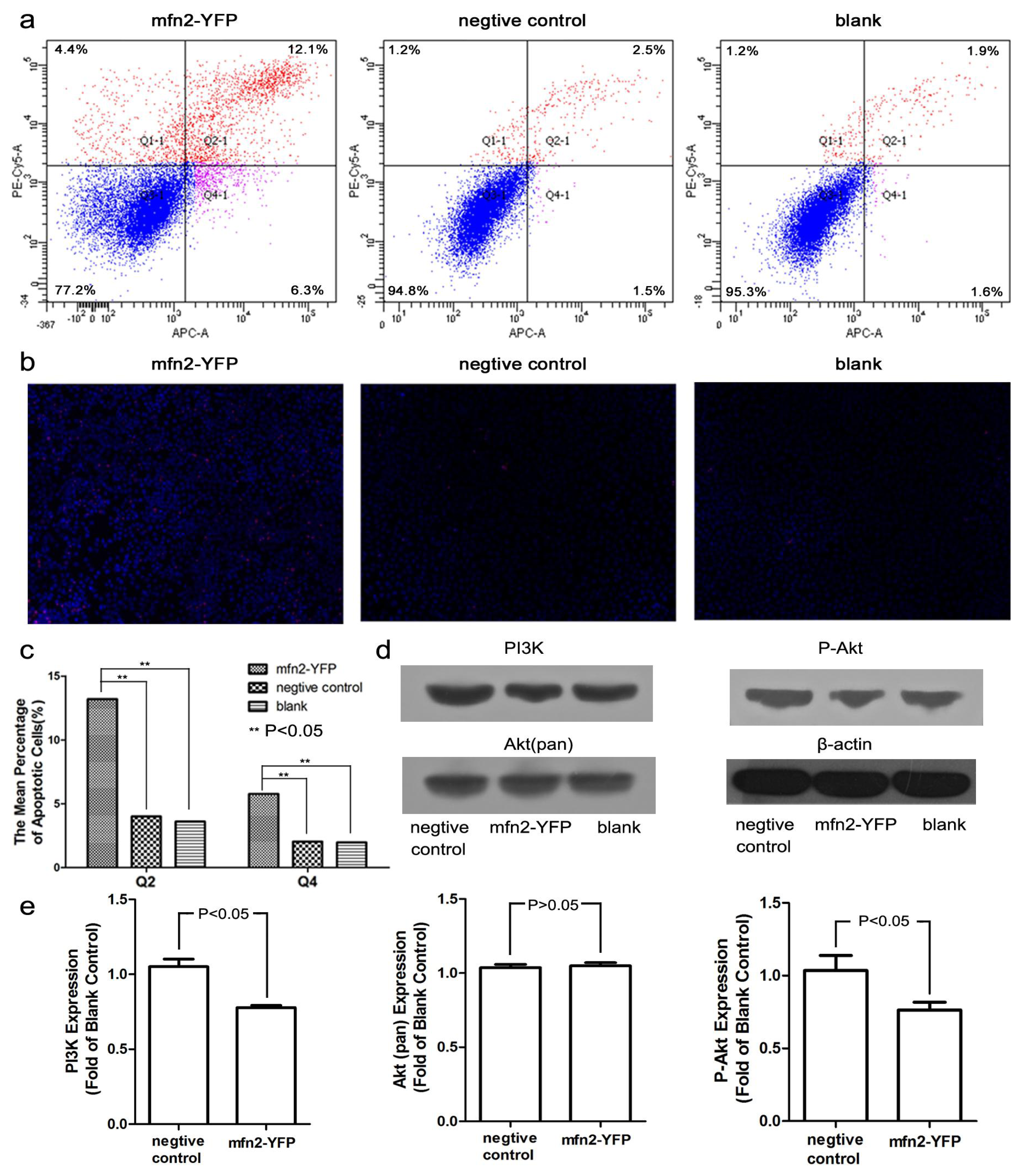
2.1.5. Mfn2 Induced Cell Apoptosis
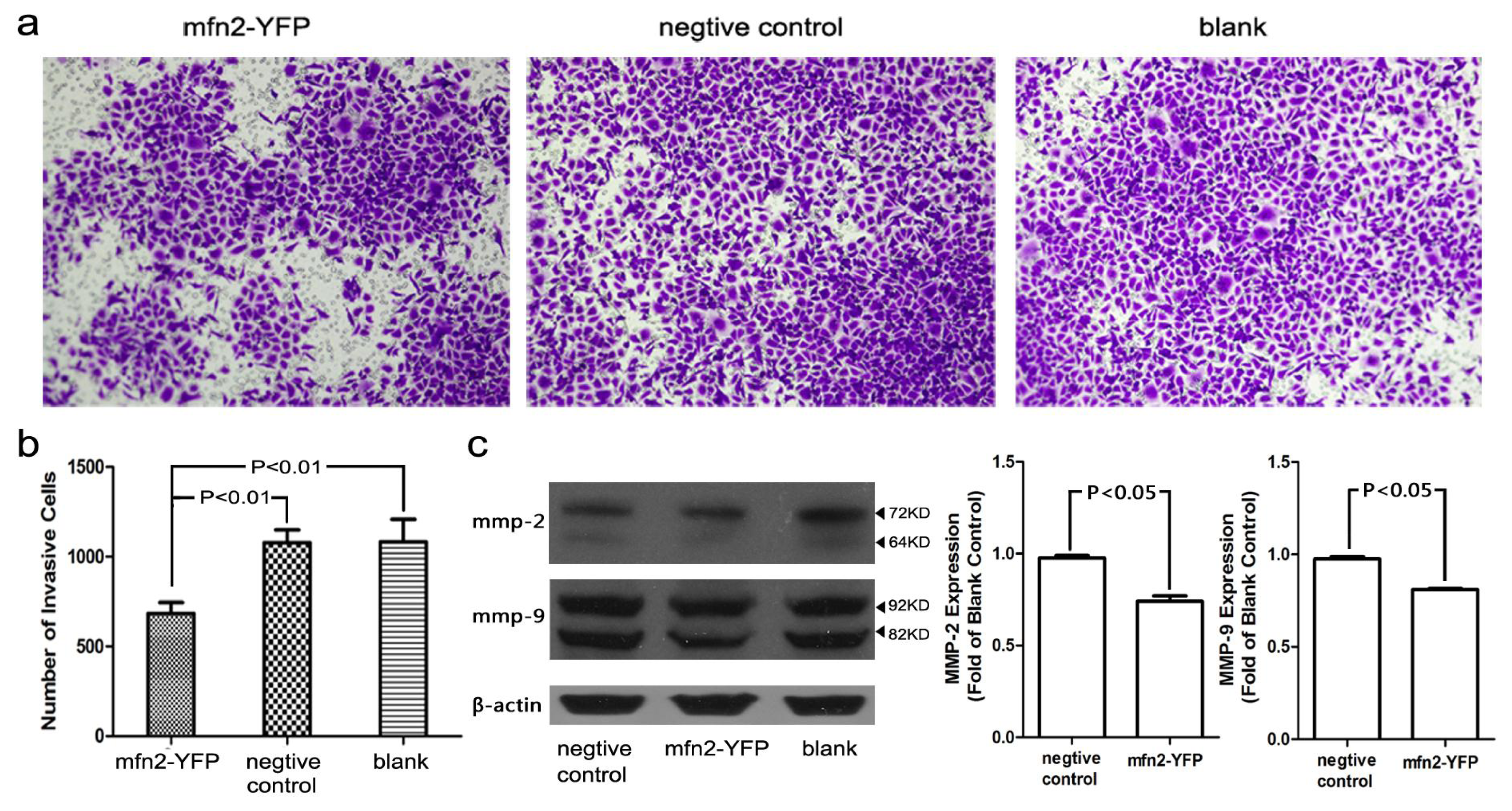
2.1.6. The Invasion and Migratory Ability of Gastric Cancer Cells Was Weakened by Mfn2 Transfection
2.1.7. Relationship between Mfn2 Expression and Clinicopathological Factors
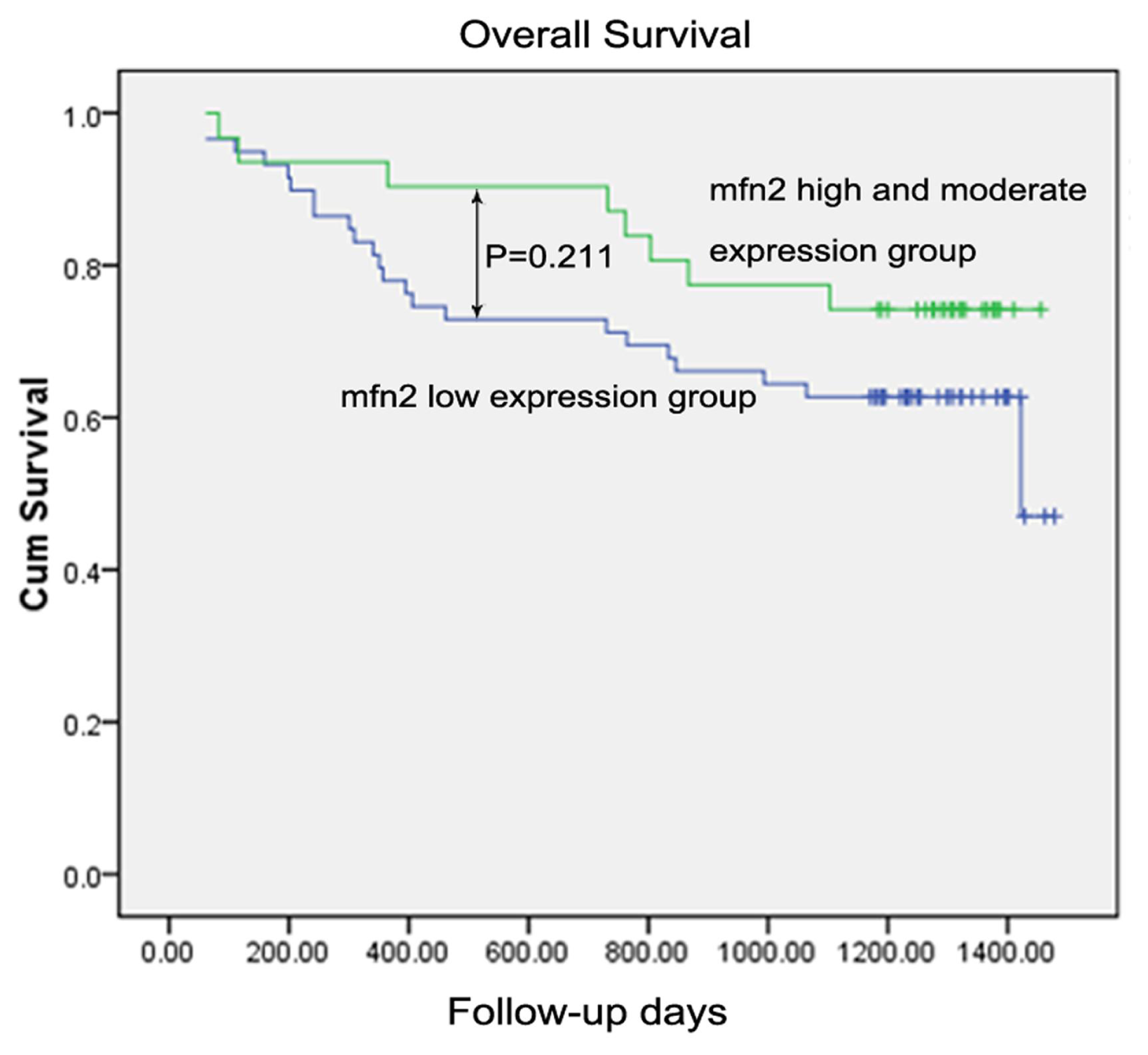
2.1.8. Survival Analysis
2.2. Discussion
3. Experimental Section
3.1. Patient Samples
3.2. Materials
3.3. Immunohistochemistry
3.4. Western Blotting
3.5. Quantitative Real-Time PCR (QRT-PCR)
3.6. Transfection
3.7. Cell Proliferation and Colony Formation
3.8. Cell Cycle and Apoptosis
3.9. Cell Migration and Invasion
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA-Cancer J. Clin 2011, 61, 69–90. [Google Scholar]
- Ren, J.S.; Li, Q.; Guan, P.; Dai, M.; Yang, L. Estimation and prediction for incidence, mortality and prevalence of common gastrointestinal tract cancers in China, in 2008. Chin. J. Epidemiol 2012, 33, 1052–1055. [Google Scholar]
- Braathen, G.J.; Sand, J.C.; Lobato, A.; Høyer, H.; Russell, M.B. Mfn2 point mutations occur in 3.4% of Charcot-Marie-Tooth families. An investigation of 232 Norwegian CMT families. BMC Med. Genet 2010, 11, 48. [Google Scholar]
- Santel, A.F. MT Control of mitochondrial morphology by a human mitofusin. J. Cell Sci. 2001, 114, 867–874. [Google Scholar]
- de Brito, O.M.; Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008, 456, 605–610. [Google Scholar]
- Chen, K.H.; Guo, X.; Ma, D.; Guo, Y.; Li, Q.; Yang, D. Dysregulation of HSG triggers vascular proliferative disorders. Nat. Cell Biol 2004, 6, 872–883. [Google Scholar]
- Wang, W.L.; Lu, J.J.; Zhu, F.; Wei, J.F.; Jia, C.K.; Zhang, Y.B.; Zhou, L.; Xie, H.Y.; Zheng, S.S. Pro-apoptotic and anti-proliferative effects of mitofusin-2 via Bax signaling in hepatocellular carcinoma cells. Med. Oncol 2010, 29, 70–76. [Google Scholar]
- Jin, B.Y.; Fu, G.H.; Pan, H.; Cheng, X.F.; Zhou, L.; Lv, J.; Chen, G.M.; Zheng, S.S. Anti-tumour efficacy of mitofusin-2 in urinary bladder carcinoma. Med. Oncol 2010, 28, 373–380. [Google Scholar]
- Wu, L.; Li, Z.X.; Zhang, Y.M.; Zhang, P.; Zhu, X.H.; Huang, J.; Ma, T.; Lu, T.; Song, Q.S.; Li, Q.; et al. Adenovirus-expressed human hyperplasia suppressor gene induces apoptosis in cancer cells. Mol. Cancer Ther 2008, 7, 222–232. [Google Scholar]
- Macleod, K.F.; Sherry, N.; Hannon, G.; Beach, D.; Tokino, T.; Kinzler, K.; Vogelstein, B.; Jacks, T. P53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev 1995, 9, 935–944. [Google Scholar]
- Wang, Y.F.; Fisher, J.C.; Mathew, R.; Ou, L.; Otieno, S.; Sublet, J.; Xiao, L.M.; Chen, J.H.; Roussel, M.F.; Kriwacki, R.W. Intrinsic disorder mediates the diverse regulatory functions of the Cdk inhibitor p21. Nat. Chem. Biol 2011, 7, 214–221. [Google Scholar]
- Yoon, M.K.; Mitrea, D.M.; Ou, L.; Kriwacki, R.W. Cell cycle regulation by the intrinsically disordered proteins p21 and p27. Biochem. Soc. Trans 2012, 40, 981–988. [Google Scholar]
- Franke, T.F.; Kaplan, D.R.; Cantley, L.C. PI3K: downstream AKTion blocks apoptosis. Cell 1997, 88, 435–437. [Google Scholar]
- Liu, H.; Chen, A.M.; Guo, F.J.; Yuan, L. A short-hairpin RNA targeting osteopontin downregulates MMP-2 and MMP-9 expressions in prostate cancer PC-3 cells. Cancer Lett 2010, 295, 27–37. [Google Scholar]
- Stetler-Stevenson, W.G.; Yu, A.E. Proteases in invasion: Matrix metalloproteinases. Semin. Cancer Biol 2001, 11, 143–152. [Google Scholar]
- Castellano, G.; Malaponte, G.; Mazzarino, M.C.; Figini, M.; Marchese, F.; Gangemi, P.; Travali, S.; Stivala, F.; Canevari, S.; Libra, M. Activation of the osteopontin/matrix metalloproteinase-9 pathway correlates with prostate cancer progression. Clin. Cancer Res 2008, 14, 7470–7480. [Google Scholar]
- Mook, O.R.F.; Frederiks, W.M.; van Noorden, C.J.F. The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta 2004, 1705, 69–89. [Google Scholar]
- Oshima, T.; Masuda, M. Molecular targeted agents for gastric and gastroesophageal junction cancer. Surg. Today 2011, 42, 313–327. [Google Scholar]
- de Brito, O.M.; Scorrano, L. Mitofusin 2: A mitochondria-shaping protein with signaling roles beyond fusion. Antioxid. Redox Signal 2008, 10, 621–634. [Google Scholar]
- Westermann, B. Molecular machinery of mitochondrial fusion and fission. J. Biol. Chem 2008, 283, 13501–13505. [Google Scholar]
- Zuchner, S.; Mersiyanova, I.V.; Muglia, M.; Bissar-Tadmouri, N.; Rochelle, J.; Dadali, E.L.; Zappia, M.; Nelis, E.; Patitucci, A.; Senderek, J.; et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet 2004, 36, 449–451. [Google Scholar]
- Baloh, R.H.; Schmidt, R.E.; Pestronk, A.; Milbrandt, J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J. Neurosci 2007, 27, 422–430. [Google Scholar]
- Mingrone, G.; Manco, M.; Calvani, M.; Castagneto, M.; Naon, D.; Zorzano, A. Could the low level of expression of the gene encoding skeletal muscle mitofusin-2 account for the metabolic inflexibility of obesity? Diabetologia 2005, 48, 2108–2114. [Google Scholar]
- Wang, W.L.; Zhu, F.; Wang, S.Q.; Wei, J.F.; Jia, C.K.; Zhang, Y.B.; Zhou, L.; Xie, H.Y.; Zheng, S.S. HSG provides antitumor efficacy on hepatocellular carcinoma both in vitro and in vivo. Oncol. Rep. 2010, 24. [Google Scholar]
- Polyak, K.; Xia, Y.; Zweier, J.L.; Kinzler, K.W.; Vogelstein, B. A model for p53-induced apoptosis. Nature 1997, 389, 300–305. [Google Scholar]
- Waldman, T.; Kinzler, K.W.; Vogelstein, B. P21 is necessary for the P53-mediated G(1) arrest in human cancer-cells. Cancer Res 1995, 55, 5187–5190. [Google Scholar]
- Gopinathan, L.; Ratnacaram, C.K.; Kaldis, P. Established and novel Cdk/cyclin complexes regulating the cell cycle and development. Results Probl. Cell Differ 2011, 53, 365–389. [Google Scholar]
- Karbowski, M. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol 2002, 159, 931–938. [Google Scholar]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar]
- Del Peso, L.; GonzalezGarcia, M.; Page, C.; Herrera, R.; Nunez, G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 1997, 278, 687–689. [Google Scholar]
- Kim, N.H.; Kim, K.; Park, W.S.; Son, H.S.; Bae, Y. PKB/Akt inhibits ceramide-induced apoptosis in neuroblastoma cells by blocking apoptosis-inducing factor (AIF) translocation. J. Cell. Biochem 2007, 102, 1160–1170. [Google Scholar]
- Zhang, Q.G.; Wu, D.N.; Han, D.; Zhang, G.Y. Critical role of PTEN in the coupling between PI3K/Akt and JNK1/2 signaling in ischemic brain injury. FEBS Lett 2007, 581, 495–505. [Google Scholar]
- Morel, C.; Carlson, S.M.; White, F.M.; Davis, R.J. Mcl-1 integrates the opposing actions of signaling pathways that mediate survival and apoptosis. Mol. Cell. Biol 2009, 29, 3845–3852. [Google Scholar]
- Kim, D.; Kim, S.; Koh, H.; Yoon, S.O.; Chung, A.S.; Cho, K.S.; Chung, J. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J 2001, 15, 1953–1962. [Google Scholar]
- Adya, R.; Tan, B.K.; Punn, A.; Chen, J.; Randeva, H.S. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: Novel insights into visfatin-induced angiogenesis. Cardiovasc. Res 2008, 78, 356–365. [Google Scholar]
- Masson, V.; de la Ballina, L.R.; Munaut, C.; Wielockx, B.; Jost, M.; Maillard, C.; Blacher, S.; Bajou, K.; Itoh, T.; Itohara, S.; et al. Contribution of host MMP-2 and MMP-9 to promote tumor vascularization and invasion of malignant keratinocytes. FASEB J 2005, 19, 234–236. [Google Scholar]

| Mfn2 expression in tumor tissue | ||||
|---|---|---|---|---|
| Clinicopathological factors | Number (n = 90 ) | Low (n = 59) | High and moderate a (n = 31) | p-value |
| Gender | ||||
| Male | 64 | 41 | 23 | 0.640 |
| Female | 26 | 18 | 8 | |
| Age | ||||
| >65 | 34 | 22 | 12 | 0.895 |
| ≤65 | 56 | 37 | 19 | |
| Tumor size | ||||
| <4 cm | 47 | 26 | 21 | 0.033 b |
| ≥4 cm | 43 | 33 | 10 | |
| Adjacent organ invasion | ||||
| Yes | 10 | 7 | 3 | 1.000 |
| No | 80 | 52 | 28 | |
| Lymphovascular invasion | ||||
| Yes | 27 | 16 | 11 | 0.411 |
| No | 63 | 43 | 20 | |
| Degree of differentiation | ||||
| Well and moderate | 18 | 12 | 6 | 0.912 |
| Poor | 72 | 47 | 25 | |
| T stagec | ||||
| T1 + T2 | 26 | 17 | 9 | 0.983 |
| T3 + T4 | 64 | 42 | 22 | |
| Lymph node metastasisc | ||||
| Yes | 65 | 44 | 21 | 0.492 |
| No | 25 | 15 | 10 | |
| Distant metastasisc | ||||
| M0 | 87 | 56 | 31 | 0.510 |
| M1 | 3 | 3 | 0 | |
| TNM stagec | ||||
| I + II | 36 | 22 | 14 | 0.469 |
| II + IV | 54 | 37 | 17 | |
| Factors | Number (n = 90) | Univariate analysis ap-value | Multivariate analysis b | |
|---|---|---|---|---|
| HR (95%CI) | p-value | |||
| Gender | ||||
| Male | 64 | 0.492 | ||
| Female | 26 | |||
| Age | ||||
| >65 | 34 | 0.021 | ||
| ≤65 | 56 | |||
| Tumor Size | ||||
| <4cm | 47 | 0.008 | ||
| ≥4cm | 43 | |||
| Adjacent organ invasion | ||||
| Yes | 10 | <0.001 | 2.554 (1.045–6.239) | 0.040 |
| No | 80 | 1 | ||
| Lymphovascular invasion | ||||
| Yes | 27 | 0.036 | 2.563 (1.204–5.459) | 0.015 |
| No | 63 | 1 | ||
| Degree of differentiationc | ||||
| Well and moderate | 18 | 0.022 | ||
| Poor | 72 | |||
| T staged | ||||
| T1 + T2 | 26 | 0.001 | ||
| T3 + T4 | 64 | |||
| Lymph node metastasisd | ||||
| Yes | 65 | 0.064 | ||
| No | 25 | |||
| Distant metastasisd | ||||
| M0 | 87 | <0.001 | 1 | |
| M1 | 3 | 8.817 (2.242–34.679) | 0.002 | |
| TNM staged | ||||
| I + II | 36 | 0.001 | 1 | |
| III + IV | 54 | 3.730 (1.395–9.970) | 0.009 | |
| Mfn2 expression in tumor tissue | ||||
| Low expression | 59 | 0.211 | ||
| High and moderate expression | 31 | |||
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, G.-E.; Jin, H.-L.; Lin, X.-K.; Chen, C.; Liu, X.-S.; Zhang, Q.; Yu, J.-R. Anti-Tumor Effects of Mfn2 in Gastric Cancer. Int. J. Mol. Sci. 2013, 14, 13005-13021. https://doi.org/10.3390/ijms140713005
Zhang G-E, Jin H-L, Lin X-K, Chen C, Liu X-S, Zhang Q, Yu J-R. Anti-Tumor Effects of Mfn2 in Gastric Cancer. International Journal of Molecular Sciences. 2013; 14(7):13005-13021. https://doi.org/10.3390/ijms140713005
Chicago/Turabian StyleZhang, Ge-Er, Hai-Long Jin, Xian-Ke Lin, Chao Chen, Xiao-Sun Liu, Qing Zhang, and Ji-Ren Yu. 2013. "Anti-Tumor Effects of Mfn2 in Gastric Cancer" International Journal of Molecular Sciences 14, no. 7: 13005-13021. https://doi.org/10.3390/ijms140713005




