New Labdane-Type Diterpenoids and Anti-Inflammatory Constituents from Hedychium coronarium
Abstract
:1. Introduction
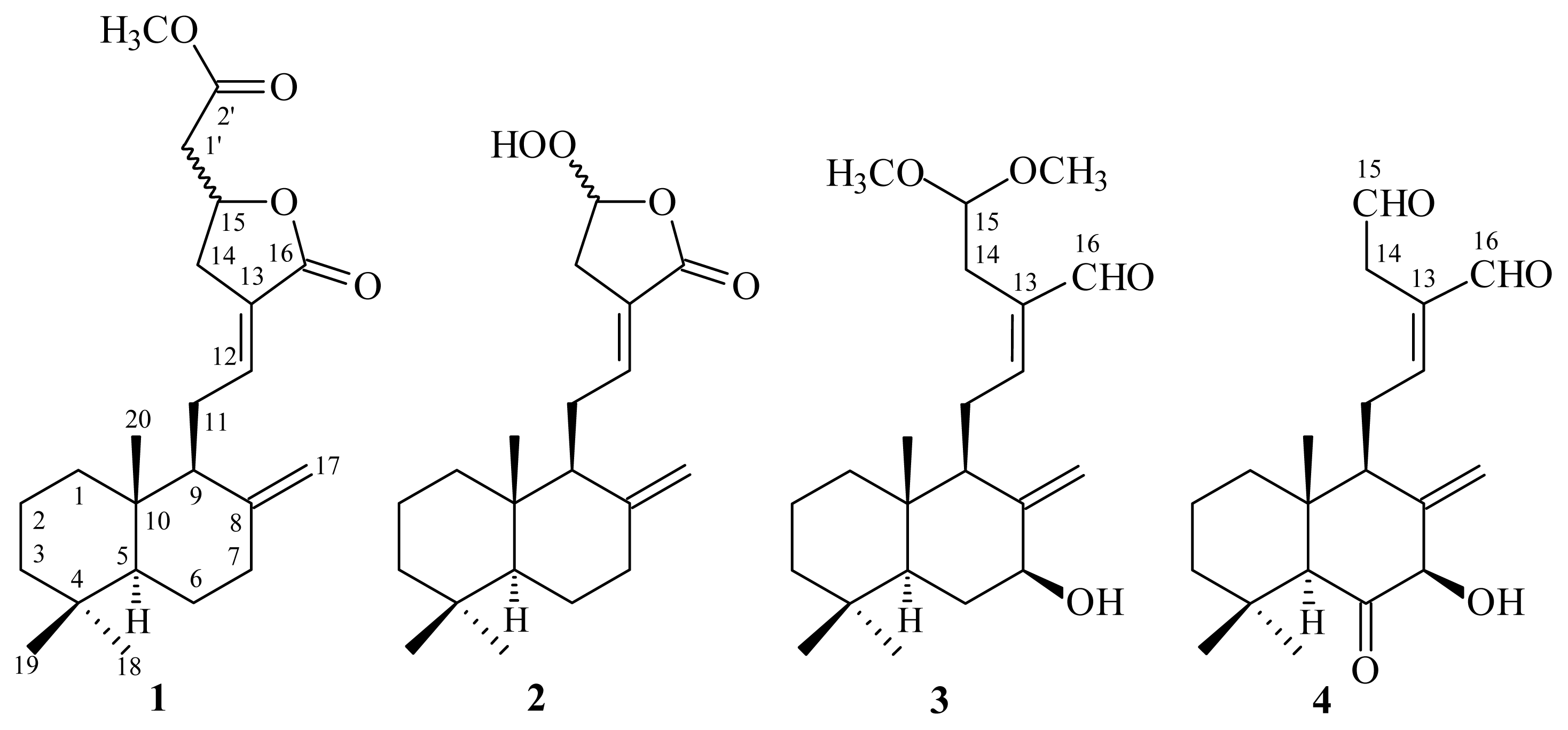
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
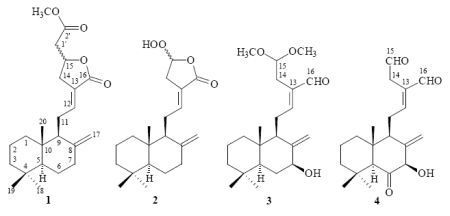
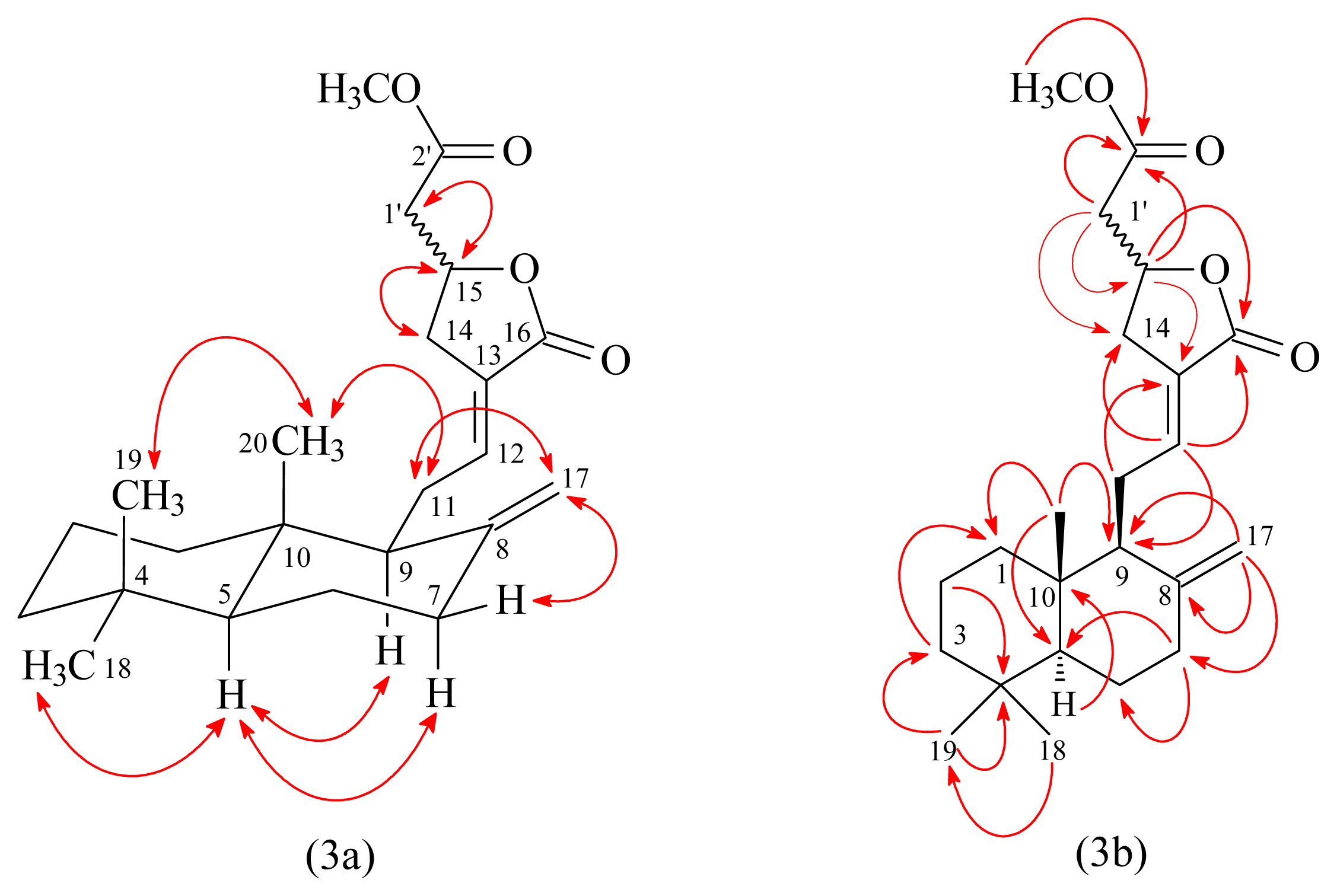
3.3.1. Hedychicoronarin (1)
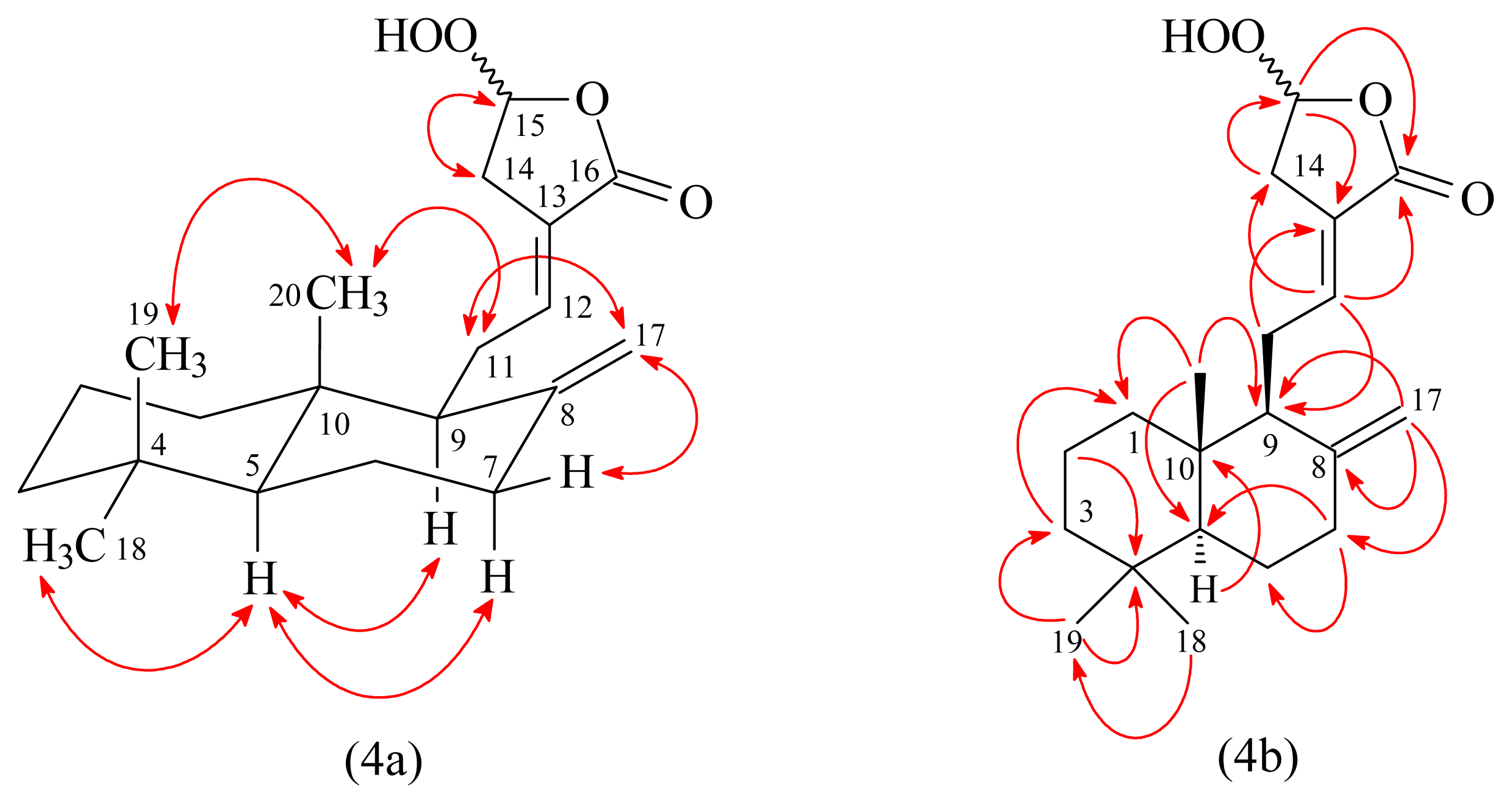
3.3.2. Peroxycoronarin D (2)
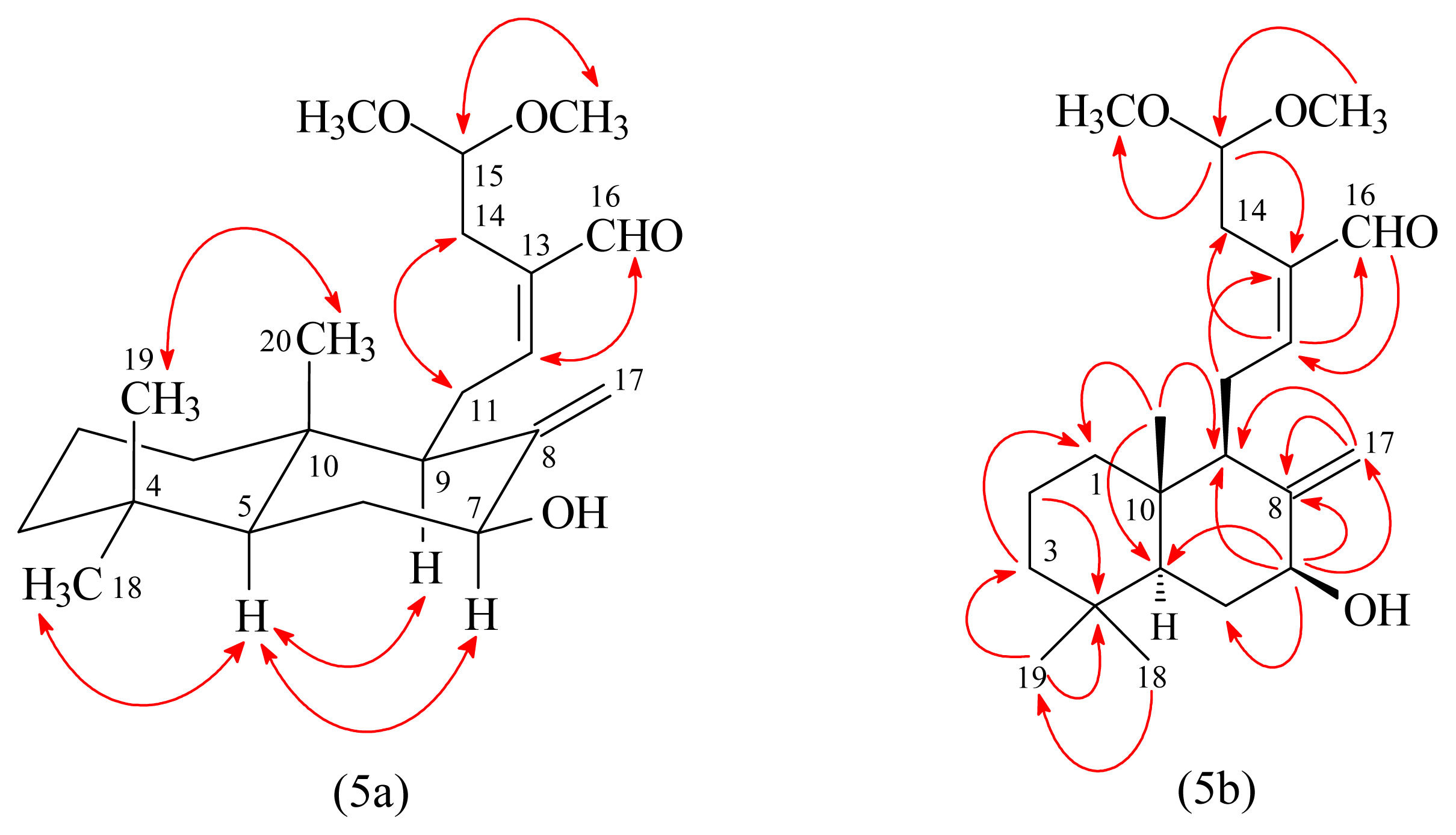
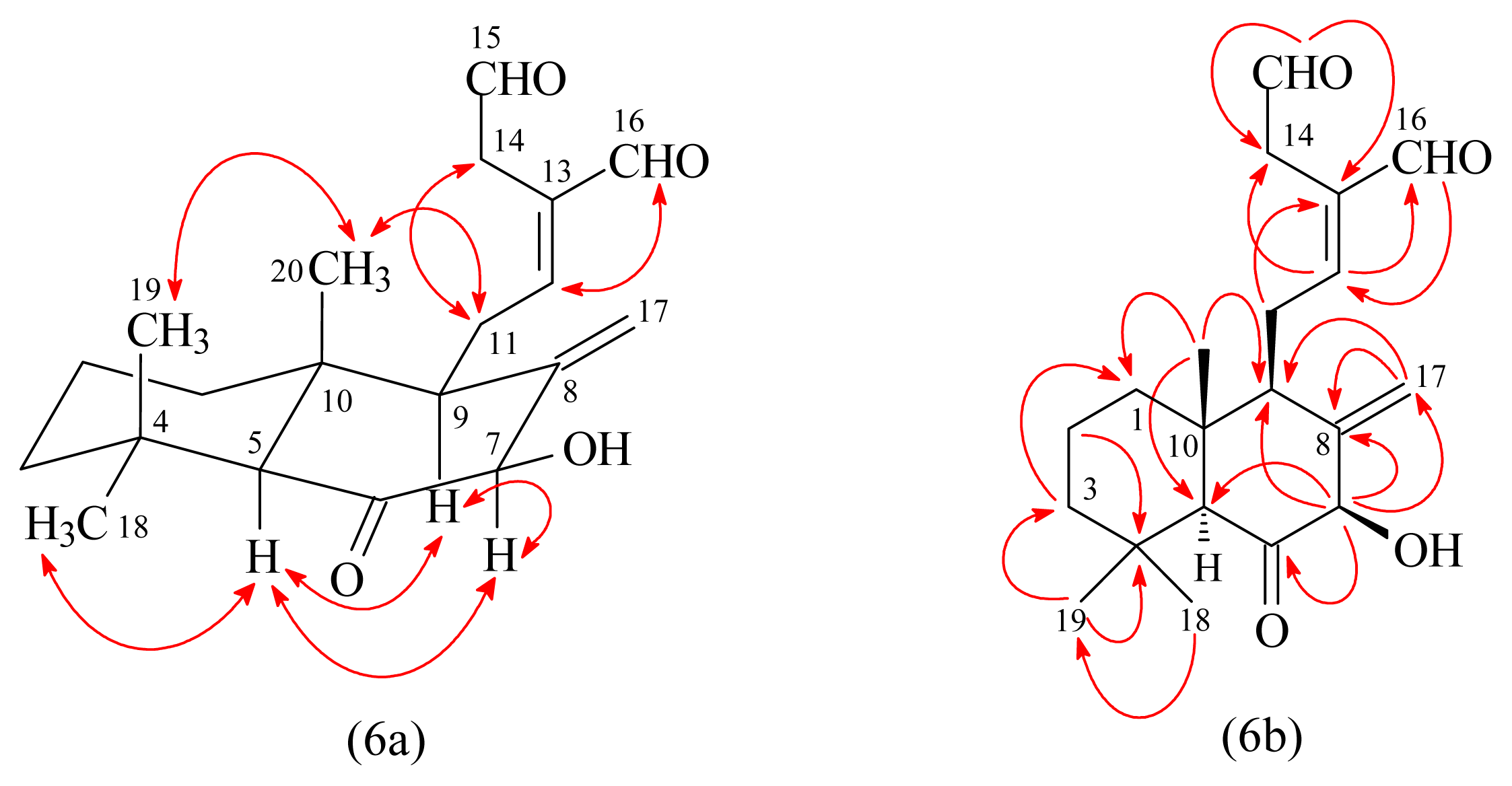
3.3.3. 7β-Hydroxycalcaratarin A (3)
3.3.4. (E)-7β-Hydroxy-6-oxo-labda-8(17),12-diene-15,16-dial (4)
3.4. Biological Assay
3.4.1. Preparation of Human Neutrophils
3.4.2. Measurement of Superoxide Anion Generation
3.4.3. Measurement of Elastase Release
3.4.4. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Wang, J.C. Zingeraceae. In Flora of Taiwan, 2nd ed; Huang, T.C., Ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 2000; Volume 5, pp. 707–724. [Google Scholar]
- Morikawa, T.; Matsuda, H.; Sakamoto, Y.; Ueda, K.; Yoshikawa, M. New farnesane-type sesquiterpenes, hedychiols A and B 8,9-diacetate, and inhibitors of degranulation in RBL-2H3 cells from the rhizome of Hedychium coronarium. Chem. Pharm. Bull 2002, 50, 1045–1049. [Google Scholar]
- Chimnoi, N.; Sarasuk, C.; Khunnawutmanotham, N.; Intachote, P.; Seangsai, S.; Saimanee, B.; Pisutjaroenpong, S.; Mahidol, C.; Techasakul, S. Phytochemical reinvestigation of labdane-type diterpenes and their cytotoxicity from the rhizomes of Hedychium coronarium. Phytochem. Lett 2009, 2, 184–187. [Google Scholar]
- Itokawa, H.; Morita, H.; Katou, I.; Takeya, K.; Cavalheiro, A.J.; de Oliveira, R.C.B.; Ishige, M.; Motidome, M. Cytotoxic diterpenes from the rhizomes of Hedychium coronarium. Planta Med 1988, 54, 311–315. [Google Scholar]
- Nakatani, N.; Kikuzaki, H.; Yamaji, H.; Yoshio, K.; Kitora, C.; Okada, K.; Padolina, W.G. Labdane diterpenes from rhizomes of Hedychium coronarium. Phytochemistry 1994, 37, 1383–1388. [Google Scholar]
- Matsuda, H.; Morikawa, T.; Sakamoto, Y.; Toguchida, I.; Yoshikawa, M. Antiinflammatory principles and three new labdane-type diterpenes, hedychilactones A, B, and C, from the rhizome of Hedychium coronarium Koeng. Heterocycles 2002, 56, 45–50. [Google Scholar]
- Matsuda, H.; Morikawa, T.; Sakamoto, Y.; Toguchida, I.; Yoshikawa, M. Labdane-type diterpenes with inhibitory effects on increase in vascular permeability and nitric oxide production from Hedychium coronarium. Bioorg. Med. Chem 2002, 10, 2527–2534. [Google Scholar]
- Nakamura, S.; Okazaki, Y.; Ninomiya, K.; Morikawa, T.; Matsuda, H.; Yoshikawa, M. Medicinal flowers. XXIV. Chemical structures and hepatoprotective effects of constituents from flowers of Hedychium coronarium. Chem. Pharm. Bull 2008, 56, 1704–1709. [Google Scholar]
- Suresh, G.; Reddy, P.P.; Babu, K.S.; Shaik, T.B.; Kalivendi, S.V. Two new cytotoxic labdane diterpenes from the rhizomes of Hedychium coronarium. Bioorg. Med. Chem. Lett 2010, 20, 7544–7548. [Google Scholar]
- Chimnoi, N.; Pisutjareonpong, S.; Ngiwsara, L.; Dechtrirut, D.; Chokchaichamnankit, D.; Khunnawutmanotham, N.; Mahidol, C.; Techasakul, S. Labdane diterpenes from the rhizomes of Hedychium coronarium. Nat. Prod. Res 2008, 22, 1249–1256. [Google Scholar]
- Taveira, F.N.; Oliveira, A.B.; Souza Filho, J.D.; Braga, F.C. Epimers of labdane diterpenes from the rhizomes of Hedychium coronarium J. Koenig. Braz. J. Pharm 2005, 15, 55–59. [Google Scholar]
- Zhao, Q.; Qing, C.; Hao, X.J.; Han, J.; Zuo, G.Y.; Zou, C.; Xu, G.L. Cytotoxicity of labdane-type diterpenoids from Hedychium forrestii. Chem. Pharm. Bull 2008, 56, 210–212. [Google Scholar]
- Mohamad, H.; Lajis, H.N.; Abas, F.; Ali, M.A.; Sukari, A.M.; Kikuzaki, H.; Nakatani, N. Antioxidative constituents of Etlingera elatior. J. Nat. Prod 2005, 68, 285–288. [Google Scholar]
- Kong, L.Y.; Qin, M.J.; Niwa, M. Diterpenoids from rhizomes of Alpinia calcarata. J. Nat. Prod 2000, 63, 939–942. [Google Scholar]
- Singh, S.; Gray, A.I.; Waterman, P.G. 14,15,16-Trinorlabda-8(17),11-(E)-dien-13-al: A trinorlabdane diterpene from the rhizome of Hedychium coronarium. Nat. Prod. Lett 1993, 3, 163–166. [Google Scholar]
- Singh, S.; Gray, A.I.; Skelton, B.W.; Waterman, P.G.; White, A.H. (+)-14β-Hydroxylabda-8(17),12-dieno-16,15-lactone [(+)-isocoronarin-D]: A new diterpene from Hedychium coronarium (Zingiberaceae). Aust. J. Chem. 1991, 44, 1789–1793. [Google Scholar]
- Price, M.J.; Worth, G.K. The occurrence of ergosta-4,6,8(14),22-tetraen-3-one in several fungi. Aust. J. Chem 1974, 27, 2505–2507. [Google Scholar]
- Chen, J.J.; Hung, H.C.; Sung, P.J.; Chen, I.S.; Kuo, W.L. Aporphine alkaloids and cytotoxic lignans from the roots of Illigera luzonensis. Phytochemistry 2011, 72, 523–532. [Google Scholar]
- Georges, P.; Sylvestre, M.; Ruegger, H.; Bourgeois, P. Ketosteroids and hydroxyketosteroids, minor metabolites of sugarcane wax. Steroids 2006, 71, 647–652. [Google Scholar]
- Lee, C.K.; Lee, P.H.; Kou, Y.H. The chemical constituents from the aril of Cassia fistula L. J. Chin. Chem. Soc 2001, 48, 1053–1058. [Google Scholar]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Invest 2000, 80, 617–653. [Google Scholar]
- Okajima, K.; Harada, N.; Uchiba, M. Ranitidine reduces ischemia/reperfusion-induced liver injury in rats by inhibiting neutrophil activation. J. Pharmacol. Exp. Ther 2002, 301, 1157–1165. [Google Scholar]
- Ennis, M. Neutrophils in asthma pathophysiology. Curr. Allergy Asthma Rep 2003, 3, 159–165. [Google Scholar]
- Vinten-Johansen, J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res 2004, 61, 481–497. [Google Scholar]
- Borregaard, N. The human neutrophil. Function and dysfunction. Eur. J. Haematol 1998, 41, 401–413. [Google Scholar]
- Roos, D.; van Bruggen, R.; Meischl, C. Oxidative killing of microbes by neutrophils. Microbes. Infect 2003, 5, 1307–1315. [Google Scholar]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest 1968, 97, 77–89. [Google Scholar]
- Jauregui, H.O.; Hayner, N.T.; Driscoll, J.L.; Williams-Holland, R.; Lipsky, M.H.; Galletti, P.M. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes-freshly isolated cells, cell suspensions, and primary monolayer cultures. In Vitro 1981, 17, 1100–1110. [Google Scholar]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest 1973, 52, 741–744. [Google Scholar]
- Hwang, T.L.; Leu, Y.L.; Kao, S.H.; Tang, M.C.; Chang, H.L. Viscolin, a new chalcone from Viscum coloratum, inhibits human neutrophil superoxide anion and elastase release via a cAMP-dependent pathway. Free Radic. Biol. Med 2006, 41, 1433–1441. [Google Scholar]
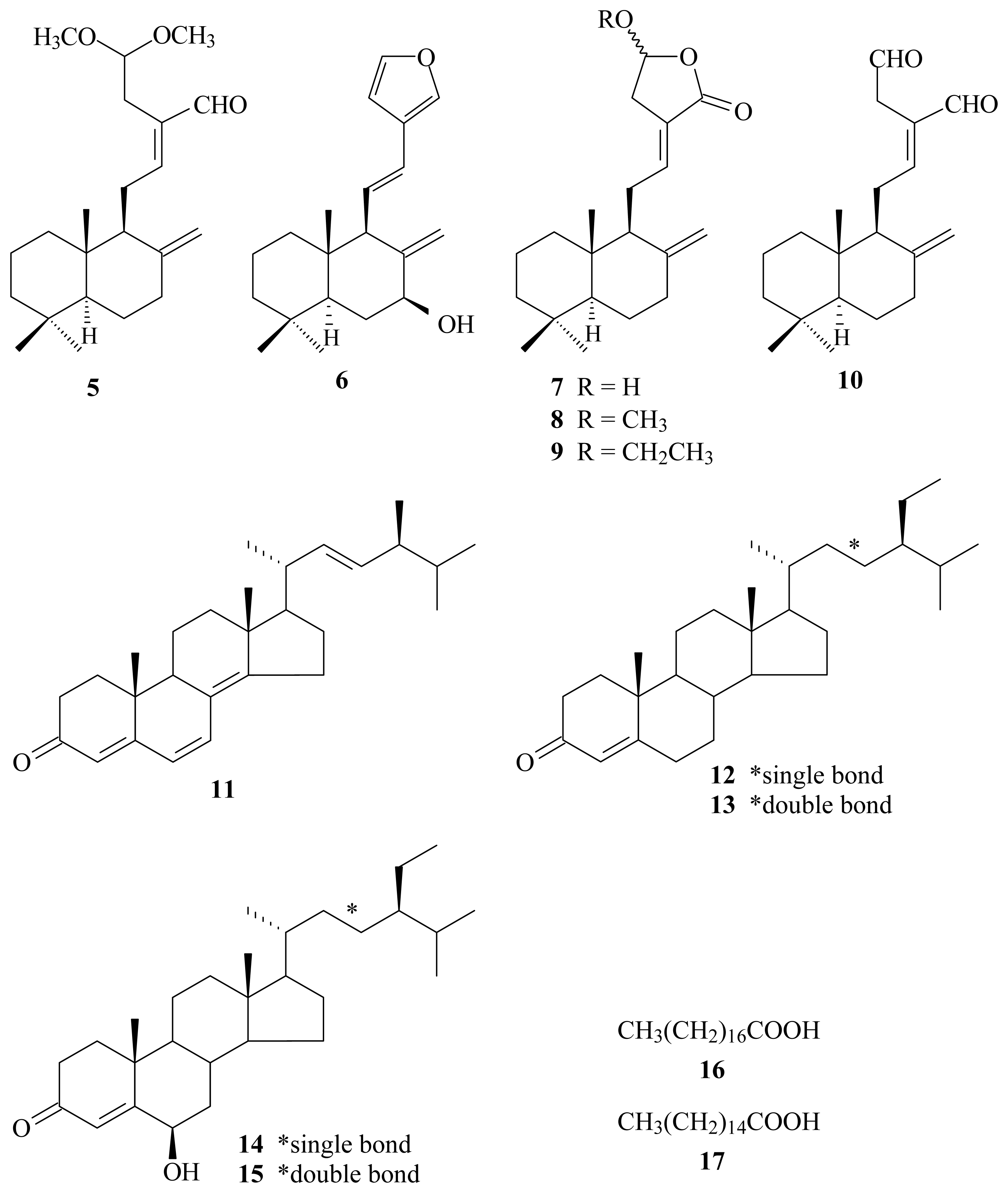
| Compounds | Superoxide anion | Elastase |
|---|---|---|
| IC50 (μg/mL) b | IC50 (μg/mL) b | |
| Hedychicoronarin (1) | >10 | >10 |
| Peroxycoronarin D (2) | >10 | >10 |
| 7β-Hydroxycalcaratarin A (3) | 4.52 ± 1.14 e | 3.86 ± 0.98 g |
| (E)-7β-Hydroxy-6-oxo-labda-8(17),12-diene-15,16-dial (4) | >10 | 5.36 ± 1.23 |
| Calcaratarin A (5) | 2.25 ± 0.42 e | 2.36 ± 0.41 e |
| Coronarin A (6) | 3.31 ± 2.41 | 2.67 ± 1.68 |
| Coronarin D (7) | >10 | >10 |
| Coronarin D methyl ether (8) | >10 | >10 |
| Coronarin D ethyl ether (9) | >10 | >10 |
| (E)-Labda-8(17),12-diene-15,16-dial (10) | 3.21 ± 1.24 | 3.70 ± 1.18 f |
| Ergosta-4,6, 8(14),22-tetraen-3-one (11) | >10 | 6.17 ± 1.30 e |
| Mixture of β-sitostenone (12) and β-stigmasta-4,22-dien-3-one (13) | >10 | >10 |
| Mixture of 6β-hydroxystigmast-4-en-3-one (14) and 6β-hydroxystigmasta-4,22-dien-3-one (15) | >10 | >10 |
| Mixture of stearic acid (16) and palmitic acid (17) | >10 | >10 |
| LY294002 c | 0.36 ± 0.04 | 0.63 ± 0.07 |
| Diphenyleneiodonium d | 0.55 ± 0.24 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, J.-J.; Ting, C.-W.; Wu, Y.-C.; Hwang, T.-L.; Cheng, M.-J.; Sung, P.-J.; Wang, T.-C.; Chen, J.-F. New Labdane-Type Diterpenoids and Anti-Inflammatory Constituents from Hedychium coronarium. Int. J. Mol. Sci. 2013, 14, 13063-13077. https://doi.org/10.3390/ijms140713063
Chen J-J, Ting C-W, Wu Y-C, Hwang T-L, Cheng M-J, Sung P-J, Wang T-C, Chen J-F. New Labdane-Type Diterpenoids and Anti-Inflammatory Constituents from Hedychium coronarium. International Journal of Molecular Sciences. 2013; 14(7):13063-13077. https://doi.org/10.3390/ijms140713063
Chicago/Turabian StyleChen, Jih-Jung, Chia-Wei Ting, Yi-Chin Wu, Tsong-Long Hwang, Ming-Jen Cheng, Ping-Jyun Sung, Tai-Chi Wang, and Jinn-Fen Chen. 2013. "New Labdane-Type Diterpenoids and Anti-Inflammatory Constituents from Hedychium coronarium" International Journal of Molecular Sciences 14, no. 7: 13063-13077. https://doi.org/10.3390/ijms140713063