Comparison of Cellular Uptake and Inflammatory Response via Toll-Like Receptor 4 to Lipopolysaccharide and Titanium Dioxide Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
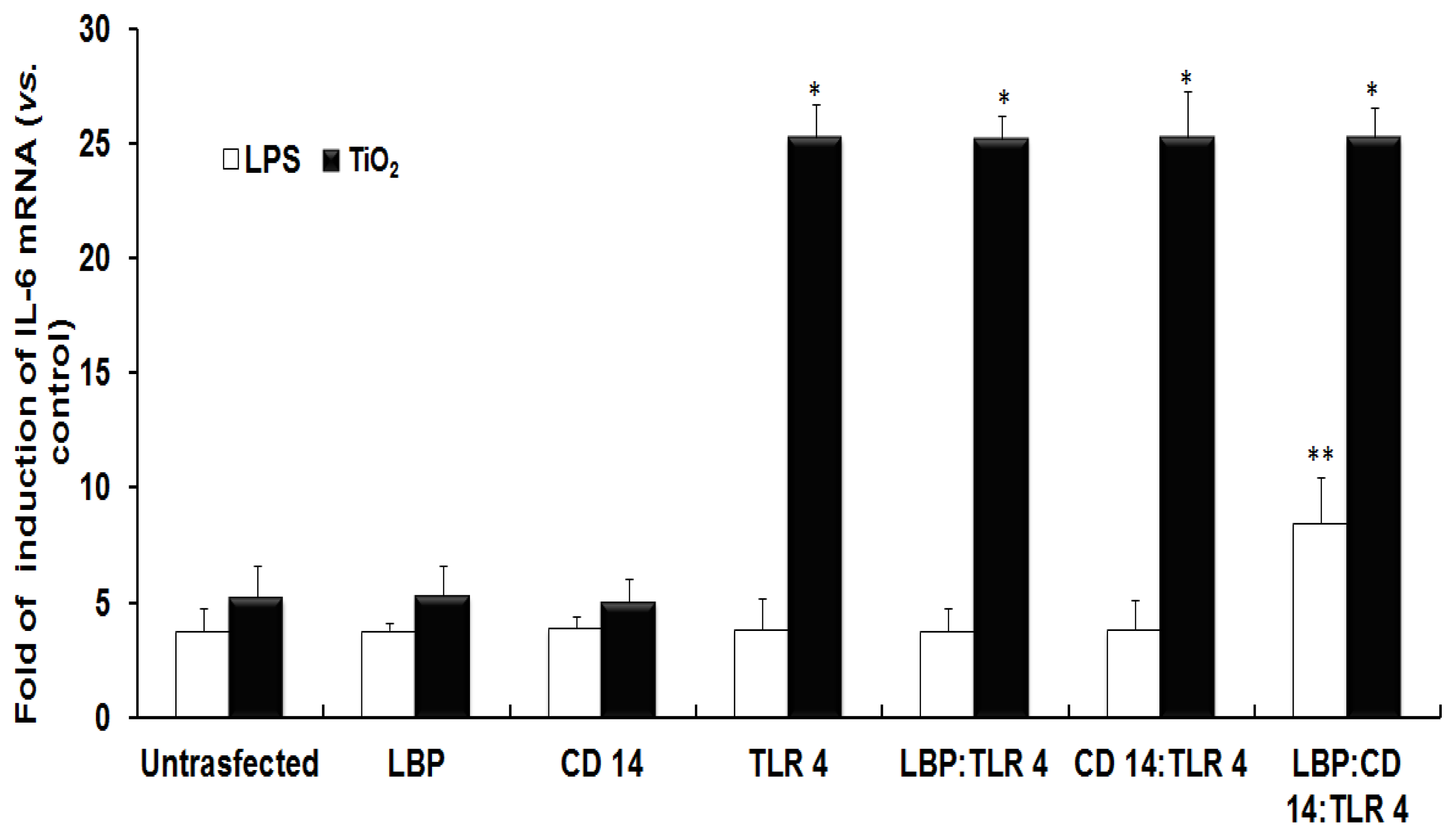
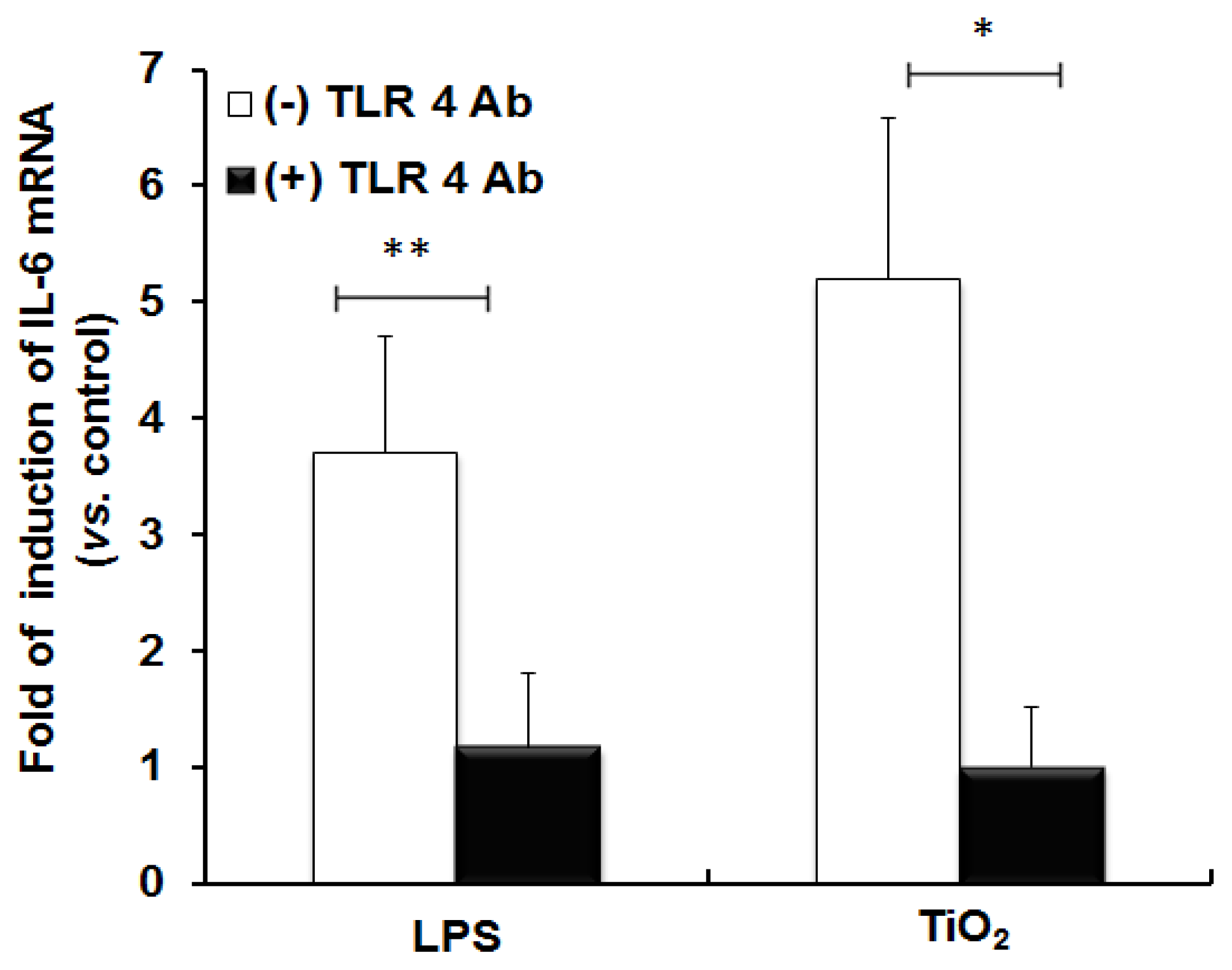
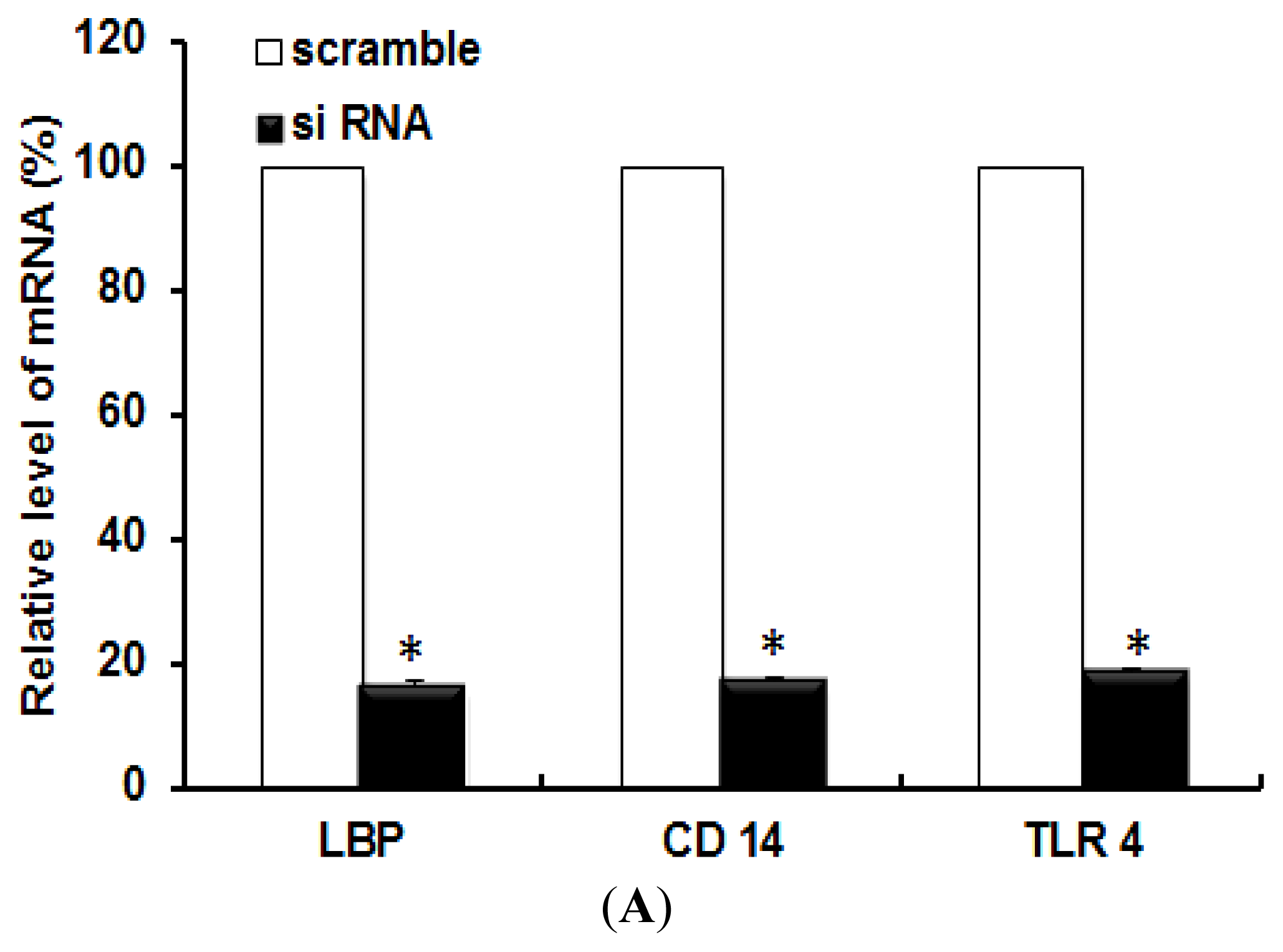
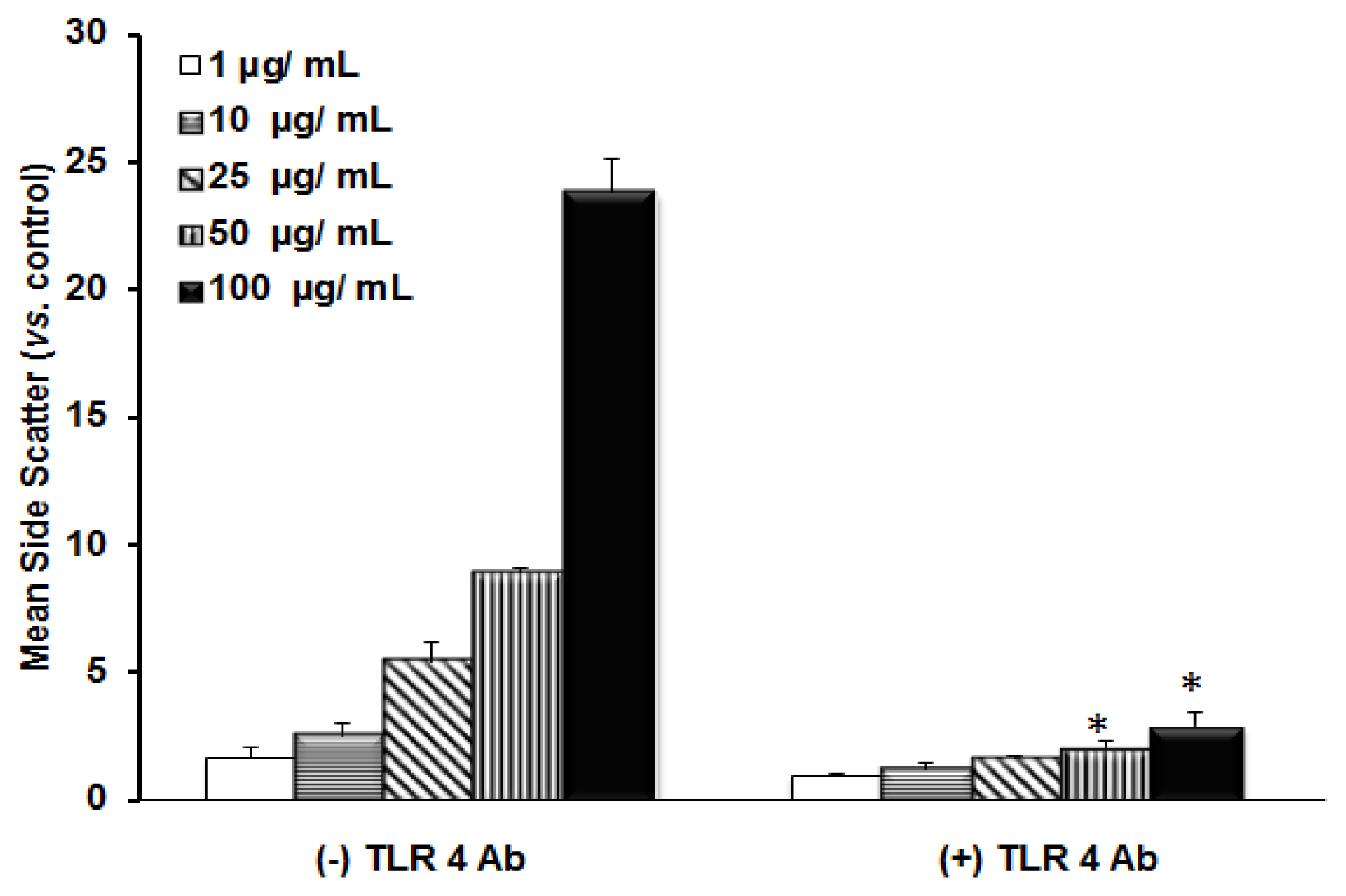
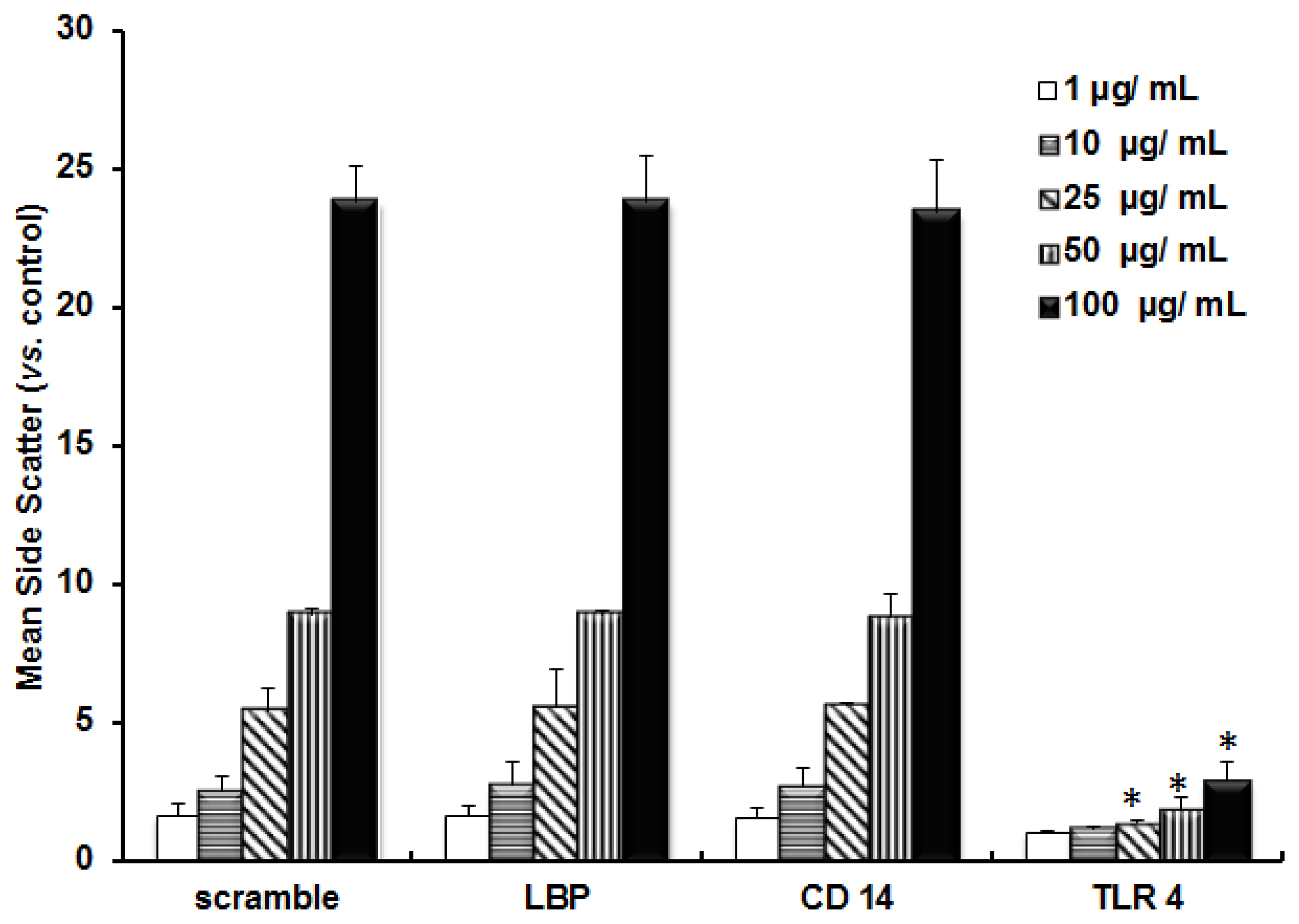
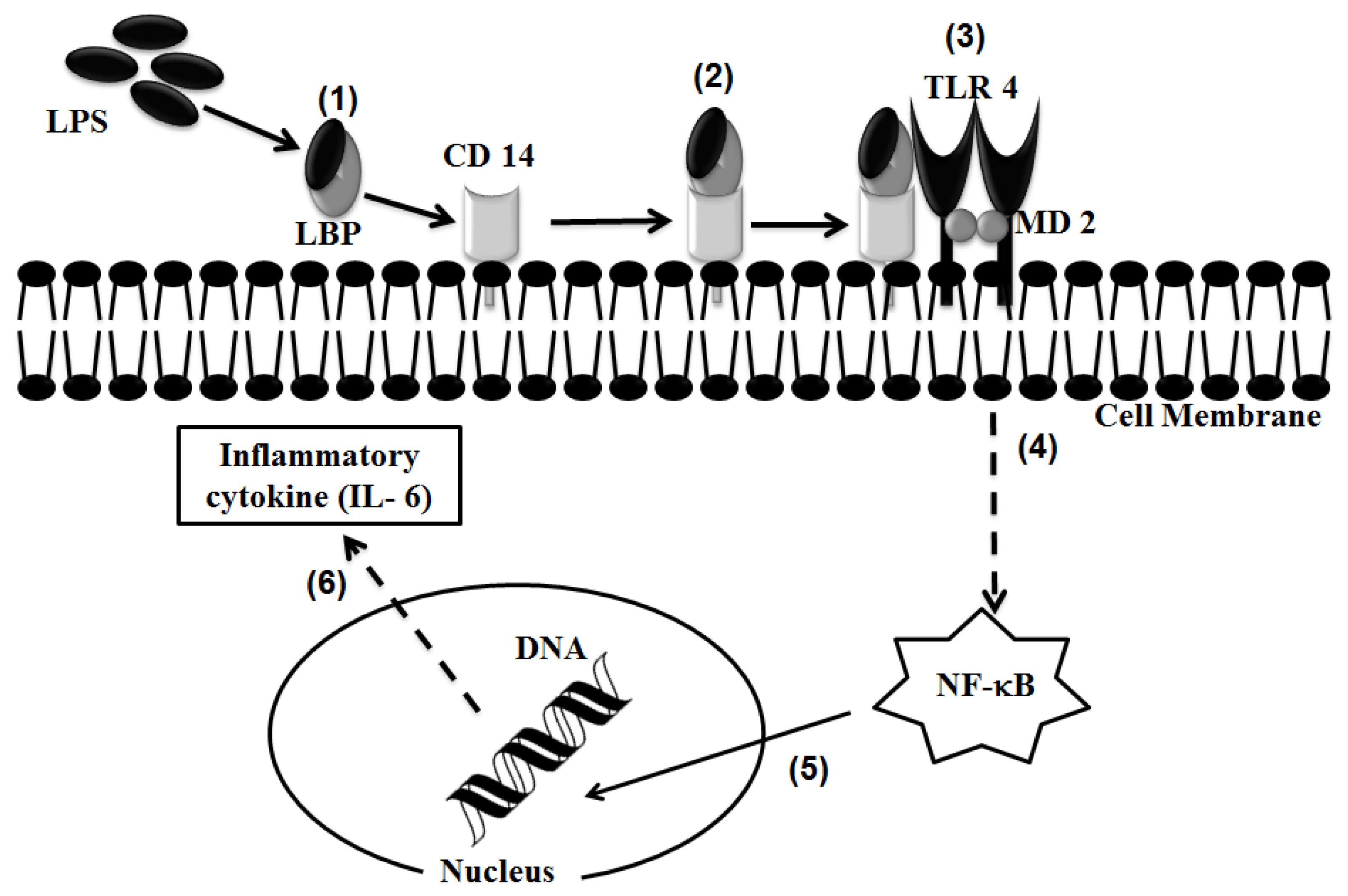
2.1. TLR 4, but Not LBP or CD 14, Is Involved in the Inflammatory Signal Transduction Mediated by TiO2 NPs
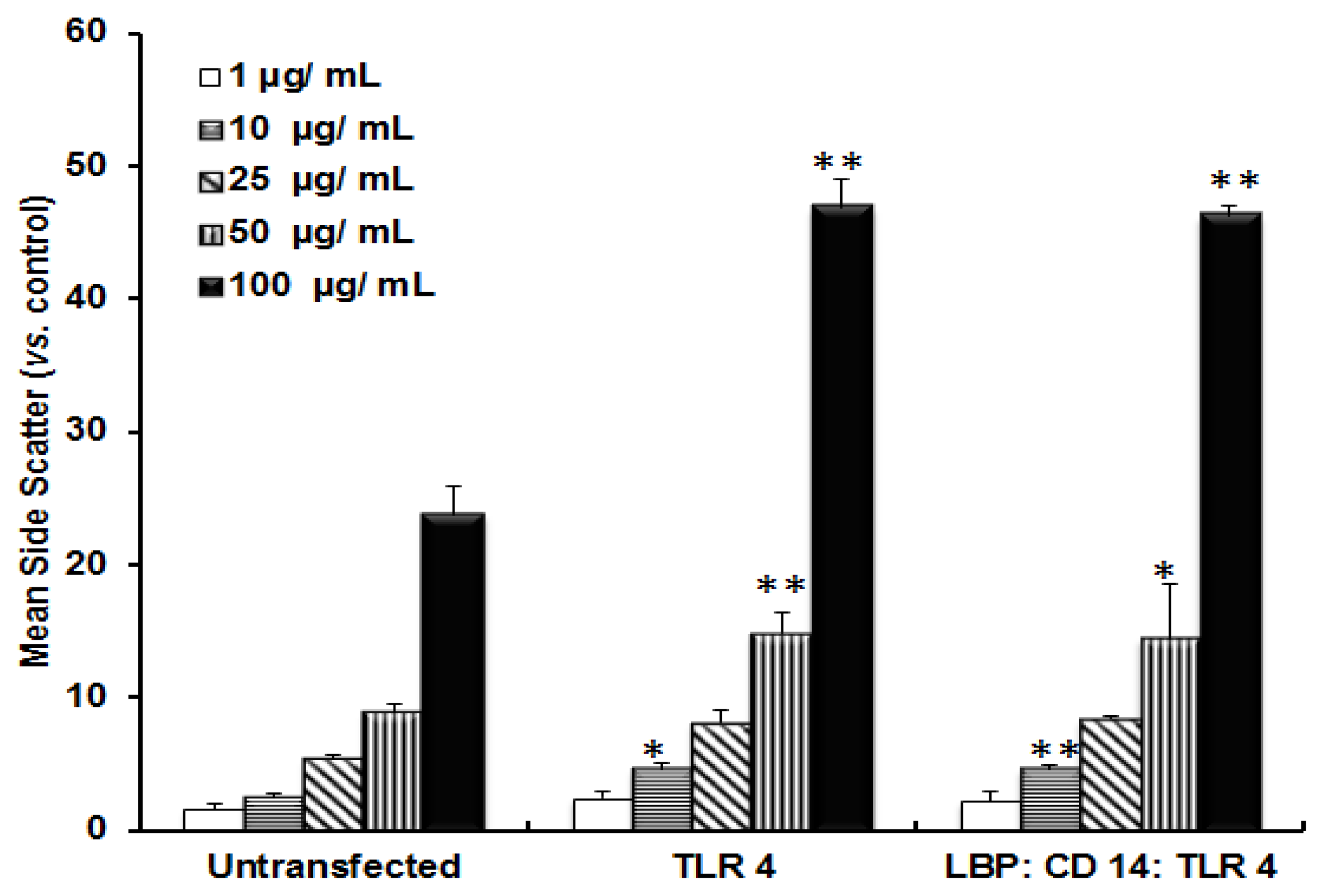
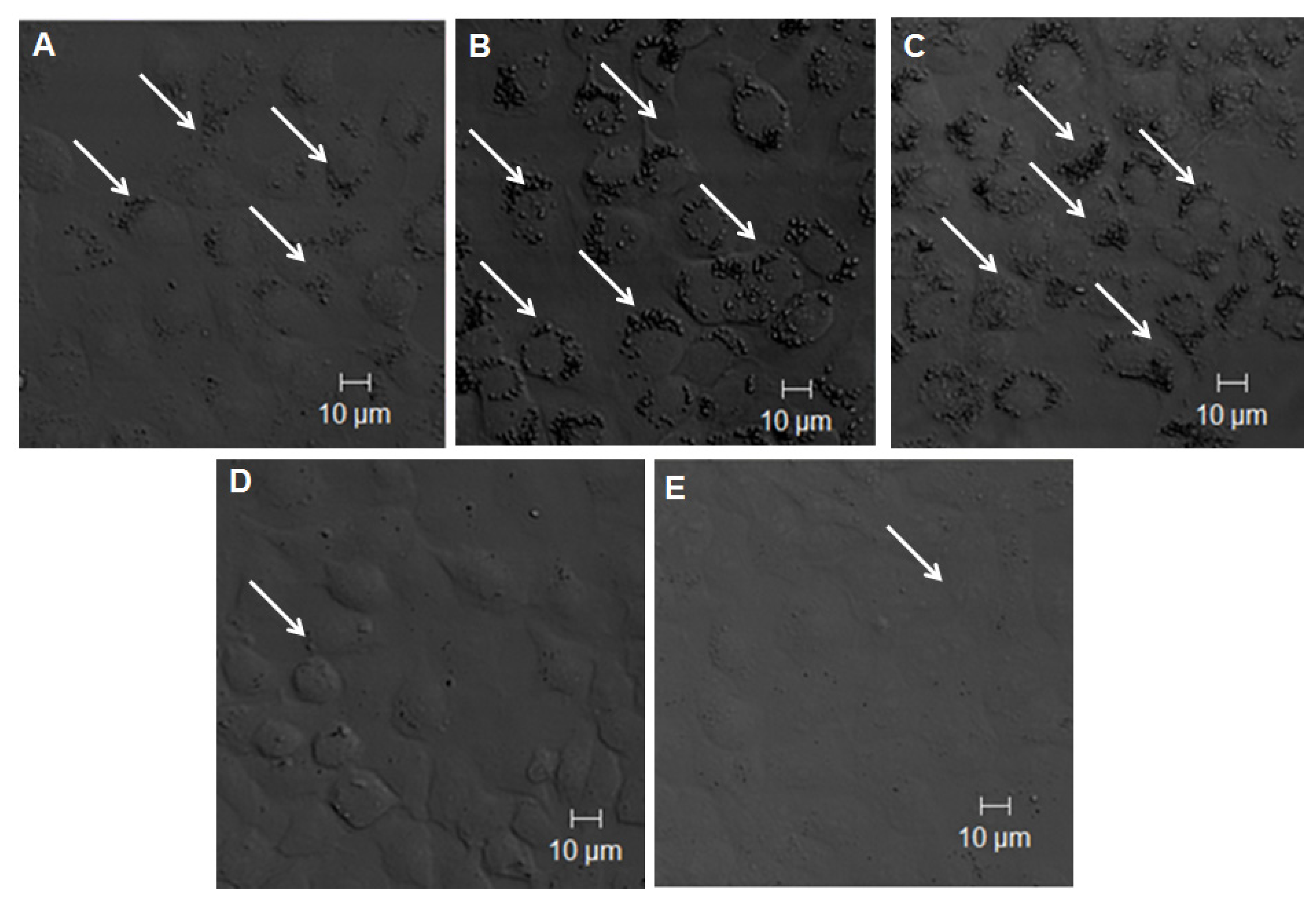
2.2. TLR 4, but Not LBP or CD 14, Is Involved in the Uptake of TiO2 NPs to Cells
2.3. Discussion
3. Experimental Section
3.1. Preparation of Titanium Dioxide Nanoparticles
3.2. Cell Culture
3.3. Quantitative Real-Time (RT) PCR
3.4. Fluorescent Activated Cell Sorter (FACS) Analysis
3.5. Confocal Laser Scanning Microscopic (CLSM) Observation
3.6. Statistical Analysis
4. Conclusions
Conflict of Interest
References
- He, Q.; Ma, M.; Wei, C.; Shi, J. Mesoporous carbon@silicon-silica nanotheranostics for synchronous delivery of insoluble drugs and luminescence imaging. Biomaterials 2012, 33, 4392–4402. [Google Scholar]
- Thurn, K.T.; Brown, E.M.B.; Wu, A.; Vogt, S.; Lai, B.; Maser, J.; Paunesku, T.; Woloschak, G.E. Nanoparticles for applications in cellular imaging. Nanoscale Res. Lett 2007, 2, 430–441. [Google Scholar]
- Kong, H.; Liu, D.; Zhang, S.; Zhang, X. Protein sensing and cell discrimination using a sensor array based on nanomaterial-assisted chemiluminescence. Anal. Chem 2011, 83, 1867–1870. [Google Scholar]
- Yang, P.; Gaib, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev 2012, 41, 3679–3698. [Google Scholar]
- Huang, S.; Li, C.; Cheng, Z.; Fan, Y.; Yang, P.; Zhang, C.; Yang, K.; Lin, J. Magnetic Fe3O4@mesoporous silica composites for drug delivery and bioadsorption. J. Colloid Intreface Sci 2012, 376, 312–321. [Google Scholar]
- Rosenholm, J.M.; Meinander, A.; Peuhu, E.; Niemi, R.; Eriksson, J.E.; Sahlgren, C.; Linden, M. Targeting of porous hybrid silica nanoparticles to cancer cells. ACS Nano 2009, 3, 197–206. [Google Scholar]
- Choi, Y.E.; Kwak, J.W.; Park, J.W. Nanotechnology for early cancer detection. Sensors 2010, 10, 428–455. [Google Scholar]
- Youns, M.; Hoheisel, J.D.; Efferth, T. Therapeutic and diagnostic applications of nanoparticles. Curr. Drug Targets 2011, 12, 357–365. [Google Scholar]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther 2008, 83, 761–769. [Google Scholar]
- Donaldson, K.; Stone, V.; Tran, C.; Kreyling, W.; Borm, P. Nanotoxicology. Occup. Environ. Med 2004, 6, 727–728. [Google Scholar]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fiber Toxicol. 2010, 7. [Google Scholar] [CrossRef]
- Alex, W.; Paul, W.; Lars, F.; Kiril, H.; Natalie, V.G. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol 2012, 46, 2242–2250. [Google Scholar]
- Baveye, P.; Laba, M. Aggregation and toxicology of titanium dioxide nanoparticles. Environ. Health Perspect 2008, 116, A152–A155. [Google Scholar]
- Jacobs, J.F.; Poel, I.V.D.; Osseweijer, P. Sunscreens with titanium dioxide (TiO2) nano-particles: A societal experiment. Nanoethics 2010, 4, 103–113. [Google Scholar]
- Valant, J.; Drobne, D. Biological reactivity of TiO2 nanoparticles assessed by ex vivo testing. Protoplasma 2011, 249, 835–842. [Google Scholar]
- Singh, S.; Shi, T.; Duffin, R.; Albrecht, C.; Berlo, D.V.; Hohr, D.; Fubini, B.; Martra, G.; Fenoglio, I.; Borm, P.J.A.; et al. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: Role of the specific surface area and of surface methylation of the particles. Toxicol. Appl. Pharmacol 2007, 222, 141–151. [Google Scholar]
- Cheyne, R.W.; Smith, T.A.D.; Trembleau, L.; Mclaughlin, A.C. Synthesis and characterisation of biologically compatible TiO2nanoparticles. Nanoscale Res. Lett. 2011, 6. [Google Scholar] [CrossRef] [Green Version]
- Lazau, C.; Mocanu, L.; Miron, I.; Sfirloaga, P.; Tanasiea, G.; Tatua, C.; Gruiab, A.; Grozescu, I. Consideration regarding the use of TiO2 doped nanoparticles in medicine. Dig. J. Nanomater. Biostruct 2007, 2, 257–263. [Google Scholar]
- Fitzgerald, K.A.; Rowe, D.C.; Barnes, B.J.; Caffrey, D.R.; Visintin, A.; Latz, E.; Monks, B.; Pitha, P.M.; Golenbock, D.T. LPS-TLR4 signaling to IRF-3/7 and NF-κB involves the toll adapters TRAM and TRIF. J. Exp. Med 2003, 6, 1043–1055. [Google Scholar]
- Mano, S.S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Toll-like receptor 4 is involved in titanium dioxide nanoparticle incorporation into cells. Sci. Adv. Mater. 2013, in press. [Google Scholar]
- Chen, P.; Migita, S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Development of sensor cells using NF-κB pathway activation for detection of nanoparticle induced inflammation. Sensors 2011, 11, 7219–7230. [Google Scholar]
- Chen, P.; Migita, S.; Kanehira, K.; Taniguchi, A. Role of toll-like receptors 3, 4 and 7 in cellular uptake and response to titanium dioxide nanoparticles. Sci. Tech. Adv. Mater. 2013, 14. [Google Scholar] [CrossRef]
- Laquerriere, A.G.; Tabary, O.; Jacquot, J.; Richard, D.; Frayssinet, P.; Guenounou, M.; Maquin, D.L.; Laquerriere, P.; Gangloff, S. Involvement of toll-like receptor 4 in the inflammatory reaction induced by hydroxyapatite particles. Biomaterials 2007, 28, 400–404. [Google Scholar]
- Chen, G.Y.; Yang, H.J.; Lu, C.H.; Chao, Y.C.; Hwang, S.M.; Chen, C.L.; Lo, K.W.; Sung, L.Y.; Luo, W.Y.; Tuan, H.Y.; et al. Simultaneous induction of autophagy and toll-like receptor signaling pathways by graphene oxide. Biomaterials 2012, 33, 6559–6569. [Google Scholar]
- Cui, Y.; Liu, H.; Zhou, M.; Duan, Y.; Li, N.; Gong, X.; Hu, R.; Hong, M.; Hong, F. Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J. Biomed. Mater. Res. A 2011, 96, 221–229. [Google Scholar]
- Darveau, R.P.; Hancock, R.E.W. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J. Bacteriol 1983, 155, 831–838. [Google Scholar]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–14333. [Google Scholar]
- Fenton, M.J.; Golenbock, D.T. LPS-binding proteins and receptors. J. Leuk. Biol 1998, 64, 25–32. [Google Scholar]
- Vreugdenhil, A.C.E.; Dentener, M.A.; Snoek, A.M.; Greve, J.M.; Buurman, W.A. Lipopolysaccharide binding protein and serum amyloid A secretion by human intestinal epithelial cells during the ccute phase response. J. Immunol 1999, 163, 2792–2798. [Google Scholar]
- Pugin, J.; Cornelia, C.; Maly, S.; Leturcq, D.; Moriarty, A. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc. Natl. Acad. Sci. USA 1993, 90, 2744–2748. [Google Scholar]
- Kogut, M.H.; He, H.; Kaiser, P. Lipopolysaccharide binding protein/CD14/TLR4-dependent recognition of salmonella LPS induces the functional activation of chicken heterophils and up-regulation of pro-inflammatory cytokine and chemokine gene expression in these cells. Anim. Biotechnol 2005, 16, 165–181. [Google Scholar]
- Shimazaki, J.O.; Takaku, S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Effects of titanium dioxide nanoparticle aggregate size on gene expression. Int. J. Mol. Sci. 2010, 11, 2383–2392. [Google Scholar]
- Mano, S.S.; Kanehira, K.; Sonezaki, S.; Taniguchi, A. Effect of polyethylene glycol modification of TiO2 nanoparticles on cytotoxicity and gene expressions in human cell lines. Int. J. Mol. Sci 2012, 13, 3703–3717. [Google Scholar]
- Buzea, C.; Blandino, I.I.P.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar]
- Stebounova, L.V.; Dodd, A.A.; Kim, J.S.; Park, H.; O’Shaughnessy, P.T.; Grassian, V.H.; Thorne, P.S. Nanosilver induces minimal lung toxicity or inflammation in a subacute murine inhalation model. Part. Fiber Toxicol. 2011, 8. [Google Scholar] [CrossRef]
- Patel, H.; Kwon, S. Multi-walled carbon nanotube-induced inflammatory response and oxidative stress in a dynamic cell growth environment. J. Biol. Eng. 2012, 6. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, H.; Kim, Y.; Yi, J.; Choi, K. Carbon fullerenes (C60s) can induce inflammatory responses in the lung of mice. Toxicol. Appl. Pharmacol 2010, 244, 226–233. [Google Scholar]
- Tsou, T.C.; Yeh, S.C.; Tsai, F.Y.; Lin, H.J.; Cheng, T.J.; Chao, H.R.; Tai, L.A. Zinc oxide particles induce inflammatory responses in vascular endothelial cells via NF-κB signaling. J. Hazard. Mater 2010, 183, 182–188. [Google Scholar]
- Ma, J.Y.; Zhao, H.; Mercer, R.R.; Barger, M.; Rao, M.; Meighan, T.; Schwegler, B.D.; Castranova, V.; Ma, J.K. Cerium oxide nanoparticle-induced pulmonary inflammation and alveolar macrophage functional change in rats. Nanotoxicology 2011, 5, 312–325. [Google Scholar]
- Sun, Q.; Tan, D.; Ze, Y.; Sang, X.; Liu, X.; Gui, S.; Cheng, Z.; Cheng, J.; Hu, R.; Gao, G.; et al. Pulmotoxicological effects caused by long-term titanium dioxide nanoparticles exposure in mice. J. Hazard. Mater. 2012, 235–236, 47–53. [Google Scholar]
- Cui, Y.; Gong, X.; Duan, Y.; Li, N.; Hu, R.; Liu, H.; Hong, M.; Zhou, M.; Wang, L.; Wang, H.; et al. Hepatocyte apoptosis and its molecular mechanisms in mice caused by titanium dioxide nanoparticles. J. Hazard. Mater. 2010, 183, 874–880. [Google Scholar]
- Linglan, M.; Jinfang, Z.; Jue, W.; Jie, L.; Yanmei, D.; Huiting, L.; Na, L.; Jingying, Y.; Jie, R.; Han, W.; et al. The acute liver injury in mice caused by nano-anatase TiO2. Nanoscale Res. Lett 2009, 4, 1275–1285. [Google Scholar]
- Yaling, C.; Huiting, L.; Yuguan, Z.; Zhang, Z.; Yuanyuan, H.; Zhe, C.; Jie, C.; Renping, H.; Guodong, G.; Ling, W.; et al. Gene expression in liver injury caused by long term exposure of TiO2 nanoparticles in mice. Toxicol. Sci 2012, 128, 171–185. [Google Scholar]
- Gui, S.; Zhang, Z.; Zheng, L.; Cui, Y.; Liu, X.; Li, N.; Sang, X.; Sun, Q.; Gao, G.; Cheng, Z.; et al. Molecular mechanism of kidney injury of mice caused by exposure to titanium dioxide nanoparticles. J. Hazard. Mater 2011, 195, 365–370. [Google Scholar]
- Qingqing, S.; Danlin, T.; Qiuping, Z.; Xiaorun, L.; Zhe, C.; Gan, L.; Min, Z.; Xuezi, S.; Suxin, G.; Jie, C.; et al. Oxidative damage of lung and its protective mechanism in mice caused by long-term exposure to titanium dioxide nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 2554–2562. [Google Scholar]
- Ze, Y.; Zheng, L.; Zhao, X.; Gui, S.; Sang, X.; Su, J.; Guan, N.; Zhu, L.; Sheng, L.; Hu, R.; et al. Molecular mechanism of titanium dioxide nanoparticles-induced oxidative injury in the brain of mice. Chemosphere 2013. [Google Scholar] [CrossRef]
- Suxin, G.; Xuezi, S.; Lei, Z.; Yuguan, Z.; Xiaoyang, Z.; Lei, S.; Qingqing, S.; Zhe, C.; Jie, C.; Renping, H.; et al. Intragastric exposure to titanium dioxide nanoparticles induced nephrotoxicity in mice, assessed by physiological and gene expression modifications. Parti. Fib. Toxi. 2013, 10. [Google Scholar] [CrossRef]
- Xiaoyang, Z.; Yuguan, Z.; Guodong, G.; Xuezi, S.; Bing, L.; Suxin, G.; Lei, S.; Qingqing, S.; Jie, C.; Zhe, C.; et al. Nanosized TiO2-induced reproductive system dysfunction and its mechanism in female mice. PLoS One 2013, 8, e59378. [Google Scholar]
- Farhat, K.; Riekenberg, S.; Heine, H.; Debarry, J.; Lang, R.; Mages, J.; Beckmann, U.B.; Roschmann, K.; Jung, G.; Wiesmuller, K.H.; et al. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J. Leukoc. Biol 2008, 83, 692–701. [Google Scholar]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–445. [Google Scholar]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar]
- Crozat, K.; Beutler, B. TLR7: A new sensor of viral infection. Proc. Natl. Acad. Sci. USA 2000, 101, 6835–6836. [Google Scholar]
- Bauer, S.; Kirschning, C.J.; Hacker, H.; Redecke, V.; Hausmann, S.; Akira, S.; Wagner, H.; Lipford, G.B. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 2001, 98, 9237–9242. [Google Scholar]
- Bhanu, P.S.; Chauhan, R.S.; Singhal, L.K. Toll-like receptors and their role in innate immunity. Curr. Sci 2003, 85, 1156–1164. [Google Scholar]
- Ungaro, R.; Fukata, M.; Hsu, D.; Hernandez, Y.; Breglio, K.; Chen, A.; Xu, R.; Sotolongo, J.; Espana, C.; Zaias, J.; et al. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am. J. Physiol. Gastrointest. Liver Physiol 2009, 296, G1167–G1179. [Google Scholar]
- Plotz, S.G.; Lentschat, A.; Behrendt, H.; Plotz, W.; Hamann, L.; Ring, J.; Rietschel, E.T.; Flad, H.D.; Ulmer, A.J. The interaction of human peripheral blood eosinophils with bacterial lipopolysaccharide is CD14 dependent. Blood 2001, 97, 235–241. [Google Scholar]
- Dunzendorfer, S.; Lee, H.K.; Soldau, K.; Tobias, P.S. TLR 4 is the signaling but not the Lipopolysaccharide uptake receptor. J. Immunol 2004, 73, 1166–1170. [Google Scholar]
- Ishibashi, Y.; Imai, S.; Inouye, Y.; Okano, T.; Taniguchi, A. Effects of carbocisteine on sialyl-Lewis x expression in NCI-H292 cells, an airway carcinoma cell line, stimulated with tumor necrosis factor-α. Eur. J. Pharmacol. 2006, 530, 223–228. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mano, S.S.; Kanehira, K.; Taniguchi, A. Comparison of Cellular Uptake and Inflammatory Response via Toll-Like Receptor 4 to Lipopolysaccharide and Titanium Dioxide Nanoparticles. Int. J. Mol. Sci. 2013, 14, 13154-13170. https://doi.org/10.3390/ijms140713154
Mano SS, Kanehira K, Taniguchi A. Comparison of Cellular Uptake and Inflammatory Response via Toll-Like Receptor 4 to Lipopolysaccharide and Titanium Dioxide Nanoparticles. International Journal of Molecular Sciences. 2013; 14(7):13154-13170. https://doi.org/10.3390/ijms140713154
Chicago/Turabian StyleMano, Sharmy Saimon, Koki Kanehira, and Akiyoshi Taniguchi. 2013. "Comparison of Cellular Uptake and Inflammatory Response via Toll-Like Receptor 4 to Lipopolysaccharide and Titanium Dioxide Nanoparticles" International Journal of Molecular Sciences 14, no. 7: 13154-13170. https://doi.org/10.3390/ijms140713154




