Involvement of Intercellular Adhesion Molecule-1 Up-Regulation in Bradykinin Promotes Cell Motility in Human Prostate Cancers
Abstract
:1. Introduction
2. Results
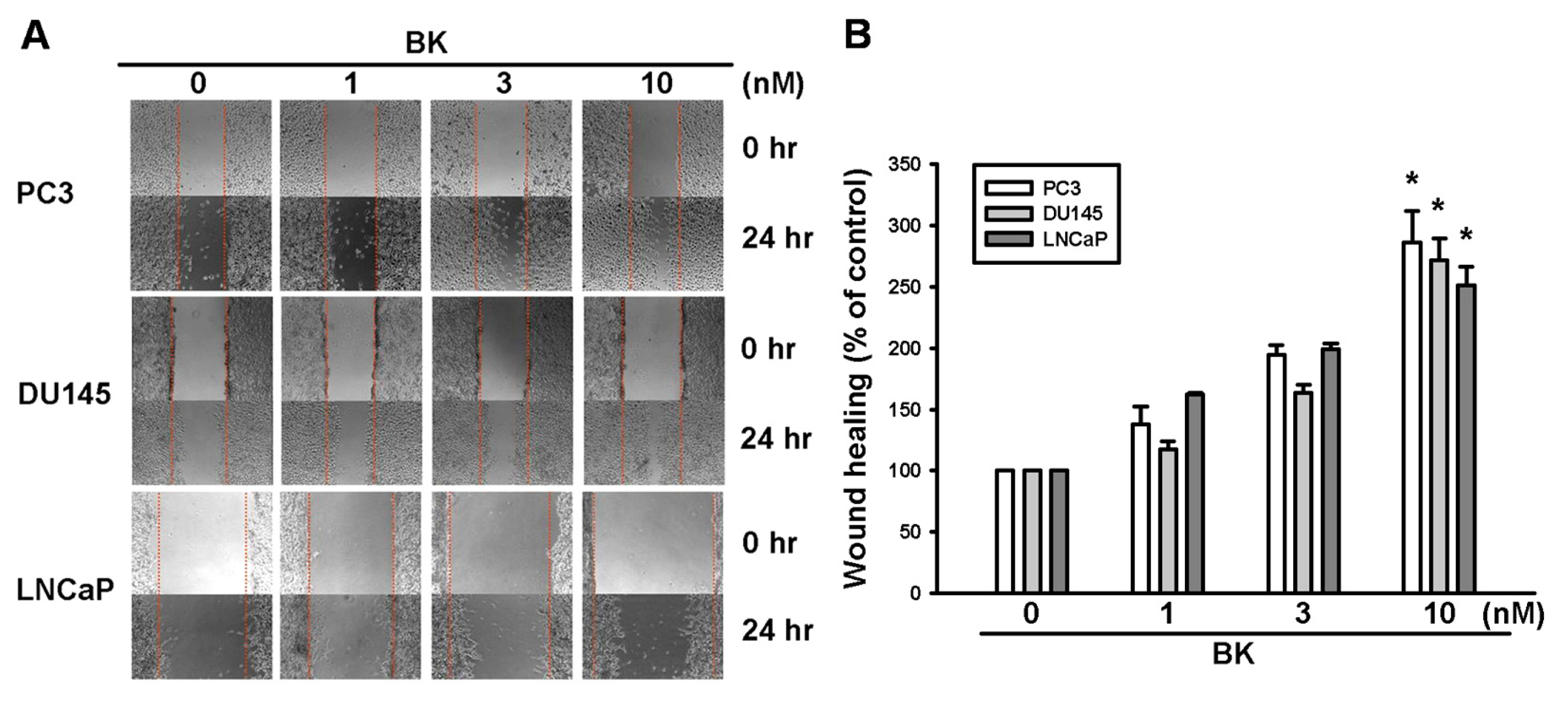
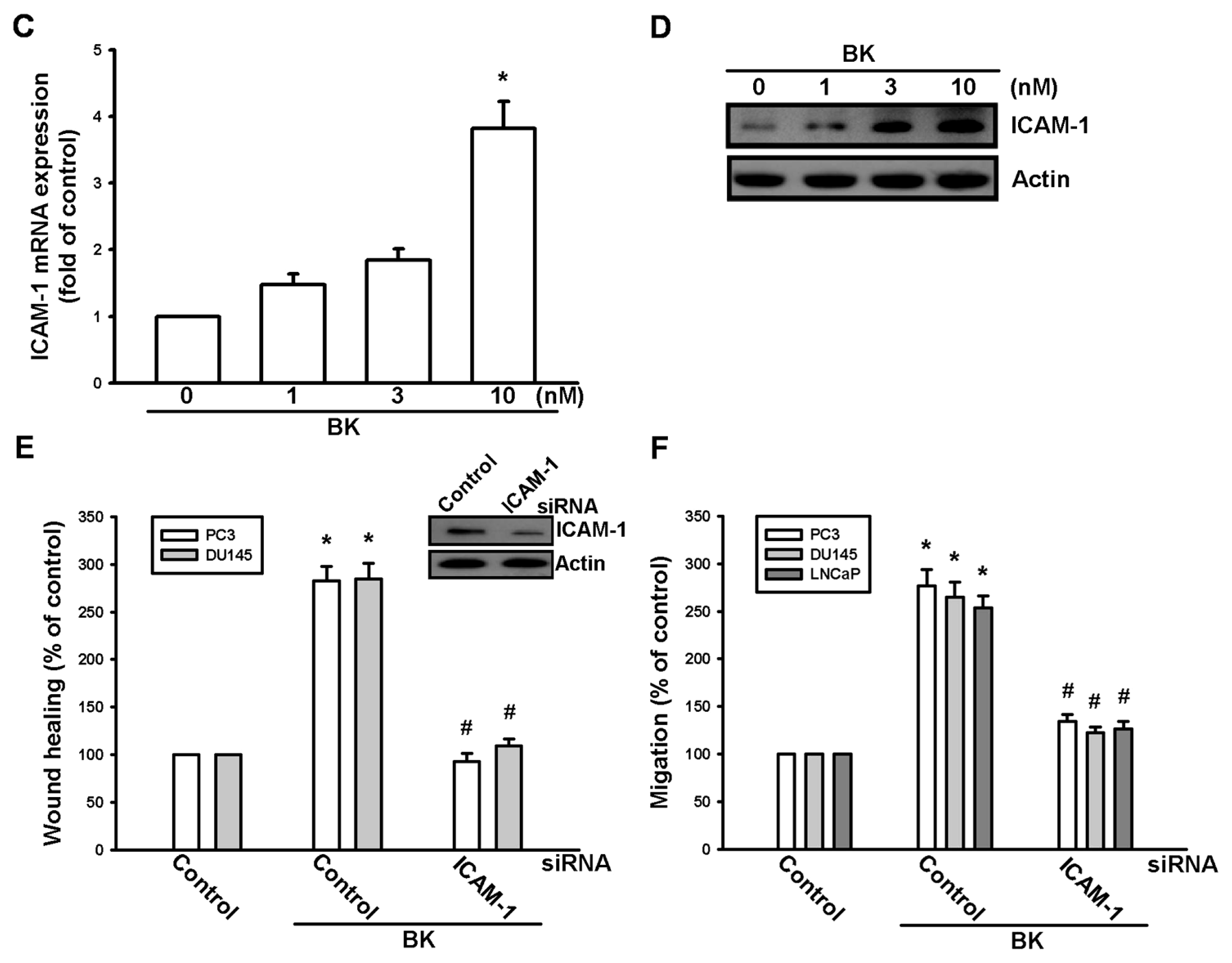
2.1. Involvement of ICAM-1 Up-Regulation in Bradykinin Promotes Cell Motility
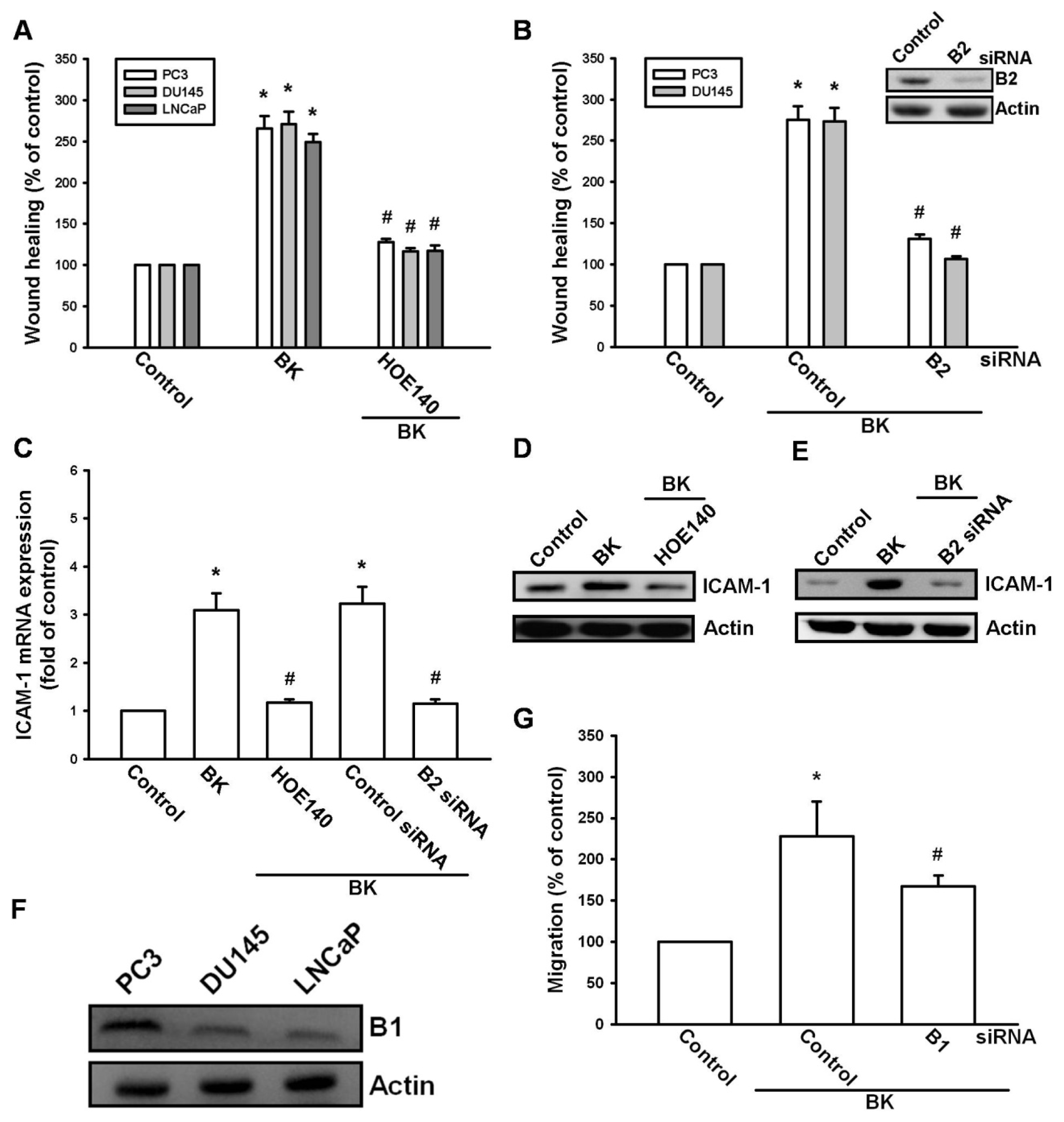
2.2. Involvement of the B2 Receptor in BK-Mediated Migration and ICAM-1 Up-Regulation of Human Prostate Cancer Cells
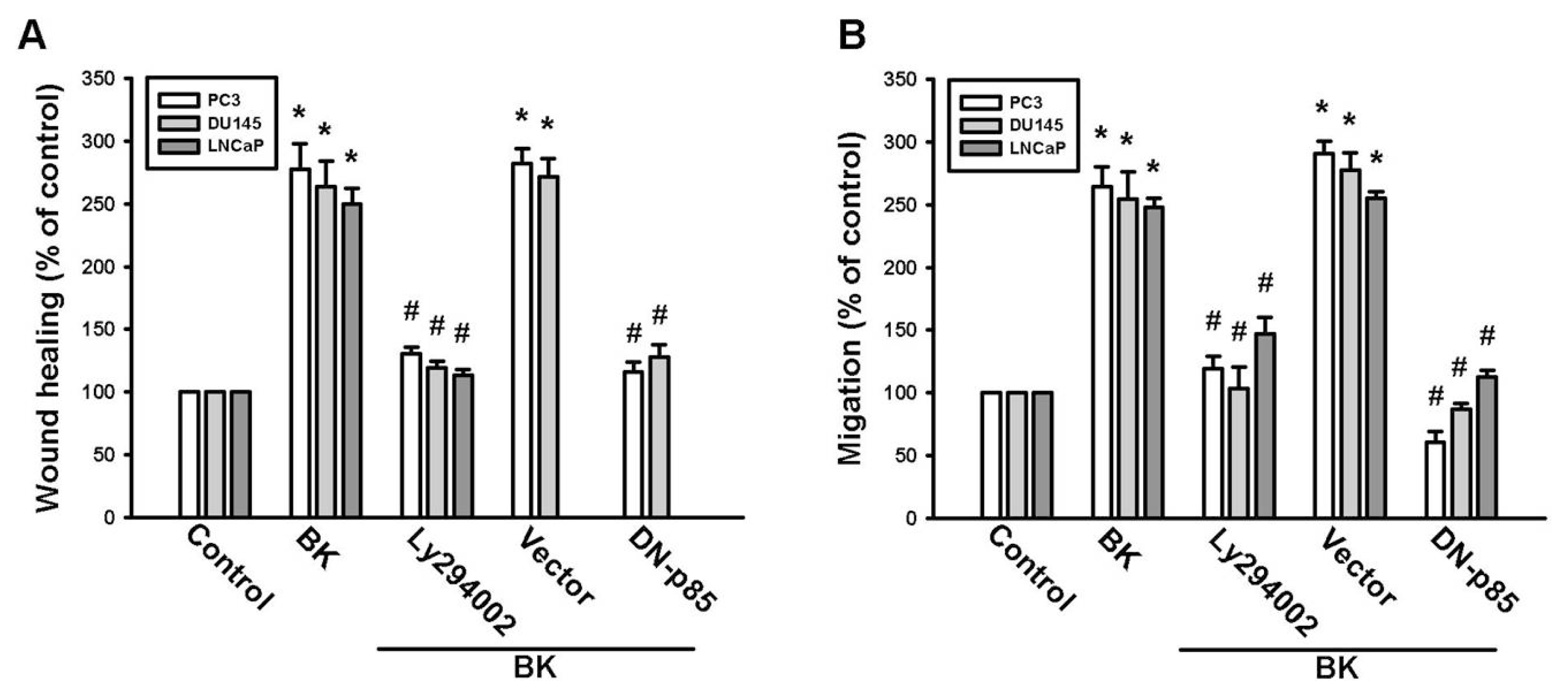
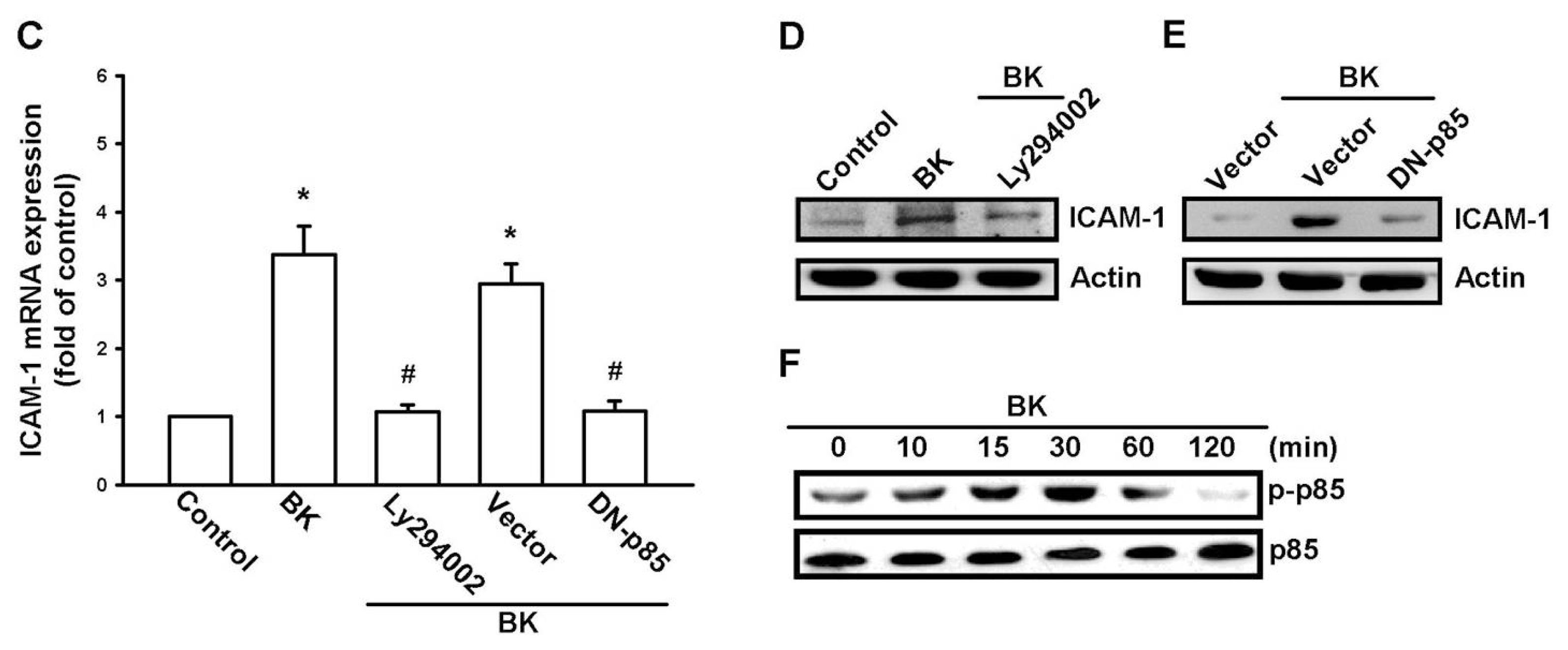
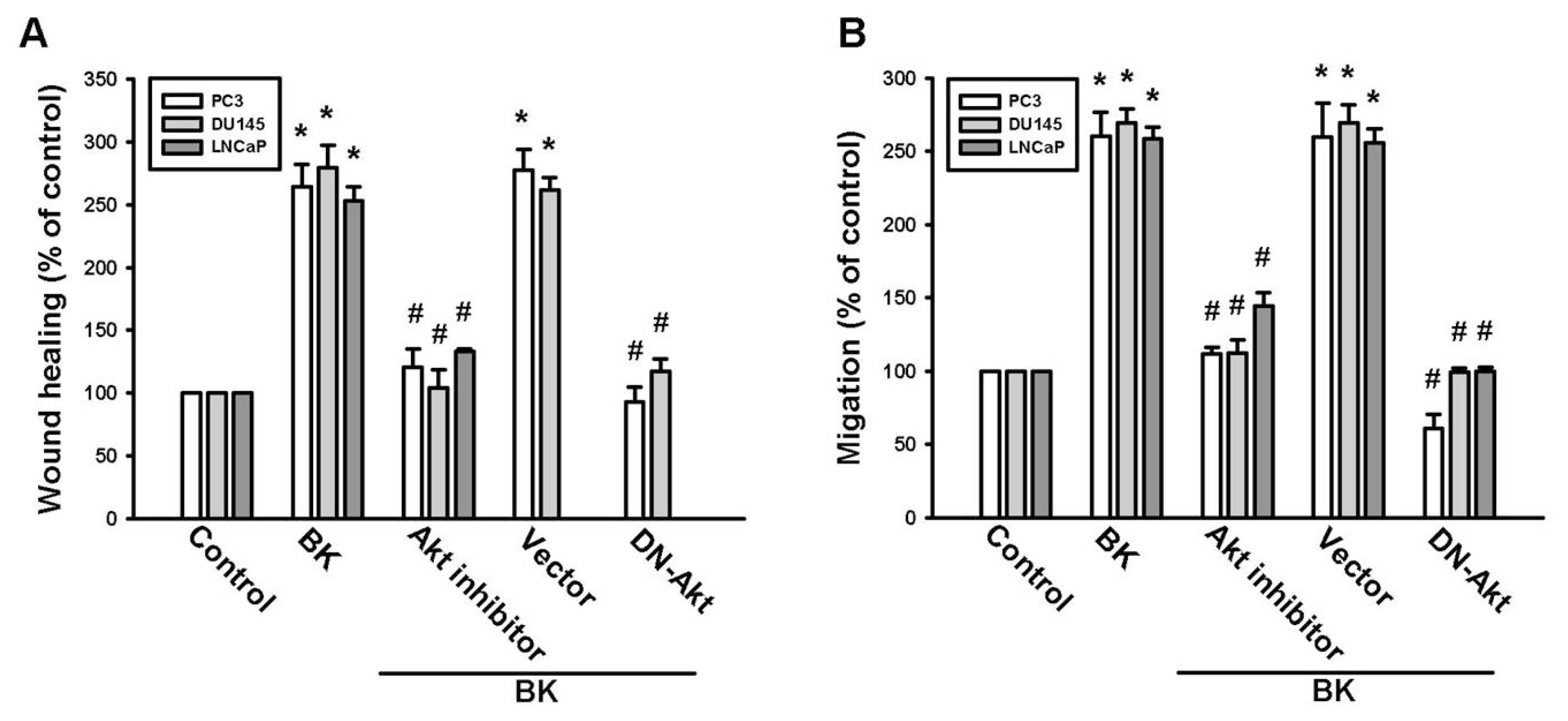
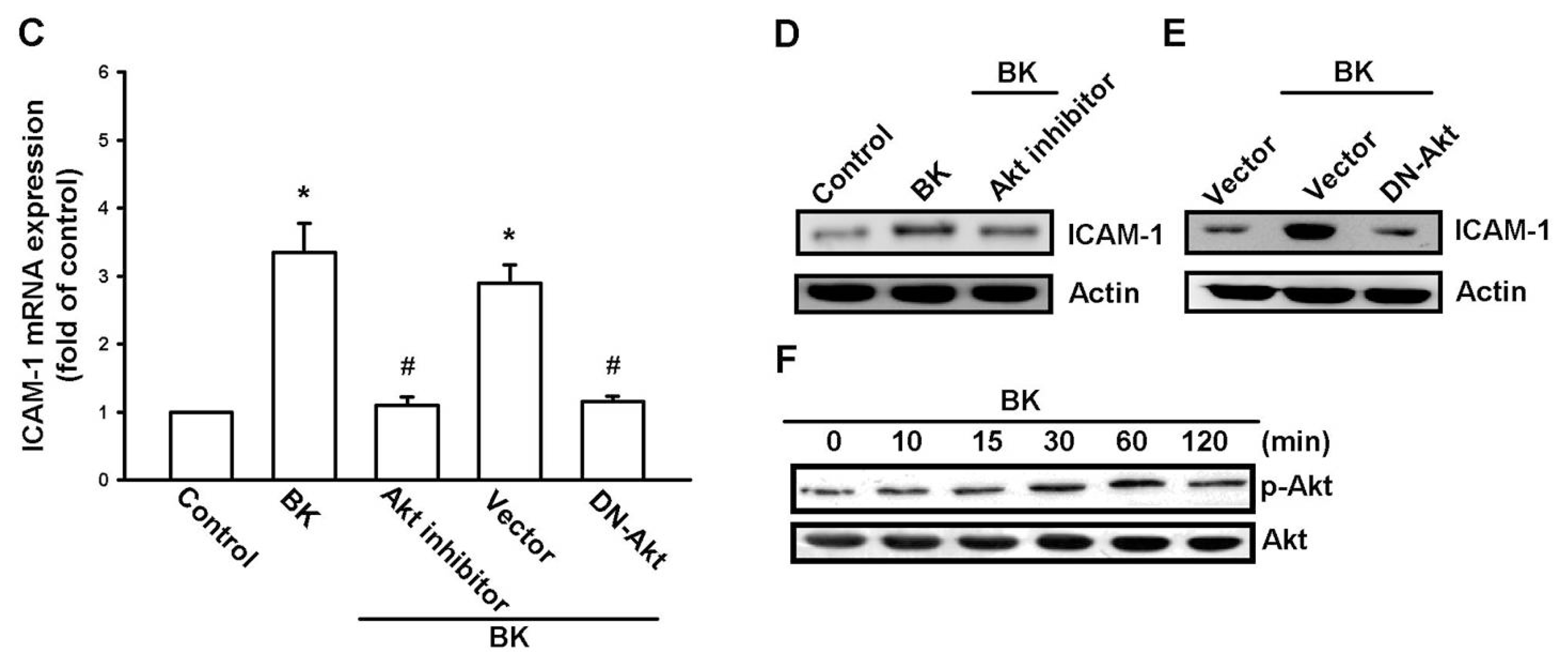
2.3. PI3K and Akt Signaling Pathways Are Involved in BK-Induced Cell Migration and ICAM-1 Expression
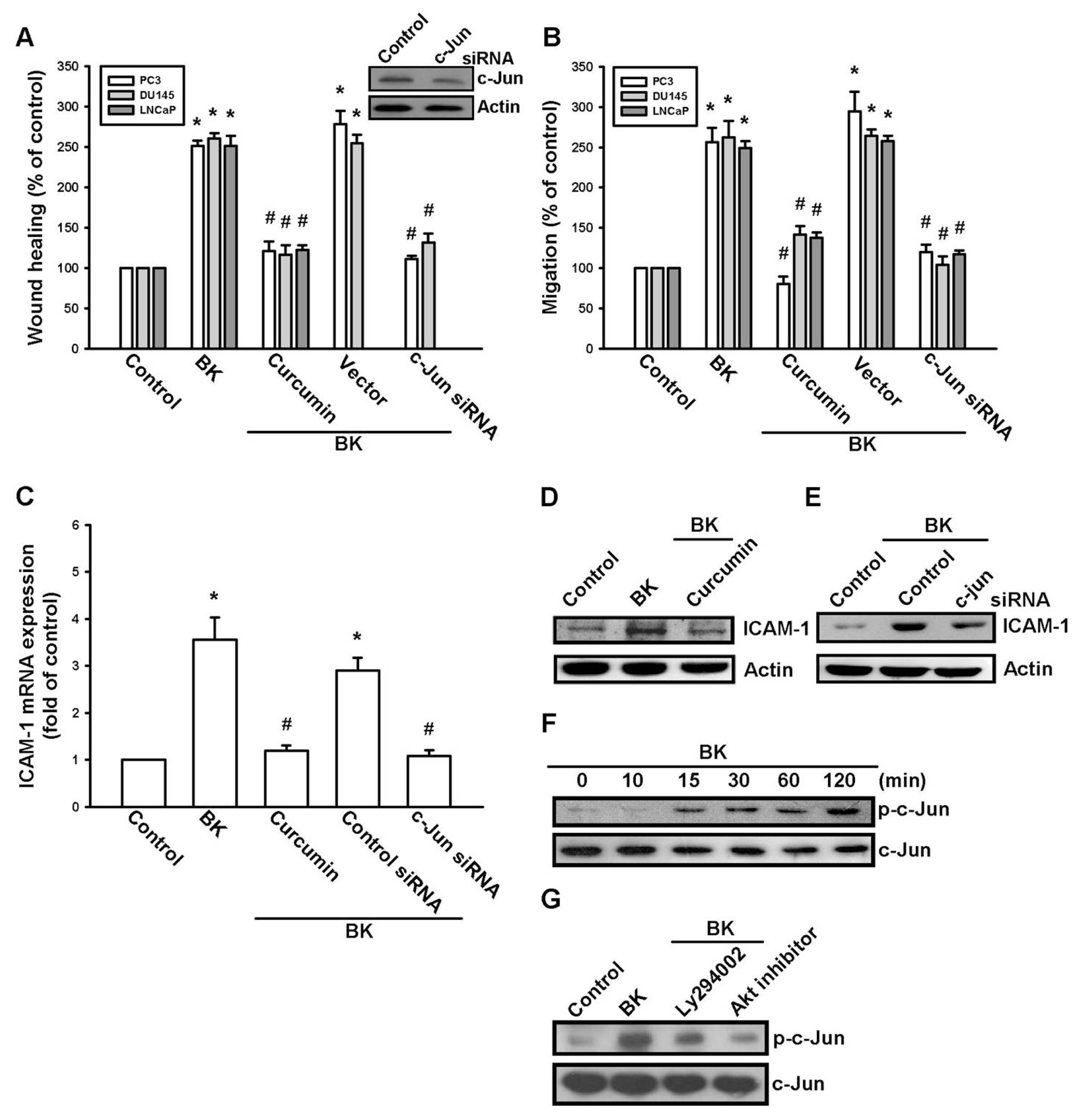
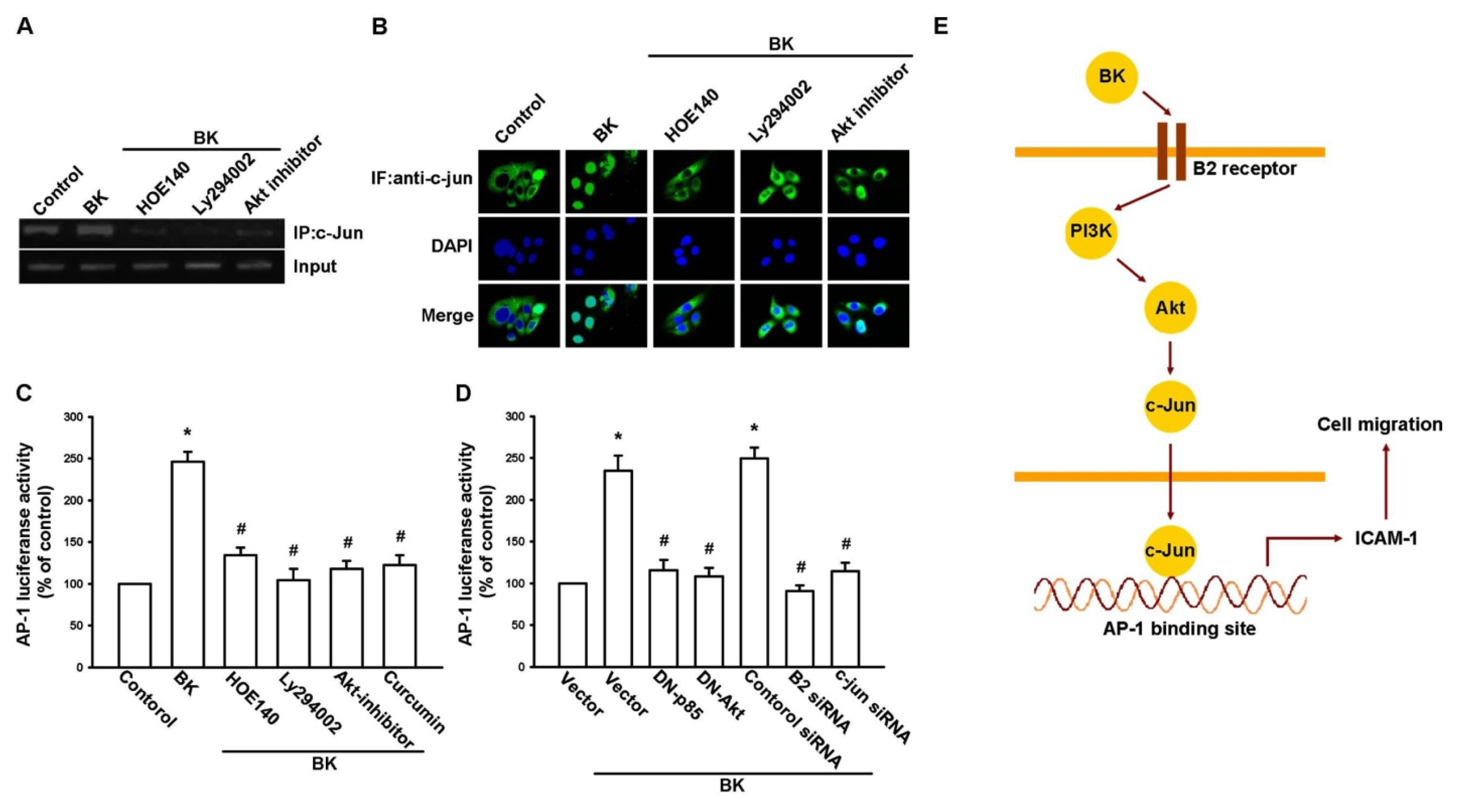
2.4. AP-1 Is Involved in BK-Mediated Migration and ICAM-1 Expression
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Cell Culture
4.3. Migration Assay
4.4. Wound-Healing Migration Assay
4.5. Quantitative Real-Time Polymerase Chain Reaction
4.6. Western Blot Analysis
4.7. Reporter Gene Assay
4.8. Chromatin Immunoprecipitation Assay
4.9. Immunofluorescence Staining
4.10. Statistics
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Bryant, R.J.; Hamdy, F.C. Screening for prostate cancer: An update. Eur. Urol 2008, 53, 37–44. [Google Scholar]
- Zhao, Y.; Xue, Y.; Liu, Y.; Fu, W.; Jiang, N.; An, P.; Wang, P.; Yang, Z.; Wang, Y. Study of correlation between expression of bradykinin B2 receptor and pathological grade in human gliomas. Br. J. Neurosurg 2005, 19, 322–326. [Google Scholar]
- Van’t Veer, L.J.; Weigelt, B. Road map to metastasis. Nat. Med 2003, 9, 999–1000. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar]
- Zimmerman, T.; Blanco, F.J. Inhibitors targeting the LFA-1/ICAM-1 cell-adhesion interaction: Design and mechanism of action. Curr. Pharm. Des 2008, 14, 2128–2139. [Google Scholar]
- Lawson, C.; Wolf, S. ICAM-1 signaling in endothelial cells. Pharmacol. Rep 2009, 61, 22–32. [Google Scholar]
- Van de Stolpe, A.; van der Saag, P.T. Intercellular adhesion molecule-1. J. Mol. Med. (Berl. ) 1996, 74, 13–33. [Google Scholar]
- Duperray, A.; Languino, L.R.; Plescia, J.; McDowall, A.; Hogg, N.; Craig, A.G.; Berendt, A.R.; Altieri, D.C. Molecular identification of a novel fibrinogen binding site on the first domain of ICAM-1 regulating leukocyte-endothelium bridging. J. Biol. Chem 1997, 272, 435–441. [Google Scholar]
- Yang, S.F.; Chen, M.K.; Hsieh, Y.S.; Chung, T.T.; Hsieh, Y.H.; Lin, C.W.; Su, J.L.; Tsai, M.H.; Tang, C.H. Prostaglandin E2/EP1 signaling pathway enhances intercellular adhesion molecule 1 (ICAM-1) expression and cell motility in oral cancer cells. J. Biol. Chem 2010, 285, 29808–29816. [Google Scholar]
- Rauch, B.H.; Muschenborn, B.; Braun, M.; Weber, A.A.; Schror, K. ICAM-1 and p38 MAPK mediate fibrinogen-induced migration of human vascular smooth muscle cells. Eur. J. Pharmacol 2007, 577, 54–57. [Google Scholar]
- Roche, Y.; Pasquier, D.; Rambeaud, J.J.; Seigneurin, D.; Duperray, A. Fibrinogen mediates bladder cancer cell migration in an ICAM-1-dependent pathway. Thromb. Haemost 2003, 89, 1089–1097. [Google Scholar]
- Rosette, C.; Roth, R.B.; Oeth, P.; Braun, A.; Kammerer, S.; Ekblom, J.; Denissenko, M.F. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis 2005, 26, 943–950. [Google Scholar]
- Grothey, A.; Heistermann, P.; Philippou, S.; Voigtmann, R. Serum levels of soluble intercellular adhesion molecule-1 (ICAM-1, CD54) in patients with non-small-cell lung cancer: Correlation with histological expression of ICAM-1 and tumour stage. Br. J. Cancer 1998, 77, 801–807. [Google Scholar]
- Proud, D.; Kaplan, A.P. Kinin formation: Mechanisms and role in inflammatory disorders. Annu. Rev. Immunol 1988, 6, 49–83. [Google Scholar]
- Hall, J.M. Bradykinin receptors: Pharmacological properties and biological roles. Pharmacol. Ther 1992, 56, 131–190. [Google Scholar]
- Regoli, D.; Barabe, J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev 1980, 32, 1–46. [Google Scholar]
- Menke, J.G.; Borkowski, J.A.; Bierilo, K.K.; MacNeil, T.; Derrick, A.W.; Schneck, K.A.; Ransom, R.W.; Strader, C.D.; Linemeyer, D.L.; Hess, J.F. Expression cloning of a human B1 bradykinin receptor. J. Biol. Chem 1994, 269, 21583–21586. [Google Scholar]
- Molina, L.; Matus, C.E.; Astroza, A.; Pavicic, F.; Tapia, E.; Toledo, C.; Perez, J.A.; Nualart, F.; Gonzalez, C.B.; Burgos, R.A.; et al. Stimulation of the bradykinin B(1) receptor induces the proliferation of estrogen-sensitive breast cancer cells and activates the ERK1/2 signaling pathway. Breast Cancer Res. Treat 2009, 118, 499–510. [Google Scholar]
- Montana, V.; Sontheimer, H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J. Neurosci 2011, 31, 4858–4867. [Google Scholar]
- Yang, W.H.; Chang, J.T.; Hsu, S.F.; Li, T.M.; Cho, D.Y.; Huang, C.Y.; Fong, Y.C.; Tang, C.H. Bradykinin enhances cell migration in human chondrosarcoma cells through BK receptor signaling pathways. J. Cell. Biochem 2010, 109, 82–92. [Google Scholar]
- Vassou, D.; Notas, G.; Hatzoglou, A.; Castanas, E.; Kampa, M. Opioids increase bladder cancer cell migration via bradykinin B2 receptors. Int. J. Oncol 2011, 39, 697–707. [Google Scholar]
- Cheng, C.Y.; Kuo, C.T.; Lin, C.C.; Hsieh, H.L.; Yang, C.M. IL-1beta induces expression of matrix metalloproteinase-9 and cell migration via a c-Src-dependent, growth factor receptor transactivation in A549 cells. Br. J. Pharmacol 2010, 160, 1595–1610. [Google Scholar]
- Yu, H.S.; Lin, T.H.; Tang, C.H. Bradykinin enhances cell migration in human prostate cancer cells through B2 receptor/PKCdelta/c-Src dependent signaling pathway. Prostate 2013, 73, 89–100. [Google Scholar]
- Stio, M.; Martinesi, M.; Simoni, A.; Zuegel, U.; Steinmeyer, A.; Santi, R.; Treves, C.; Nesi, G. The novel vitamin D analog ZK191784 inhibits prostate cancer cell invasion. Anticancer Res 2011, 31, 4091–4098. [Google Scholar]
- Chen, P.C.; Lin, T.H.; Cheng, H.C.; Tang, C.H. CCN3 increases cell motility and ICAM-1 expression in prostate cancer cells. Carcinogenesis 2012, 33, 937–945. [Google Scholar]
- Bell, R.M.; Yellon, D.M. Bradykinin limits infarction when administered as an adjunct to reperfusion in mouse heart: The role of PI3K, Akt and eNOS. J. Mol. Cell. Cardiol 2003, 35, 185–93. [Google Scholar]
- Xu, X.; Tu, L.; Jiang, W.; Feng, W.; Zhao, C.X.; Wang, D.W. Bradykinin prevents the apoptosis of NIT-1 cells induced by TNF-alpha via the PI3K/Akt and MAPK signaling pathways. Int. J. Mol. Med 2012, 29, 891–898. [Google Scholar]
- Dugourd, C.; Gervais, M.; Corvol, P.; Monnot, C. Akt is a major downstream target of PI3-kinase involved in angiotensin II-induced proliferation. Hypertension 2003, 41, 882–890. [Google Scholar]
- Uzzo, R.G.; Crispen, P.L.; Golovine, K.; Makhov, P.; Horwitz, E.M.; Kolenko, V.M. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: Implications for prostate cancer progression. Carcinogenesis 2006, 27, 1980–1990. [Google Scholar]
- Hahm, E.R.; Cheon, G.; Lee, J.; Kim, B.; Park, C.; Yang, C.H. New and known symmetrical curcumin derivatives inhibit the formation of Fos-Jun-DNA complex. Cancer Lett 2002, 184, 89–96. [Google Scholar]
- Huang, W.C.; Chen, C.C. Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol. Cell. Biol 2005, 25, 6592–6602. [Google Scholar]
- Robinson, D.R.; Zylstra, C.R.; Williams, B.O. Wnt signaling and prostate cancer. Curr. Drug Targets 2008, 9, 571–580. [Google Scholar]
- Fiore, E.; Fusco, C.; Romero, P.; Stamenkovic, I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene 2002, 21, 5213–5223. [Google Scholar]
- Lu, D.Y.; Leung, Y.M.; Huang, S.M.; Wong, K.L. Bradykinin-induced cell migration and COX-2 production mediated by the bradykinin B1 receptor in glioma cells. J. Cell. Biochem 2010, 110, 141–150. [Google Scholar]
- Wai Wong, C.; Dye, D.E.; Coombe, D.R. The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int. J. Cell Biol 2012, 2012, 340296. [Google Scholar]
- Tang, C.H.; Lu, M.E. Adiponectin increases motility of human prostate cancer cells via adipoR, p38, AMPK, and NF-kappaB pathways. Prostate 2009, 69, 1781–1789. [Google Scholar]
- Lin, Y.M.; Chang, Z.L.; Liao, Y.Y.; Chou, M.C.; Tang, C.H. IL-6 promotes ICAM-1 expression and cell motility in human osteosarcoma. Cancer Lett 2013, 328, 135–143. [Google Scholar]
- Hall, J.M. Bradykinin receptors. General Pharmacol 1997, 28, 1–6. [Google Scholar]
- Blaes, N.; Pecher, C.; Mehrenberger, M.; Cellier, E.; Praddaude, F.; Chevalier, J.; Tack, I.; Couture, R.; Girolami, J.P. Bradykinin inhibits high glucose- and growth factor-induced collagen synthesis in mesangial cells through the B2-kinin receptor. Am. J. Physiol. Renal Physiol 2012, 303, F293–F303. [Google Scholar]
- Hsieh, H.L.; Sun, C.C.; Wang, T.S.; Yang, C.M. PKC-delta/c-Src-mediated EGF receptor transactivation regulates thrombin-induced COX-2 expression and PGE(2) production in rat vascular smooth muscle cells. Biochim. Biophys. Acta 2008, 1783, 1563–1575. [Google Scholar]
- Hsieh, H.L.; Tung, W.H.; Wu, C.Y.; Wang, H.H.; Lin, C.C.; Wang, T.S.; Yang, C.M. Thrombin induces EGF receptor expression and cell proliferation via a PKC(delta)/c-Src-dependent pathway in vascular smooth muscle cells. Arterioscler. Thrombosis Vasc. Biol 2009, 29, 1594–1601. [Google Scholar]
- Hsieh, M.T.; Hsieh, C.L.; Lin, L.W.; Wu, C.R.; Huang, G.S. Differential gene expression of scopolamine-treated rat hippocampus-application of cDNA microarray technology. Life Sci 2003, 73, 1007–1016. [Google Scholar]
- Wang, Y.C.; Lee, P.J.; Shih, C.M.; Chen, H.Y.; Lee, C.C.; Chang, Y.Y.; Hsu, Y.T.; Liang, Y.J.; Wang, L.Y.; Han, W.H. Damage formation and repair efficiency in the p53 gene of cell lines and blood lymphocytes assayed by multiplex long quantitative polymerase chain reaction. Anal. Biochem 2003, 319, 206–215. [Google Scholar]
- Huang, H.C.; Shi, G.Y.; Jiang, S.J.; Shi, C.S.; Wu, C.M.; Yang, H.Y.; Wu, H.L. Thrombomodulin-mediated cell adhesion: Involvement of its lectin-like domain. J. Biol. Chem 2003, 278, 46750–46759. [Google Scholar]
- Tseng, C.P.; Huang, C.L.; Huang, C.H.; Cheng, J.C.; Stern, A.; Tseng, C.H.; Chiu, D.T. Disabled-2 small interfering RNA modulates cellular adhesive function and MAPK activity during megakaryocytic differentiation of K562 cells. FEBS Lett 2003, 541, 21–27. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, H.-S.; Lin, T.-H.; Tang, C.-H. Involvement of Intercellular Adhesion Molecule-1 Up-Regulation in Bradykinin Promotes Cell Motility in Human Prostate Cancers. Int. J. Mol. Sci. 2013, 14, 13329-13345. https://doi.org/10.3390/ijms140713329
Yu H-S, Lin T-H, Tang C-H. Involvement of Intercellular Adhesion Molecule-1 Up-Regulation in Bradykinin Promotes Cell Motility in Human Prostate Cancers. International Journal of Molecular Sciences. 2013; 14(7):13329-13345. https://doi.org/10.3390/ijms140713329
Chicago/Turabian StyleYu, Hsin-Shan, Tien-Huang Lin, and Chih-Hsin Tang. 2013. "Involvement of Intercellular Adhesion Molecule-1 Up-Regulation in Bradykinin Promotes Cell Motility in Human Prostate Cancers" International Journal of Molecular Sciences 14, no. 7: 13329-13345. https://doi.org/10.3390/ijms140713329




