A Labile Pool of IQGAP1 Disassembles Endothelial Adherens Junctions
Abstract
:1. Introduction
2. Results and Discussion
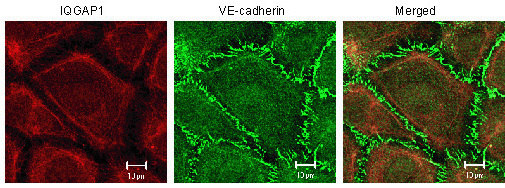
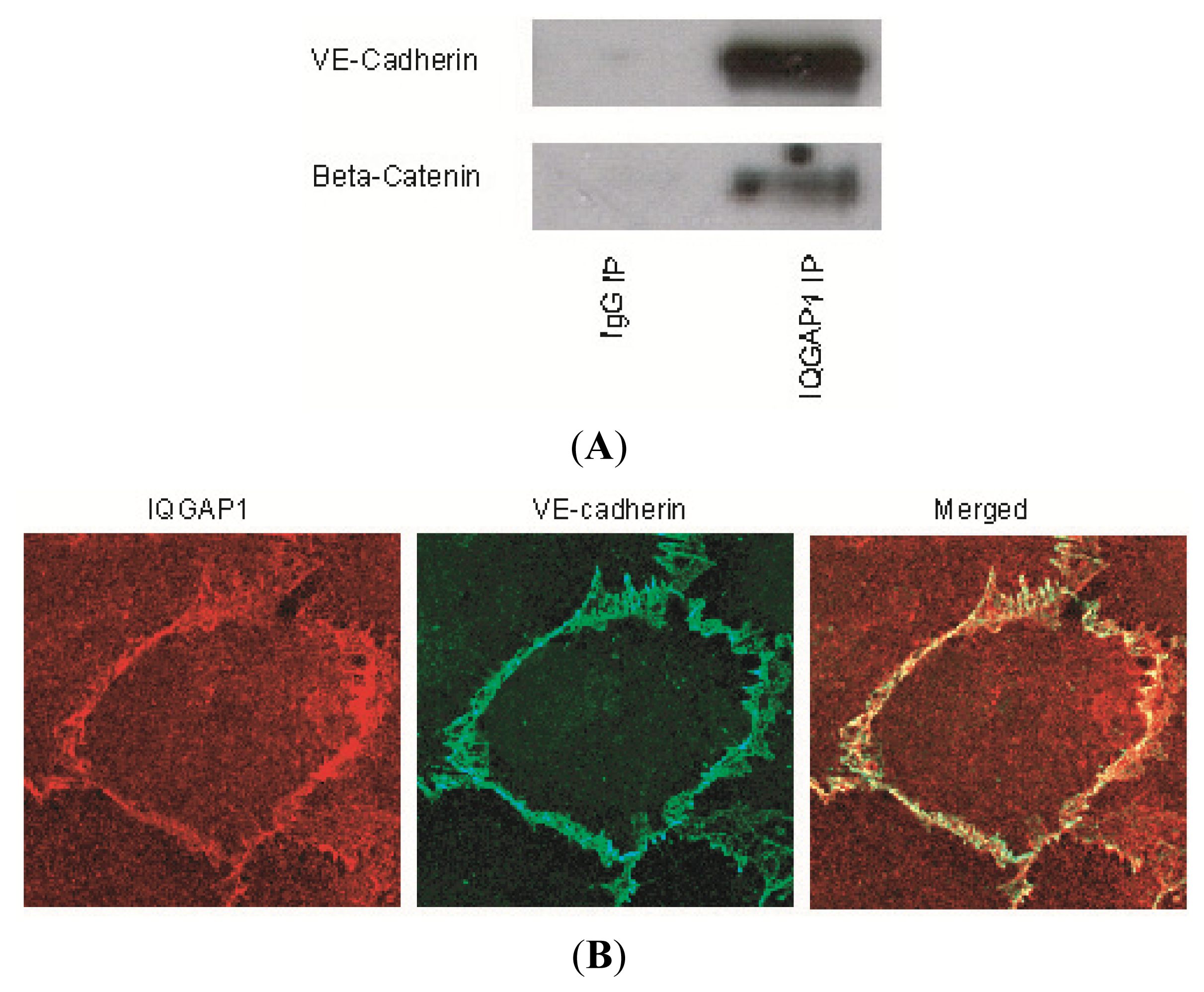
2.1. IQGAP1 Interacts with Proteins of Adherens Junction
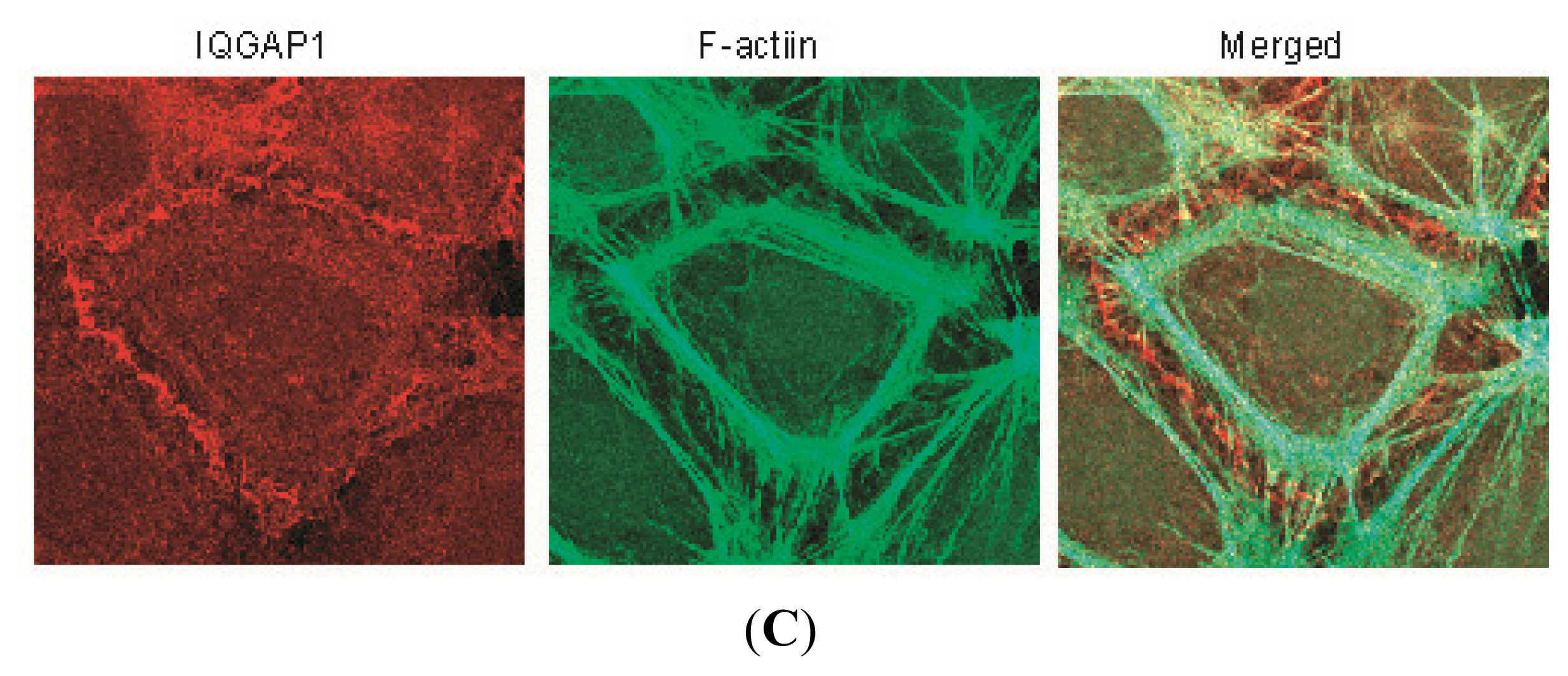
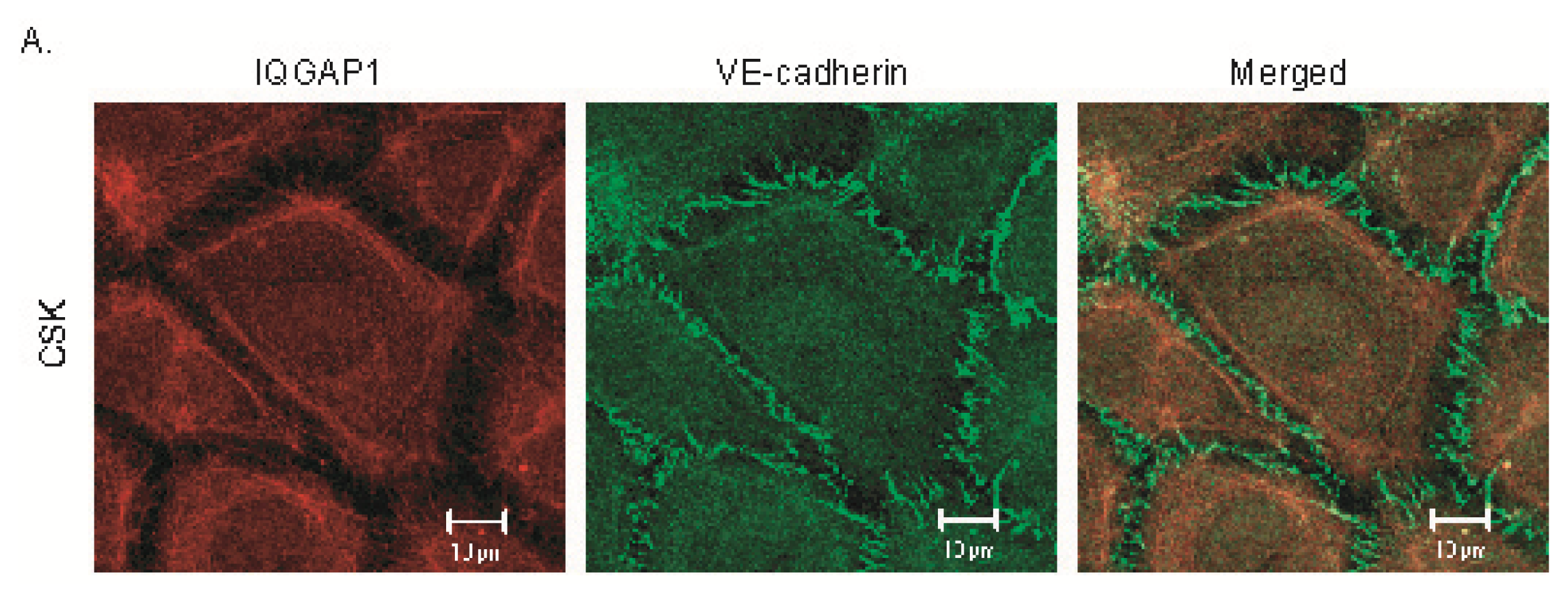
2.2. IQGAP1 Is Labile at Intercellular Junctions and Stable in the Cortex
2.3. Expression of GFP Labeled VE-α-catenin Diminishes Endogenous IQGAP1 at the Intercellular Junctions
2.4. Expression of GFP Labeled VE-α-catenin Increases the Electrical Resistance across HUVEC Monolayers
2.5. Knock-Down of IQGAP1 by siRNA Increases the Electrical Resistance across HUVEC Monolayers
2.6. IQGAP1 Knockdown Increased Association of VE-Cadherin with p120- and β-Catenins; Opposite Response Occurred with N-Cadherin
2.7. Discussion
3. Experimental Section
3.1. Materials
3.2. Cell Culture
3.3. Transfection
3.4. Infection
3.5. Assessment of Endothelial Barrier Function by ECIS
3.6. Immunoprecipitation and Immunoblotting
3.7. Immunocytochemistry and Confocal Microscopy
3.8. Statistics
4. Conclusions
Supplementary Information
ijms-14-13377-s001.pdfAcknowledgments
Conflict of Interest
References
- Urao, N.; Razvi, M.; Oshikawa, J.; McKinney, R.D.; Chavda, R.; Bahou, W.F.; Fukai, T.; Ushio-Fukai, M. IQGAP1 is involved in post-ischemic neovascularization by regulating angiogenesis and macrophage infiltration. PLoS One 2010, 5, e13440. [Google Scholar]
- Nakhaei-Nejad, M.; Zhang, Q.X.; Murray, A.G. Endothelial IQGAP1 regulates efficient lymphocyte transendothelial migration. Eur. J. Immunol 2010, 40, 204–213. [Google Scholar]
- Meyer, R.D.; Sacks, D.B.; Rahimi, N. IQGAP1-dependent signaling pathway regulates endothelial cell proliferation and angiogenesis. PLoS One 2008, 3, e3848. [Google Scholar]
- Bazzoni, G.; Dejana, E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol. Rev 2004, 84, 869–901. [Google Scholar]
- Corada, M.; Mariotti, M.; Thurston, G.; Smith, K.; Kunkel, R.; Brockhaus, M.; Lampugnani, M.G.; Martin-Padura, I.; Stoppacciaro, A.; Ruco, L.; et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc. Natl. Acad. Sci. USA 1999, 96, 9815–9820. [Google Scholar]
- Iyer, S.; Ferreri, D.M.; DeCocco, N.C.; Minnear, F.L.; Vincent, P.A. VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am. J. Physiol. Lung Cell. Mol. Physiol 2004, 286, L1143–L1153. [Google Scholar]
- Wu, Y.; Vendome, J.; Shapiro, L.; Ben-Shaul, A.; Honig, B. Transforming binding affinities from three dimensions to two with application to cadherin clustering. Nature 2011, 475, 510–513. [Google Scholar]
- Dejana, E.; Giampietro, C. Vascular endothelial-cadherin and vascular stability. Curr. Opin. Hematol 2012, 19, 218–223. [Google Scholar]
- Dejana, E.; Vestweber, D. The role of VE-cadherin in vascular morphogenesis and permeability control. Prog. Mol. Biol. Transl. Sci 2013, 116, 119–144. [Google Scholar]
- Ohkubo, T.; Ozawa, M. p120(ctn) binds to the membrane-proximal region of the E-cadherin cytoplasmic domain and is involved in modulation of adhesion activity. J. Biol. Chem 1999, 274, 21409–21415. [Google Scholar]
- Yamada, S.; Pokutta, S.; Drees, F.; Weis, W.I.; Nelson, W.J. Deconstructing the cadherin-cateninactin complex. Cell 2005, 123, 889–901. [Google Scholar]
- Kuroda, S.; Fukata, M.; Nakagawa, M.; Fujii, K.; Nakamura, T.; Ookubo, T.; Izawa, I.; Nagase, T.; Nomura, N.; Tani, H.; et al. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science 1998, 281, 832–835. [Google Scholar]
- Kuroda, S.; Fukata, M.; Nakagawa, M.; Kaibuchi, K. Cdc42, Rac1, and their effector IQGAP1 as molecular switches for cadherin-mediated cell-cell adhesion. Biochem. Biophys. Res. Commun 1999, 262, 1–6. [Google Scholar]
- Briggs, M.W.; Sacks, D.B. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep 2003, 4, 571–574. [Google Scholar]
- Bashour, A.M.; Fullerton, A.T.; Hart, M.J.; Bloom, G.S. IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments. J. Cell Biol 1997, 137, 1555–1566. [Google Scholar]
- Mateer, S.C.; Morris, L.E.; Cromer, D.A.; Benseñor, L.B.; Bloom, G.S. Actin filament binding by a monomeric IQGAP1 fragment with a single calponin homology domain. Cell Motil. Cytoskeleton 2004, 58, 231–241. [Google Scholar]
- Fey, E.G.; Wan, K.M.; Penman, S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: Three-dimensional organization and protein composition. J. Cell. Biol 1984, 98, 1973–1984. [Google Scholar]
- Kuroda, S.; Fukata, M.; Kobayashi, K.; Nakafuku, M.; Nomura, N.; Iwamatsu, A.; Kaibuchi, K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J. Biol. Chem 1996, 271, 23363–23367. [Google Scholar]
- Yamaoka-Tojo, M.; Ushio-Fukai, M.; Hilenski, L.; Dikalov, S.I.; Chen, Y.E.; Tojo, T.; Fukai, T.; Fujimoto, M.; Patrushev, N.A.; Wang, N.; et al. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species—Dependent endothelial migration and proliferation. Circ. Res 2004, 95, 276–283. [Google Scholar]
- Yamaoka-Tojo, M.; Tojo, T.; Kim, H.W.; Hilenski, L.; Patrushev, N.A.; Zhang, L.; Fukai, T.; Ushio-Fukai, M. IQGAP1 mediates VE-cadherin-based cell-cell contacts and VEGF signaling at adherence junctions linked to angiogenesis. Arterioscler. Thromb. Vasc. Biol 2006, 26, 1991–1997. [Google Scholar]
- Weissbach, L.; Settleman, J.; Kalady, M.F.; Snijders, A.J.; Murthy, A.E.; Yan, Y.X.; Bernards, A. Identification of a human rasGAP-related protein containing calmodulin-binding motifs. J. Biol. Chem 1994, 269, 20517–20521. [Google Scholar]
- Fukata, M.; Nakagawa, M.; Itoh, N.; Kawajiri, A.; Yamaga, M.; Kuroda, S.; Kaibuchi, K. Involvement of IQGAP1, an effector of Rac1 and Cdc42 GTPases, in cell-cell dissociation during cell scattering. Mol. Cell. Biol 2001, 21, 2165–2183. [Google Scholar]
- Garcia, J.G.; Liu, F.; Verin, A.D.; Birukova, A.; Dechert, M.A.; Gerthoffer, W.T.; Bamberg, J.R.; English, D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest 2001, 108, 689–701. [Google Scholar]
- Wegener, J.; Keese, C.R.; Giaever, I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp. Cell Res 2000, 259, 158–166. [Google Scholar]





© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yuan, Z.; Zhang, W.; Tan, W. A Labile Pool of IQGAP1 Disassembles Endothelial Adherens Junctions. Int. J. Mol. Sci. 2013, 14, 13377-13390. https://doi.org/10.3390/ijms140713377
Yuan Z, Zhang W, Tan W. A Labile Pool of IQGAP1 Disassembles Endothelial Adherens Junctions. International Journal of Molecular Sciences. 2013; 14(7):13377-13390. https://doi.org/10.3390/ijms140713377
Chicago/Turabian StyleYuan, Zhiguo, Wentao Zhang, and Wen Tan. 2013. "A Labile Pool of IQGAP1 Disassembles Endothelial Adherens Junctions" International Journal of Molecular Sciences 14, no. 7: 13377-13390. https://doi.org/10.3390/ijms140713377