Inland Treatment of the Brine Generated from Reverse Osmosis Advanced Membrane Wastewater Treatment Plant Using Epuvalisation System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Characteristics of RO Brine
2.1.2. Brine Quality during Epuvalisation Treatment
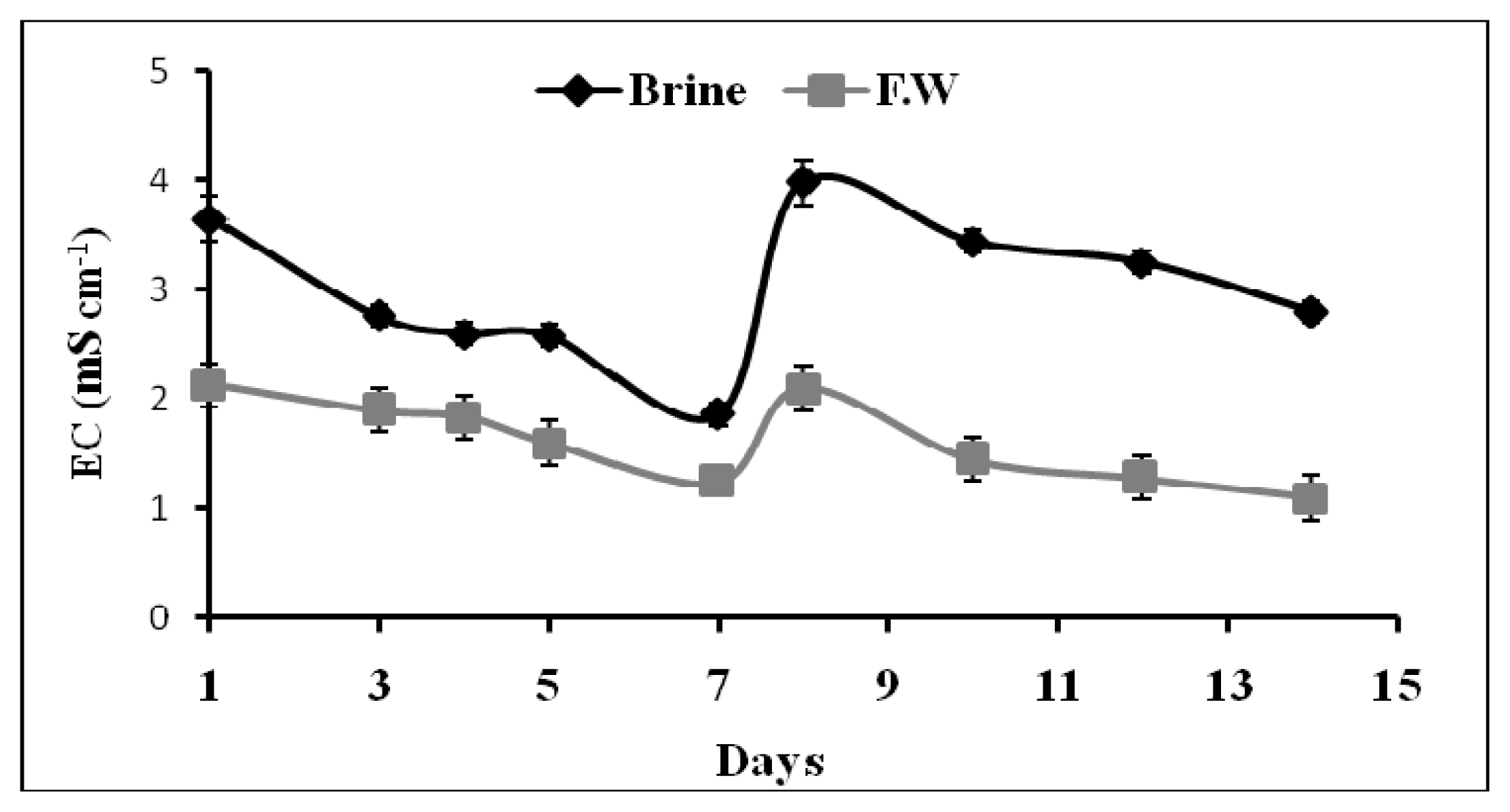
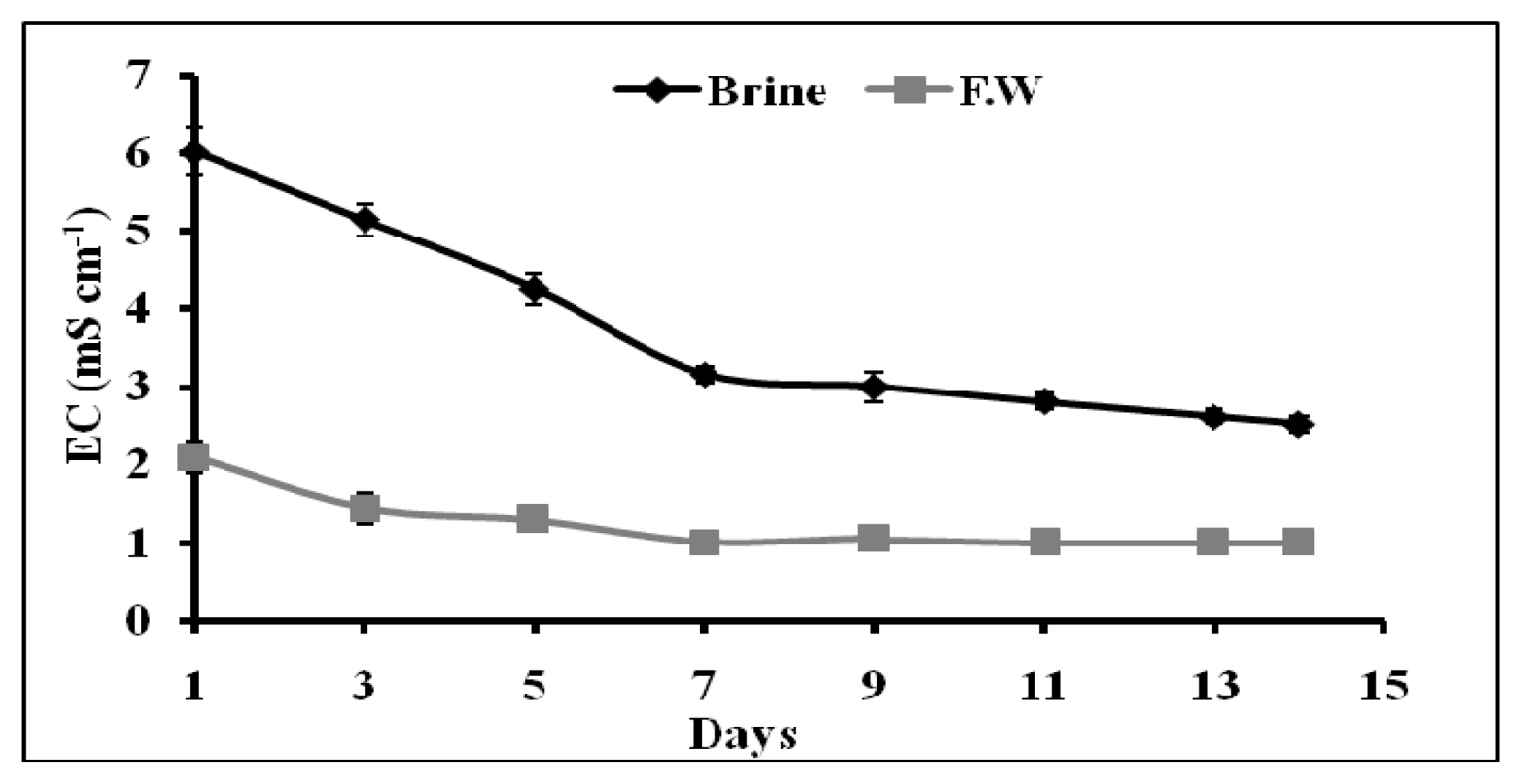
2.1.3. Real Time Analysis of EC
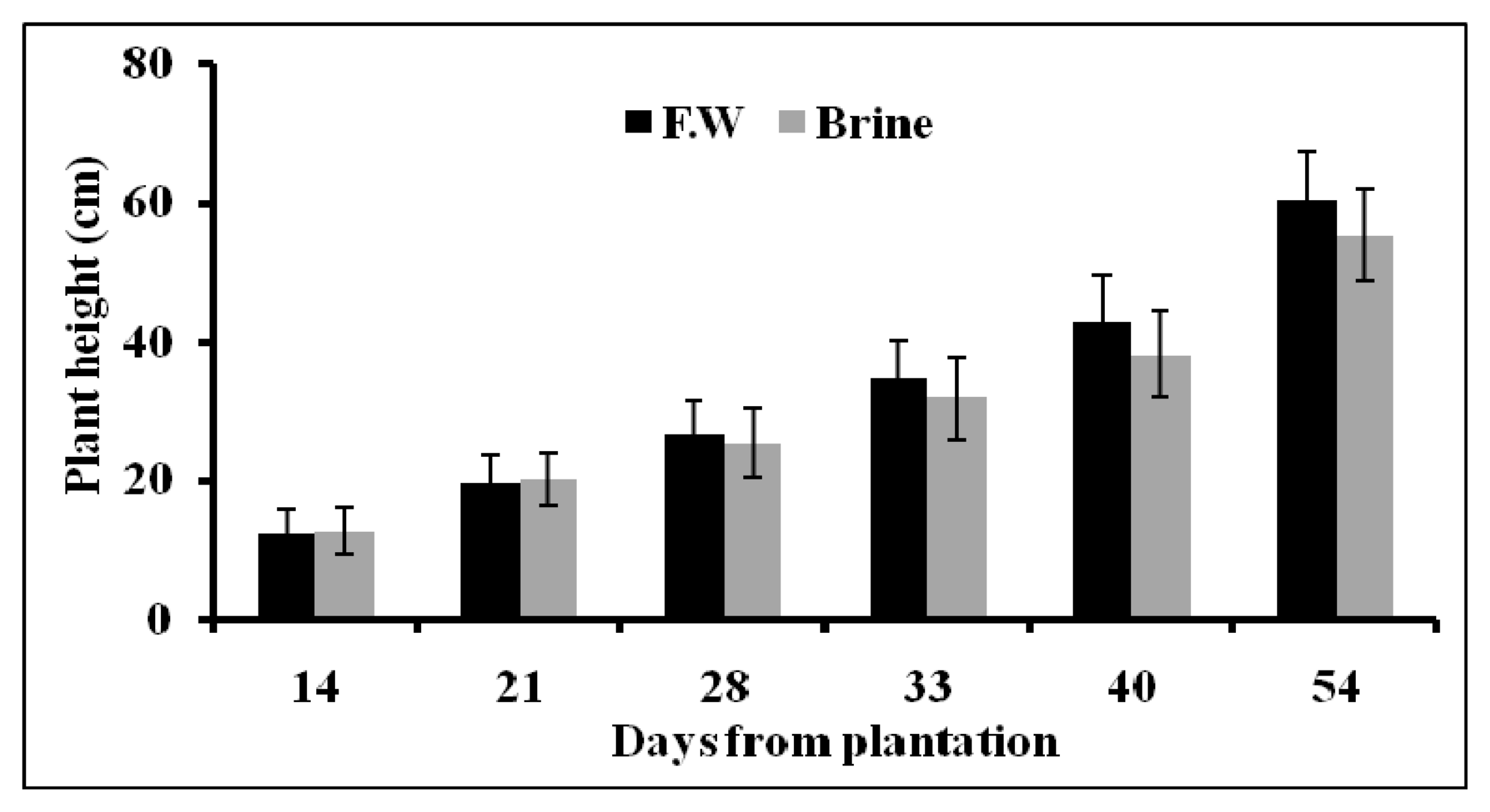
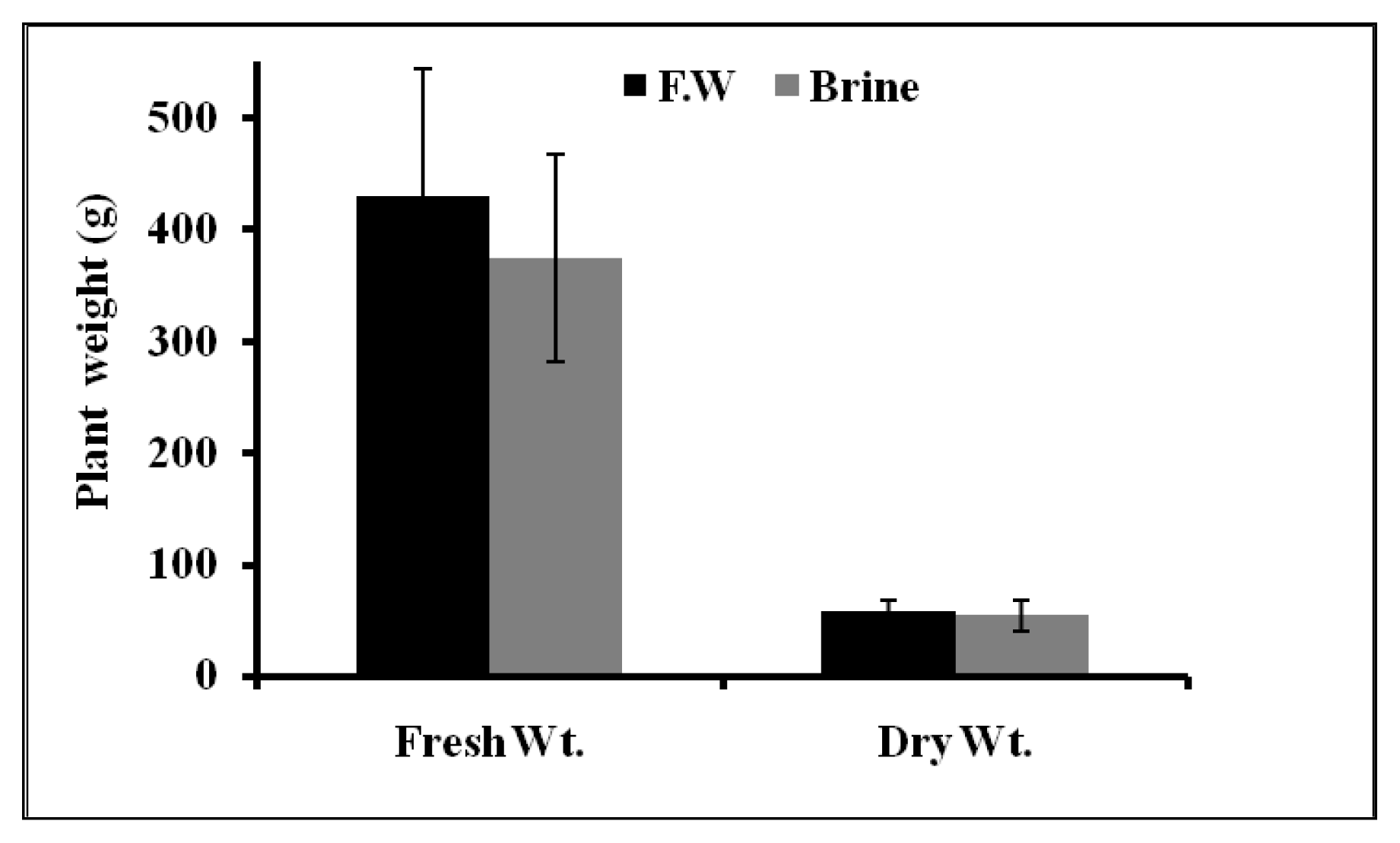
2.1.4. Plant Growth Parameters
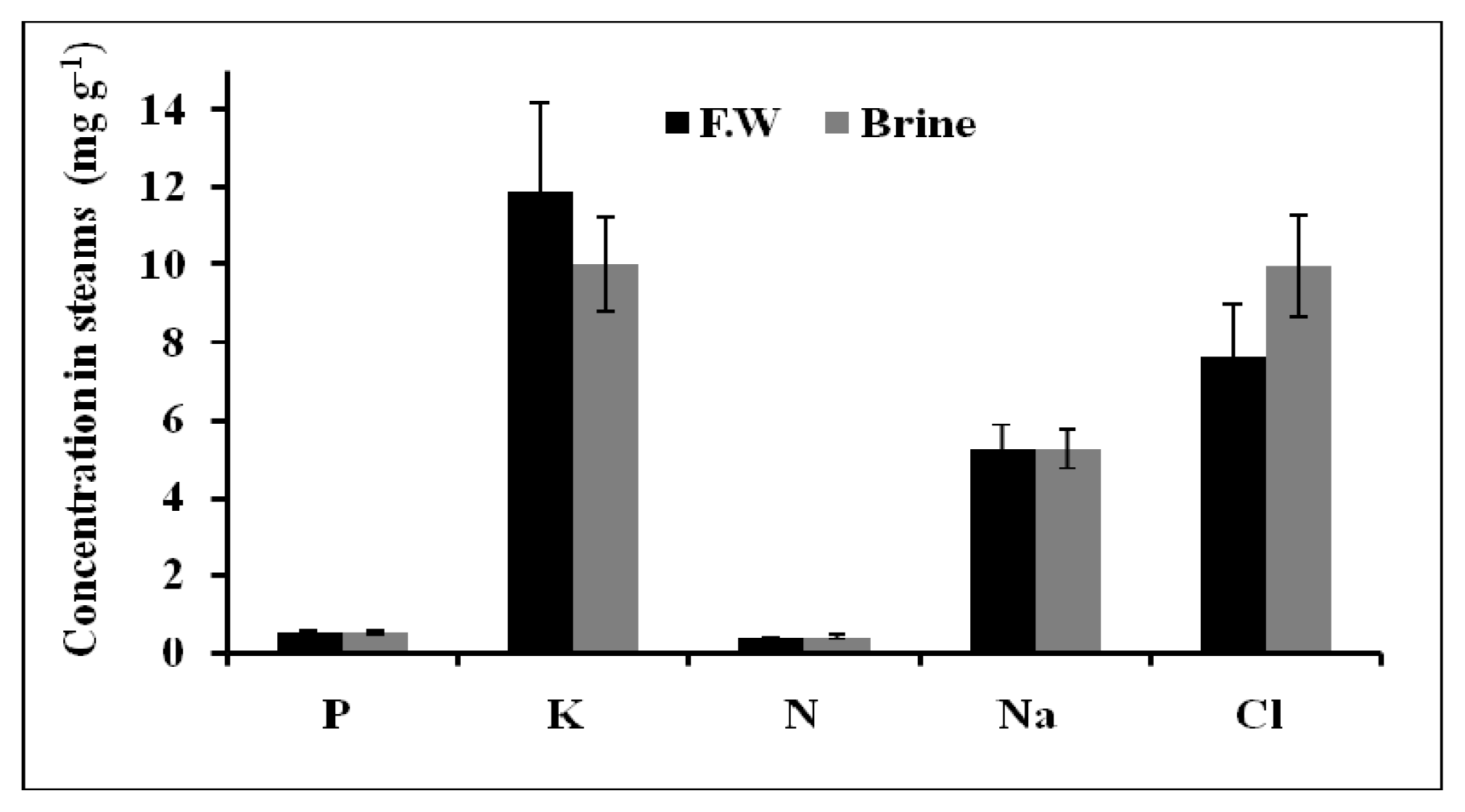
2.1.5. Chemical Composition of Plant Tissues
2.2. Discussion
3. Experimental Section
3.1. Wastewater Treatment System and Site
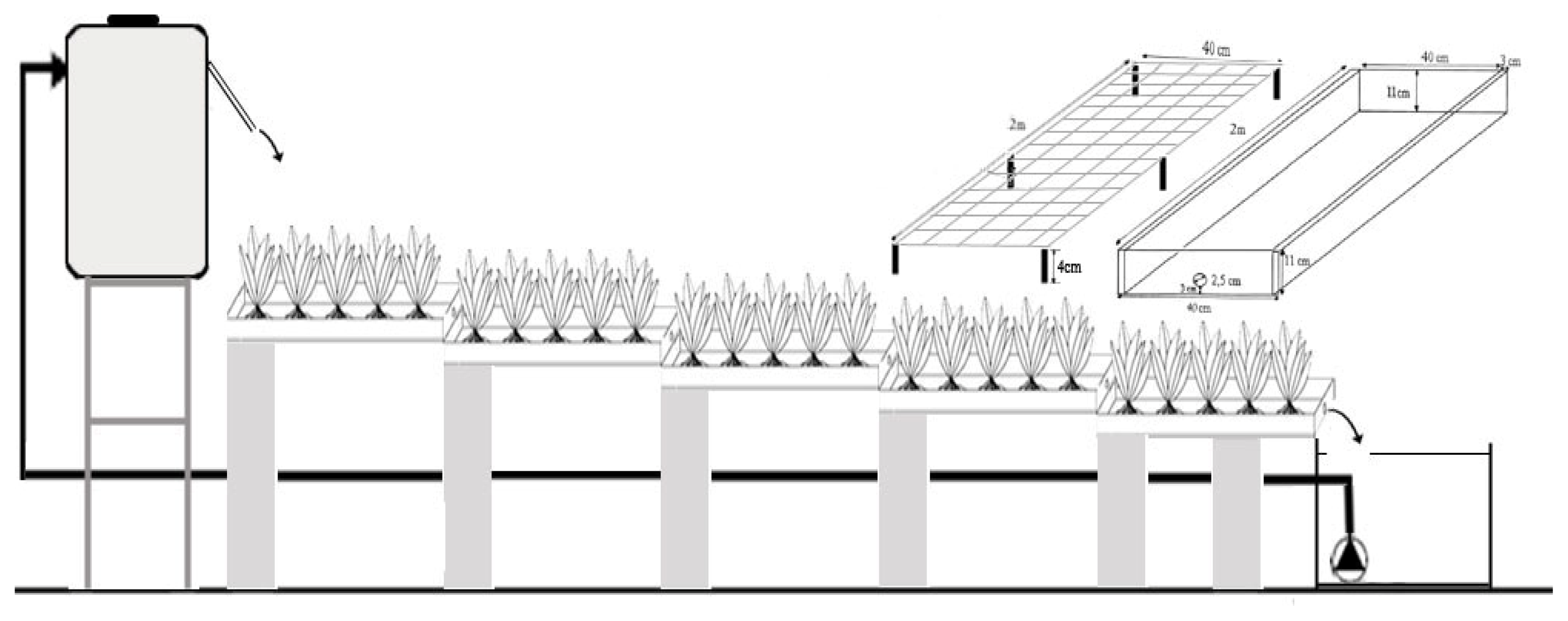
3.2. Epuvalisation System
3.3. Plant Selection
3.4. Plantation and Growth
3.5. Water Analysis
3.6. Harvesting and Analytical Procedures
3.7. Statistics and Yield Component Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Oron, G.; Gillerman, L.; Bick, A.; Mnaor, Y.; Buriakovsky, N.; Hagin, J. Advanced low quality waters treatment for unrestricted use purposes:imminent challenges. Desalination 2007, 213, 189–198. [Google Scholar]
- Al-Sajwani, T.M.A.; Lawrence, R.J. Proceedings of International Conference on Water Resources Management in Arid Countries, Ministry of Water Resources, Sultanate of Oman, Muscat, 12–16 March 1995; pp. 617–626.
- Oron, G.; Gillerman, L.; Bick, A.; Buriakovsky, N.; Mnaor, Y.; Yitshak, E.B.; Katz, L.; Hagin, J. A two stage membrane treatment of secondary effluent for unrestricted reuse and sustainable agriculture production. Desalination 2006, 187, 335–345. [Google Scholar]
- Gillermana, L.; Bick, A.; Buriakovskya, N.; Oron, G. Secondary wastewater polishing with ultrafiltration membranes for unrestricted reuse: Fouling and flushing modeling. Environ. Sci. Technol. 2006, 40, 6830–6836. [Google Scholar]
- Trivedy, R.K. Low cost and energy saving technologies for water and wastewater treatment. J. Ind. Pollut. Control 2007, 23, 403–411. [Google Scholar]
- Zhou, H.; Smith, D.W. Advanced technologies in water and wastewater treatment. J. Environ. Eng. Sci 2002, 1, 247–264. [Google Scholar]
- Goto, T. East and South East Asia. Inst. Cheme 2002, 12, 28–30. [Google Scholar]
- Oron, G.; Gillermana, L.; Buriakovskya, N.; Bickd, A.; Gargirb, M.; Dolanb, Y.; Manore, Y.; Katz, L.; Hagin, J. Membrane technology for advanced wastewater reclamation for sustainable agriculture production. Desalination 2008, 218, 170–180. [Google Scholar]
- Ng, H.Y.; Lee, L.Y.; Ong, S.L.; Tao, G.; Viawanath, B.; Kekre, K.; Lay, W.; Seah, H. Treatment of RO brine-towards sustainable water reclamation practice. Water Sci. Technol 2008, 58, 931–936. [Google Scholar]
- Arnal, J.M.; Sancho, M.; Iborra, I.; Gozalvez, J.M.; Santafe, A.; Lora, J. Concentration of brines from RO desalination plants by natural evaporation. Desalination 2005, 182, 435–439. [Google Scholar]
- Mushtaque, A.; Shayya, W.H.; Hoey, D.; Al-Handaly, J. Brine Disposal from reverse osmosis desalination plants in Oman and United Arab Emirates. Desalination 2001, 133, 135–147. [Google Scholar]
- Glater, J.; Cohen, Y. Brine disposal from land based membrane desalination plants: A critical assessment. Available online: http://www.twdb.state.tx.us/wrpi/rwp/3rdRound/2011_RWP/RegionK/Files/Reference_Docs/Brackish_Desal/BRINE%20DISPOSAL.pdf (on accessed 10 October 2011).
- Smith, D.J.; Humphreys, L. CSIRO Land and Water Sustainable Irrigated Agriculture Griffith 2000 Research Report; CSIRO Land and Water: Griffith, Australia, 2001; pp. 1–91. [Google Scholar]
- Mushtaque, A.; Arakel, A.; Hoey, D.; Thumarukudy, M.R.; Goosen, M.; Al-Haddabi, M.; Al-Belushi, A. Feasibility of salt production from inland RO desalination plant reject brine: A case study. Desalination 2003, 158, 109–117. [Google Scholar]
- Reimold, R.J.; Loland-McLaughlin, G.; Bloetscher, F. An innovative opportunity for water reuse. Florida Water Resour. J 1996, 26–28. [Google Scholar]
- Mickley, M. Environmental Considerations for the Disposal of Desalination Concentrate. Proceedings of the IDA World Congress on Desalination and Water Sciences, Abu Dhabi, UAE, 18–24 November 1995; VII, pp. 351–363.
- Muchuweti, M.; Birkett, J.W.; Chinyanga, E.; Zvauya, R.; Scrimshaw, M.D.; Lester, J.N. Heavy metal content of vegetables irrigated with mixture of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agric. Ecosyst. Environ 2006, 112, 41–48. [Google Scholar]
- Bahemuka, T.E.; Mubofu, E.B. Heavy metals in edible green vegetables grown along the sites of the Sinza and Msimbazi rivers in Dar es Salaam, Tanzania. Food Chem 1991, 66, 63–66. [Google Scholar]
- Mapanda, F.; Mangwayana, E.N.; Nyamangara, J.; Giller, K.E. The effects of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agric. Ecosyst. Environ 2005, 107, 151–156. [Google Scholar]
- Everest, W.; Murphree, T. Desalting residuals: A problem or a beneficial resource? Desalination 1995, 102, 107–117. [Google Scholar]
- Ahmed, M.; Shayya, W.H.; Hoey, D.; Mahendran, A.; Morris, R.; Al-Handaly, J. Use of evaporation ponds for brine disposal in desalination plants. Desalination 2000, 130, 155–168. [Google Scholar]
- Papadopoulos, I.; Chimonidou, D.; Savvides, S.; Polycarpou, P. Optimization of Irrigation with Treated Wastewater on Flower Cultivations. Proceeding of the Non-Conventional Water Use 3rd WASAMED (WaterSAving in MEDiterranean agriculture) Workshop, Cairo, Egypt, 7–10 December 2004; pp. 227–235.
- Xanthoulis, D.; Dumont, P.; Wauthelet, M. Epuvalisation: A Developing Technique. Experiences, results in different countries. Proceeding of the 2nd International Symposium on Ecological Sanitation, Lübeck, Germany, 7–11 April 2003; pp. 527–530.
- Simon, J.; Bruce, J. Membranes for Industrial Wastewater Recovery and Re-Use; Elsevier Advanced Technology: Kidlington, UK, 2003. [Google Scholar]
- Kowalski, J.A.; Palada, M.C. Responce of selected vegetable crops to saline water in U.S Virgin Island. Proceedings of the Thirtieth Annual Meeting of the Caribbean Food Crops Society, St. Thomas (United States Virgin Islands), 31 July–5 August 1994; Caribbean Food Crops Society (CFCS): St. Croix, United States Virgin Islands (USVI), 1995; pp. 232–246. [Google Scholar]
- Ghaly, A.E.; Kamal, M.; Mahmoud, N.S. Phytoremediation of aquaculture wastewater for water recycling and production of fish feed. Environ. Int 2005, 31, 1–13. [Google Scholar]
- Mathieu, J.J.; Wang, J.K. The Effect of Water Velocity and Nutrient Concentration on Plant Nutrient Uptake: A Review. In Aquacultural Engineering and Waste Management. Proceedings from the Aquaculture Proceedings from the Aquaculture Expo VIII and Aquaculture in the Mid-Atlantic Conference; Timmons, M.B., Ed.; Northeast Regional Agricultural Engineering Service: Washington, DC, USA, 1995; pp. 187–211. [Google Scholar]
- Rackocy, J.E. The Role of Plant Crop Production in Aquaculture Waste Management. In Aquaculutral Engineering and Waste Management: Proceedings from the Aquaculture Expo VIII and Aquaculture in the Mid-Atlantic Conference; Timmons, M.B., Ed.; Northeast Regional Agricultural Engineering Service: Washington, DC, USA, 1995; pp. 349–364. [Google Scholar]
- Rackocy, J.E.; Hargreaves, J.A. Integration of Vegetable Hydroponics with Fish Culture: A Review. In Techniques for Modern Aquaculture. Proceeding of an Aquacultural Engineering Conference; Wang, J.K., Ed.; Spokane: Washington, DC, USA, 1993; pp. 112–136. [Google Scholar]
- Jiang, Z.; Xinyuan, Z. Treatment and utilization of wastewater in the Beijing zoo by an aquatic macrophyte system. Ecol. Eng 1998, 11, 101–110. [Google Scholar]
- Batoul, M.A.; Nouf, A.S. Evaluation of essential elements of sweet Basil (Ocimum. Basilicum) at different growth stages under deficit irrigation. Int. J. Appl. Biol. Pharm. Technol 2012, 3, 56–62. [Google Scholar]
- Naegel, L.C.A. Combined production of fish and plants in recirculating water. Aquaculture 1997, 20, 17–24. [Google Scholar]
- Mant, C.; Peterkin, J.; May, E.; Butler, J. A feasibility study of a Salix viminalis gravel hydroponic system to renovate primary settled wastewater. Bioresour. Technol 2003, 90, 19–25. [Google Scholar]
- Marschner, M. Mineral Nutrition of High Plants; Academic Press: New York, NY, USA, 1995. [Google Scholar]
- Holtan, H.; Kamp-Nielsen, L.; Shuanes, A.O. Phosphorous in soil, water and sediment: An overview. Hydrobiologia 1988, 170, 19–34. [Google Scholar]
- Abbadi, J.; Gerendás, J. Phosphorous use efficiency of Safflower and Sunflower studied in nutrient solutions. J. Agric. Sci. Technol 2012, A2, 1260–1280. [Google Scholar]
- Brown, J.J.; Glenn, E.P.; Fitzsimmons, K.M.; Smith, S.E. Halophytes for the treatment of saline aquaculture effluent. Aquaculture 1999, 75, 255–268. [Google Scholar]
- Gloger, K.C.; Rakocy, J.E.; Conter, J.B.; Bailey, D.S.; Cole, W.M.; Shultz, K.A. Contribution of lettuce to wastewater treatment capacity of raft hydroponics in a closed recirculating fish culture system. In Aquacultural Engineering and Waste Management. Proceedings from the Aquaculture Expo VIII and Aquaculture in the Mid-Atlantic Conference; Timmons, M.B., Ed.; Northeast Regional Agricultural Engineering Service: Washington, DC, USA, 1995; pp. 272–300. [Google Scholar]
- Dontje, J.H.; Clanton, C.J. Nutrient fate in aquacultural systems for waste treatment. Trans. ASAE 1999, 42, 1073–1085. [Google Scholar]
- Khalid, K.A. Influence of water stress on growth, essential oil and chemical composition of herb (Ocimum. basilicum L.). Int. Agrophys 2006, 20, 289–296. [Google Scholar]
- Westgate, M.E.; Grant, L.T. Water deficits and reproduction in maize. Plant. Physiol 1989, 91, 862–867. [Google Scholar]
- Khamis, M.; Karaman, R.; Ayyash, F.; Qtait, A.; Deeb, O.; Manassra, A. Efficiency of advanced membrane wastewater treatment plant towards removal of aspirin, salicylic acid, paracetamol and p-aminophenol. J. Environ. Sci. Eng 2011, 5, 121–137. [Google Scholar]
- Karaman, R.; Khamis, M.; Qurie, M.; Halabieh, R.; Makharzeh, I.; Mannassra, A.; Abbadi, J.; Qtait, A.; Bufo, S.A.; Nasser, A.; et al. Removal of diclofenac potassium from wastewater using clay-micelle complex. Environ. Technol 2012, 33, 1279–1287. [Google Scholar]
- Shekoofeh, E.; Sepideh, H.; Roya, R. Role of mycorrhizal fungi and salicylic acid in salinity tolerance of Ocimum basilicum resistance to salinity. Afr. J. Biotechnol 2012, 11, 2223–2235. [Google Scholar]
- Marotti, M.; Piccaglia, R.; Giovanelli, E. Differences in essential oil composition of Basil (Ocimum. basilicum L.) Italian cultivars related to morfological characteristics. J. Agric. Food Chem 1996, 44, 3926–3929. [Google Scholar]
- American Public Health Association (APHA), Standard Methods for Examination of Water and Wastewater Analysis, 21st ed; APHA: Washington DC, USA, 2005.
- Ryan, J.; Harmsen, S.G.; Rashid, A. A Soil and Plant Analysis Manual Adapted for the West Asia and North Africa Region; ICARDA: Aleppo, Syria, 1996. [Google Scholar]
- Gericke, S.; Kurmies, B. Die kalorimetrische phosphorsäurebestimmung mit ammonium-vanadat-molybdat und ihre anwendung bei der pflanzenanalyse. Z pflanzenern düng Bodenkd 1952, 59, 235–247. [Google Scholar]
- Searle, P.L. The Berthelot or indophenol reaction and its use in the analytical chemistry of Nitrogen. Analyst 1984, 109, 549–568. [Google Scholar]


| Parameter | Mean value ± SD (different units) a | Ions | Mean value ± SD (mg L−1) |
|---|---|---|---|
| pH | 7.70 ± 0.30 | Cl− | 2,560 ± 80 |
| EC | 4.50 ± 0.50 | NO3− | 95.0 ± 5.0 |
| TDS | 2,250 ± 500 | PO43− | 2.30 ± 0.70 |
| COD | 330 ± 55 | NH4+ | 720 ± 20 |
| BOD | 120 ± 20 | Na+ | 330 ± 25 |
| FC | 0 | K+ | 81.0 ± 10 |
| TC | 0 | Ca2+ | 154 ± 15 |
| Mg2+ | 59.0 ± 13 |
| Parameter | Influent a | AS b | UF-HF | UF-SW | RO |
|---|---|---|---|---|---|
| pH | 7.50 ± 0.30 | 7.12 ± 0.47 | 7.50 ± 0.07 | 7.45 ± 0.08 | 6.20 ± 0.15 |
| EC (mS cm−1) | 1.99 ± 0.23 | 1.90 ± 0.22 | 1.53 ± 0.01 | 1.53 ± 0.01 | 0.03 ± 0.01 |
| TDS (mg L−1) | 966 ± 105 | 912 ± 92 | 760 ± 10 | 750 ± 16 | 30 ± 12 |
| COD (mg L−1) | 380 ± 150 | 182 ± 96 | 90 ± 20 | 46 ± 13 | 20 ± 11 |
| BOD (mg L−1) | 242 ± 138 | 107 ± 49 | 56 ± 25 | 41 ± 14 | 10 ± 5 |
| Cl− (mg L−1) | 268 ± 62 | 192 ± 100 | 246 ± 20 | 246 ± 10 | 13.50 ± 1.05 |
| NO3− (mg L−1) | 15.50 ± 10 | 12.40 ± 10 | 10.02 ± 5.02 | 13.03 ± 3.06 | 2.50 ± 0.10 |
| PO43−(mg L−1) | 25.69 ± 3.15 | 14.70 ± 2.2 | 10.16 ± 1.50 | 3.14 ± 0.50 | 0.23 ± 0.05 |
| NH4+ (mg L−1) | 91 ± 64 | 89 ± 56 | 85 ± 11 | 23.30 ± 3.11 | 5.20 ± 0.21 |
| Na+ (mg L−1) | 128 ± 45 | 86 ± 50 | 112 ± 14 | 114 ± 10 | 7.30 ± 2.12 |
| K+ (mg L−1) | 41 ± 30 | 36 ± 13 | 38.8 ± 10 | 19.70 ± 5.05 | 1.38 ± 1.03 |
| Ca2+ (mg L−1) | 65 ± 21 | 60 ± 15 | 65 ± 12 | 65.70 ± 13 | 1.75 ± 0.51 |
| Mg2+ (mg L−1) | 30 ± 10 | 24 ± 20 | 24 ± 11 | 27.30 ± 5.01 | 0.68 ± 0.11 |
| TC (cfu/mL) | (6 × 105) ± 102 | (2 × 104) ± 101 | 120 ± 50 | 0 | 0 |
| FC (cfu/mL) | (5 × 103) ± 102 | (2 × 102) ± 101 | 40 ± 20 | 0 | 0 |
| Brine: Fresh Water 1:1 (v:v) a | Fresh Water a | |||||
|---|---|---|---|---|---|---|
| Parameter | Influent | Effluent | % removal | Influent | Effluent | % removal |
| pH | 7.30 ± 0.1 | 7.8 ± 0.2 | 7.40 ± 0.1 | 7.5 ± 0.10 | ||
| EC (mS cm−1) | 3.82 ± 0.2 | 1.91 ± 0.2 | 50.0 | 1.74 ± 0.1 | 1.10 ± 0.1 | 37.0 |
| TDS (mg L−1) | 1910 ± 120 | 930 ± 113 | 51.0 | 905 ± 63.0 | 520 ± 40.0 | 43.0 |
| COD (mg L−1) | 141 ± 15.0 | 75 ± 10.0 | 47.0 | 73.0 ± 10.0 | 38 ± 10.0 | 48.0 |
| BOD (mg L−1) | 61 ± 10.0 | 20 ± 10.0 | 67.0 | 40.0 ± 10.0 | 19 ± 10.0 | 53.0 |
| Cl− (mg L−1) | 1631 ± 500 | 1081 ± 200 | 34.0 | 420 ± 80.0 | 285 ± 60.0 | 32.0 |
| NO3− (mg L−1) | 838 ± 231 | 624 ± 19.0 | 26.0 | 645 ± 10.0 | 332 ± 74.0 | 49.0 |
| PO43− (mg L−1) | 2.15 ± 0.1 | 0.50 ± 0.1 | 77.0 | 2.15 ± 0.1 | 0.50 ±0.10 | 77.0 |
| NH4+ (mg L−1) | 292 ± 80.0 | 12 ± 4.0 | 96.0 | 44.0 ± 4.00 | 10 ± 4.0 | 77.0 |
| Na+ (mg L−1) | 192 ± 7.0 | 100 ± 49.0 | 48.0 | 54.0 ± 2.0 | 43 ± 2.0 | 20.0 |
| K+ (mg L−1) | 123 ± 19.0 | 93 ± 6.0 | 25.0 | 110 ± 2.0 | 66 ± 26.0 | 40.0 |
| Ca2+ (mg L−1) | 117 ± 2.0 | 101 ± 1.0 | 14.0 | 79.0 ± 2.0 | 80 ± 1.0 | 0.0 |
| Mg2+ (mg L−1) | 55 ± 1.00 | 46 ± 4.0 | 17.0 | 37.0 ± 1.0 | 32 ± 1.0 | 14.0 |
| 100% Brine a | Fresh Water a | |||||
|---|---|---|---|---|---|---|
| Parameter | Influent b | Effluent c | % removal | Influent | Effluent | % removal |
| pH | 7.20 ± 0.2 | 7.1 ± 0.1 | 7.02 ± 0.1 | 7.50 ± 0.1 | ||
| EC (mS cm−1) | 6.04 ± 0.2 | 2.51 ± 0.1 | 58.0 | 2.1 ± 0.1 | 1.05 ± 0.1 | 50.0 |
| TDS (mg L−1) | 3,000 ± 100 | 1,560 ± 50.0 | 48.0 | 1,050 ± 50.0 | 510 ± 15.0 | 51.0 |
| COD (mg L−1) | 180 ± 20.0 | 60 ± 20.0 | 67.0 | 70 ± 15.0 | 38 ± 10.0 | 46.0 |
| BOD (mg L−1) | 85 ± 10.0 | 57 ± 10.0 | 12.0 | 38 ± 10.0 | 12 ± 5.00 | 68.0 |
| Cl− (mg L−1) | 2,463 ± 200 | 1,676 ± 200 | 32.0 | 460 ± 10.0 | 337 ± 10.0 | 27.0 |
| NO3− (mg L−1) | 1,017 ± 50.0 | 921 ± 15.0 | 10.0 | 545 ±25.0 | 44 ± 25.0 | 92.0 |
| PO43− (mg L−1) | 3.59 ± 0.3 | 0.94 ± 0.2 | 74.0 | 2.59 ± 0.1 | 0.94 ± 0.1 | 64.0 |
| NH4+ (mg L−1) | 688 ± 10.0 | 16 ± 10.0 | 98.0 | 41 ± 10.0 | 6 ± 4.00 | 85.0 |
| Na+ (mg L−1) | 329 ± 10.0 | 55 ± 5.0 | 83.0 | 58 ± 5.0 | 35 ± 5.0 | 40.0 |
| K+ (mg L−1) | 111 ± 25.0 | 45 ± 5.0 | 60.0 | 98 ± 1.0 | 9 ± 5.0 | 91.0 |
| Ca2+ (mg L−1) | 184 ± 10.0 | 163 ± 5.0 | 11.0 | 83 ± 2.0 | 78 ± 5.0 | 6.0 |
| Mg2+ (mg L−1) | 62 ± 5.0 | 46 ± 2.0 | 26.0 | 33 ± 5.0 | 31 ± 2.0 | 6.0 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qurie, M.; Abbadi, J.; Scrano, L.; Mecca, G.; Bufo, S.A.; Khamis, M.; Karaman, R. Inland Treatment of the Brine Generated from Reverse Osmosis Advanced Membrane Wastewater Treatment Plant Using Epuvalisation System. Int. J. Mol. Sci. 2013, 14, 13808-13825. https://doi.org/10.3390/ijms140713808
Qurie M, Abbadi J, Scrano L, Mecca G, Bufo SA, Khamis M, Karaman R. Inland Treatment of the Brine Generated from Reverse Osmosis Advanced Membrane Wastewater Treatment Plant Using Epuvalisation System. International Journal of Molecular Sciences. 2013; 14(7):13808-13825. https://doi.org/10.3390/ijms140713808
Chicago/Turabian StyleQurie, Mohannad, Jehad Abbadi, Laura Scrano, Gennaro Mecca, Sabino A. Bufo, Mustafa Khamis, and Rafik Karaman. 2013. "Inland Treatment of the Brine Generated from Reverse Osmosis Advanced Membrane Wastewater Treatment Plant Using Epuvalisation System" International Journal of Molecular Sciences 14, no. 7: 13808-13825. https://doi.org/10.3390/ijms140713808






