Recent Progress in Pharmaceutical Therapies for Castration-Resistant Prostate Cancer
Abstract
:1. Introduction
2. CYP17 Inhibitor Abiraterone
3. AR Antagonist Enzalutamide
4. Cytotoxic Cabazitaxel
5. Vaccine Sipuleucel-T
6. RANKL Antibody Denosumab
7. Radiopharmaceutical Alpharadin
8. Other Drug Candidates in Clinical Trials and Novel Strategies
9. Conclusions
Conflict of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin 2012, 62, 10–29. [Google Scholar]
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer treatment and survivorship statistics. CA Cancer J. Clin 2012, 62, 220–241. [Google Scholar]
- Attard, G.; Reid, A.H.M.; Yap, T.A.; Raynaud, F.; Dowsett, M.; Settatree, S.; Barrett, M.; Parker, C.; Martins, V.; Folkerd, E.; et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol 2008, 26, 4563–4571. [Google Scholar]
- Weinzimer, S.A.; Gibson, T.B.; Collett-Solberg, P.F.; Khare, A.; Liu, B.; Cohen, P. Transferrin is an insulin-like growth factor-binding protein-3 binding protein. J. Clin. Endocrinol. Metab 2011, 86, 1806–1813. [Google Scholar]
- Geller, J. Basis for hormonal management of advanced prostate cancer. Cancer 1993, 71, 1039–1045. [Google Scholar]
- Titus, M.A.; Schell, M.J.; Lih, F.B.; Tomer, K.B.; Mohler, J.L. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res 2005, 11, 4653–4657. [Google Scholar]
- Hu, Q.; Negri, M.; Olgen, S.; Hartmann, R.W. The role of fluorine substitution in biphenyl methylene imidazole type CYP17 inhibitors for the treatment of prostate carcinoma. ChemMedChem 2010, 5, 899–910. [Google Scholar]
- Hu, Q.; Yin, L.; Jagusch, C.; Hille, U.E.; Hartmann, R.W. Isopropylidene substitution increases activity and selectivity of biphenyl methylene 4-pyridine type CYP17 inhibitors. J. Med. Chem 2010, 53, 5049–5053. [Google Scholar]
- Hu, Q.; Negri, M.; Jahn-Hoffmann, K.; Zhuang, Y.; Olgen, S.; Bartels, M.; Müller-Vieira, U.; Lauterbach, T.; Hartmann, R.W. Synthesis, biological evaluation, and molecular modeling studies of methylene imidazole substituted biaryls as inhibitors of human 17α-hydroxylase-17,20-lyase (CYP17)—Part II: Core rigidification and influence of substituents at the methylene bridge. Bioorg. Med. Chem 2008, 16, 7715–7727. [Google Scholar]
- Hille, U.E.; Hu, Q.; Vock, C.; Negri, M.; Bartels, M.; Mueller-Vieira, U.; Lauterbach, T.; Hartmann, R.W. Novel CYP17 inhibitors: Synthesis, biological evaluation, structure-activity relationships and modeling of methoxy- and hydroxy-substituted methyleneimidazolyl biphenyls. Eur. J. Med. Chem 2009, 44, 2765–2775. [Google Scholar]
- Pinto-Bazurco Mendieta, M.A.E.; Negri, M.; Hu, Q.; Hille, U.E.; Jagusch, C.; Jahn-Hoffmann, K.; Müller-Vieira, U.; Schmidt, D.; Lauterbach, T.; Hartmann, R.W. CYP17 inhibitors—Annulations of additional rings in methylene imidazole substituted biphenyls: Synthesis, biological evaluation and molecular modeling. Arch. Pharm 2008, 341, 597–609. [Google Scholar]
- Hille, U.E.; Hu, Q.; Pinto-Bazurco Mendieta, M.A.E.; Bartels, M.; Vock, C.A.; Lauterbach, T.; Hartmann, R.W. Steroidogenic cytochrome P450 (CYP) enzymes as drug targets: Combining substructures of known CYP inhibitors leads to compounds with different inhibitory profile. C. R. Chim 2009, 12, 1117–1126. [Google Scholar]
- Jagusch, C.; Negri, M.; Hille, U.E.; Hu, Q.; Bartels, M.; Jahn-Hoffmann, K.; Pinto-Bazurco Mendieta, M.A.E.; Rodenwaldt, B.; Müller-Vieira, U.; Schmidt, D.; et al. Synthesis, biological evaluation and molecular modeling studies of methyleneimidazole substituted biaryls as inhibitors of human 17α-hydroxylase-17,20-lyase (CYP17)—Part I: Heterocyclic modifications of the core structure. Bioorg. Med. Chem 2008, 16, 1992–2010. [Google Scholar]
- Krug, S.J.; Hu, Q.; Hartmann, R.W. Hits identified in library screening demonstrate selective CYP17A1 lyase inhibition. J. Steroid Biochem. Mol. Biol 2013, 134, 75–79. [Google Scholar]
- Abadi, A.H.; Abou-Seri, S.M.; Hu, Q.; Negri, M.; Hartmann, R.W. Synthesis and biological evaluation of imidazolylmethylacridones as cytochrome P-450 enzymes inhibitors. Med. Chem. Commun 2012, 3, 663–666. [Google Scholar]
- Gobbi, S.; Cavalli, A.; Rampa, A.; Belluti, F.; Piazzi, L.; Paluszcak, A.; Hartmann, R.W.; Recanatini, M.; Bisi, A. Lead optimization providing a series of flavone derivatives as potent nonsteroidal inhibitors of the cytochrome P450 aromatase enzyme. J. Med. Chem 2006, 49, 4777–4780. [Google Scholar]
- Leze, M.P.; Palusczak, A.; Hartmann, R.W.; le Borgne, M. Synthesis of 6- or 4-functionalized indoles via a reductive cyclization approach and evaluation as aromatase inhibitors. Bioorg. Med. Chem. Lett 2008, 18, 4713–4715. [Google Scholar]
- Al-Soud, Y.A.; Heydel, M.; Hartmann, R.W. Design and synthesis of 1,3,5-trisubstituted 1,2,4-triazoles as CYP enzyme inhibitors. Tetrahedron Lett 2011, 52, 6372–6375. [Google Scholar]
- Yin, L.; Hu, Q. Drug discovery for breast cancer and coinstantaneous cardiovascular disease: What is the future? Future Med. Chem 2013, 5, 359–362. [Google Scholar]
- Yin, L.; Lucas, S.; Maurer, F.; Kazmaier, U.; Hu, Q.; Hartmann, R.W. Novel imidazol-1-ylmethyl substituted 1,2,5,6-tetrahydropyrrolo[3,2,1-ij]quinolin-4-ones as potent and selective CYP11B1 inhibitors for the treatment of Cushing’s syndrome. J. Med. Chem 2012, 55, 6629–6633. [Google Scholar]
- Emmerich, J.; Hu, Q.; Hanke, N.; Hartmann, R.W. Cushing’s syndrome: Development of highly potent and selective CYP11B1 inhibitors of the (pyridylmethyl)pyridine type. J. Med. Chem 2013, in press. [Google Scholar]
- Gobbi, S.; Hu, Q.; Negri, M.; Zimmer, C.; Belluti, F.; Rampa, A.; Hartmann, R.W.; Bisi, A. Modulation of cytochromes P450 with xanthone-based molecules: From aromatase to aldosterone synthase and steroid 11β-hydroxylase inhibition. J. Med. Chem 2013, 56, 1723–1729. [Google Scholar]
- Yin, L.; Hu, Q.; Hartmann, R.W. 3-Pyridinyl substituted aliphatic cycles as CYP11B2 inhibitors: Aromaticity abolishment of the core significantly increased selectivity over CYP1A2. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Grombein, C.M.; Hu, Q.; Heim, R.; Hartmann, R.W. Unexpected results of a SN,Ar-reaction—A novel synthetic approach to 1-arylthio- naphthalen-2-ols. Adv. Synth. Catal. 2013, in press. [Google Scholar]
- Hu, Q.; Yin, L.; Hartmann, R.W. Novel heterocycle substituted 4,5-dihydro-[1,2,4]triazolo[4,3-a]quinolines as potent and selective aldosterone synthase inhibitors for the treatment of aldosterone-related cardiovascular diseases. J. Med. Chem. 2013, in press. [Google Scholar]
- Grombein, C.M.; Hu, Q.; Heim, R.; Rau, S.; Zimmer, C.; Hartmann, R.W. 1-Phenylsulfinyl-3- (pyridin-3-yl)naphthalen-2-ols: A new class of potent and selective aldosterone synthase inhibitors. J. Med. Chem. 2013, in press. [Google Scholar]
- Yin, L.; Hu, Q.; Hartmann, R.W. Novel pyridyl or isoquinolinyl substituted indolines and indoles as potent and selective aldosterone synthase inhibitors. J. Med. Chem. 2013, in press. [Google Scholar]
- Hu, Q.; Yin, L.; Hartmann, R.W. Selective dual inhibitors of CYP19 and CYP11B2: Targeting cardiovascular diseases hiding in the shadow of breast cancer. J. Med. Chem 2012, 55, 7080–7089. [Google Scholar]
- Yin, L.; Hu, Q.; Hartmann, R.W. Tetrahydropyrroloquinolinone type dual inhibitors of aromatase/aldosterone synthase as a novel strategy for breast cancer patients with elevated cardiovascular risks. J. Med. Chem 2013, 56, 460–470. [Google Scholar]
- Potter, G.A.; Banie, S.E.; Jarman, M.; Rowlands, M.G. Novel steroidal inhibitors of human cytochrome P45017α (l7α-hydroxylase-Cl7,20-lyase): Potential agents for the treatment of prostatic cancer. J. Med. Chem 1995, 38, 2463–2471. [Google Scholar]
- Yin, L.; Hu, Q. CYP17 inhibitors: From promiscuous abiraterone to selective C17-20 lyase inhibitors and multi-targeting agents. Nat. Rev. Urol. 2013, in press. [Google Scholar]
- Soifer, H.S.; Souleimanian, N.; Wu, S.; Voskresenskiy, A.M.; Collak, F.K.; Cinar, B.; Stein, C.A. Direct regulation of androgen receptor activity by potent CYP17 inhibitors in prostate cancer cells. J. Bio. Chem 2012, 287, 3777–3787. [Google Scholar]
- Zytiga prescribing information. Available online: http://www.zytigahcp.com/pdf/full_prescribing_info.pdf (on accessed 8 November 2012).
- Ryan, C.J.; Smith, M.R.; Fong, L.; Rosenberg, J.E.; Kantoff, P.; Raynaud, F.; Martins, V.; Lee, G.; Kheoh, T.; Kim, J.; et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J. Clin. Oncol 2010, 28, 1481–1488. [Google Scholar]
- Acharya, M.; Gonzalez, M.; Mannens, G.; de Vries, R.; Lopez, C.; Griffin, T.; Tran, N. A phase I, open-label, single-dose, mass balance study of 14C-labeled abiraterone acetate in healthy male subjects. Xenobiotica 2013, 43, 379–389. [Google Scholar]
- Efstathiou, E.; Titus, M.; Tsavachidou, D.; Tzelepi, V.; Wen, S.; Hoang, A.; Molina, A.; Chieffo, N.; Smith, L.A.; Karlou, M.; et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J. Clin. Oncol 2012, 30, 637–643. [Google Scholar]
- Attard, G.; Reid, A.H.M.; A’Hern, R.; Parker, C.; Oommen, N.B.; Folkerd, E.; Messiou, C.; Molife, L.R.; Maier, G.; Thompson, E.; et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J. Clin. Oncol 2009, 27, 3742–3748. [Google Scholar]
- Ryan, C.J.; Shah, S.; Efstathiou, E.; Smith, M.R.; Taplin, M.E.; Bubley, G.J.; Logothetis, C.J.; Kheoh, T.; Kilian, C.; Haqq, C.M.; et al. Phase II study of abiraterone acetate in chemotherapy-naïve metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin. Cancer Res 2011, 17, 4854–4861. [Google Scholar]
- Danila, D.C.; Morris, M.J.; de Bono, J.S.; Ryan, C.S.; Denmeade, S.R.; Smith, M.R.; Taplin, M.E.; Bubley, G.J.; Kheoh, T.; Haqq, C.; et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J. Clin. Oncol 2010, 28, 1496–1501. [Google Scholar]
- Reid, A.H.M.; Attard, G.; Danila, D.C.; Oommen, N.B.; Olmos, D.; Fong, P.C.; Molife, L.R.; Hunt, J.; Messiou, C.; Parker, C.; et al. Significant and sustained antitumor activity in postdocetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J. Clin. Oncol 2010, 28, 1489–1495. [Google Scholar]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med 2013, 368, 138–148. [Google Scholar]
- Logothetis, C.J.; Basch, E.; Molina, A.; Fizazi, K.; North, S.A.; Chi, K.N.; Jones, R.J.; Goodman, O.B.; Mainwaring, P.N.; Sternberg, C.N.; et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: Exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol 2012, 13, 1210–1217. [Google Scholar]
- Sternberg, C.N.; Molina, A.; North, S.; Mainwaring, P.; Fizazi, K.; Hao, Y.; Rothman, M.; Gagnon, D.D.; Kheoh, T.; Haqq, C.M.; et al. Effect of abiraterone acetate on fatigue in patients with metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Ann. Oncol 2012, 24, 1017–1025. [Google Scholar]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012, 13, 983–992. [Google Scholar]
- Zagar, Y.; Chaumaz, G.; Lieberherr, M. Signaling cross-talk from Gbeta4 subunit to Elk-1 in the rapid action of androgens. J. Bio. Chem 2004, 279, 2403–2413. [Google Scholar]
- Kampa, M.; Papakonstanti, E.A.; Hatzoglou, A.; Stathopoulos, E.N.; Stournaras, C.; Castanas, E. The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors, which increase PSA secretion and modify actin cytoskeleton. FASEB J 2002, 16, 1429–1431. [Google Scholar]
- Chen, C.D.; Welsbie, D.S.; Tran, C.; Baek, S.H.; Chen, R.; Vessella, R.; Rosenfeld, M.G.; Sawyers, C.L. Molecular determinants of resistance to antiandrogen therapy. Nat. Med 2004, 10, 33–39. [Google Scholar]
- Small, E.J.; Srinivas, S. The antiandrogen withdrawal syndrome—Experience in a large cohort of unselected patients with advanced prostate cancer. Cancer 1995, 76, 1428–1434. [Google Scholar]
- Taplin, M.E.; Bubley, G.J.; Shuster, T.D.; Frantz, M.E.; Spooner, A.E.; Ogata, G.K.; Keer, H.N.; Balk, S.P. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N. Engl. J. Med 1995, 332, 1393–1398. [Google Scholar]
- Hara, T.; Miyazaki, J.; Araki, H.; Yamaoka, M.; Kanzaki, N.; Kusaka, M.; Miyamoto, M. Novel mutations of androgen receptor: A possible mechanism of bicalutamide withdrawal syndrome. Cancer Res 2003, 63, 149–153. [Google Scholar]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar]
- Belikov, S.; Öberg, C.; Jääskeläinen, T.; Rahkama, V.; Palvimo, J.J.; Wrange, Ö. FoxA1 corrupts the antiandrogenic effect of bicalutamide but only weakly attenuates the effect of MDV3100 (Enzalutamide™). Mol. Cell. Endocrinol. 2013, 365, 95–107. [Google Scholar]
- Scher, H.I.; Beer, T.M.; Higano, C.S.; Anand, A.; Taplin, M.E.; Efstathiou, E.; Rathkopf, D.; Shelkey, J.; Yu, E.Y.; Alumkal, J.; et al. Antitumour activity of MDV3100 in castration- resistant prostate cancer: A phase 1–2 study. Lancet 2010, 375, 1437–1446. [Google Scholar]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med 2012, 367, 1187–1197. [Google Scholar]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med 2004, 351, 1502–1512. [Google Scholar]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar]
- Mita, A.C.; Denis, L.J.; Rowinsky, E.K.; de Bono, J.S.; Goetz, A.D.; Ochoa, L.; Forouzesh, B.; Beeram, M.; Patnaik, A.; Molpus, K.; et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin. Cancer Res 2009, 15, 723–730. [Google Scholar]
- Kearns, B.; Lloyd, J.M.; Stevenson, M.; Littlewood, C. Cabazitaxel for the second-line treatment of metastatic hormone-refractory prostate cancer: A NICE single technology appraisal. Pharmacoeconomics 2013, in press. [Google Scholar]
- Provenge—FDA full prescribing information. Available online: http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM210031.pdf (on accessed 4 May 2013).
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med 2010, 363, 411–422. [Google Scholar]
- Higano, C.S.; Schellhammer, P.F.; Small, E.J.; Burch, P.A.; Nemunaitis, J.; Yuh, L.; Provost, N.; Frohlich, M.W. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009, 115, 3670–3679. [Google Scholar]
- Small, E.J.; Schellhammer, P.F.; Higano, C.S.; Redfern, C.H.; Nemunaitis, J.J.; Valone, F.H.; Verjee, S.S.; Jones, L.A.; Hershberg, R.M. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol 2006, 24, 3089–3094. [Google Scholar]
- Saylor, P.J.; Lee, R.J.; Smith, M.R. Emerging therapies to prevent skeletal morbidity in men with prostate cancer. J. Clin. Oncol 2011, 29, 3705–3714. [Google Scholar]
- Pfeilschifter, J.; Mundy, G.R. Modulation of type beta transforming growth factor activity in bone cultures by osteotropic hormones. Proc. Natl. Acad. Sci. USA 1987, 84, 2024–2028. [Google Scholar]
- Brown, J.M.; Corey, E.; Lee, Z.D.; True, L.D.; Yun, T.J.; Tondravi, M.; Vessella, R.L. Osteoprotegerin and rank ligand expression in prostate cancer. Urology 2001, 57, 611–616. [Google Scholar]
- Smith, M.R.; Egerdie, B.; Hernández, T.N.; Feldman, R.; Tammela, T.L.; Saad, F.; Heracek, J.; Szwedowski, M.; Ke, C.; Kupic, A.; et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 2009, 361, 745–755. [Google Scholar]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar]
- Fizazi, K.; Bosserman, L.; Gao, G.; Skacel, T.; Markus, R. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: Results of a randomized phase II trial. J. Urol 2009, 182, 509–515. [Google Scholar]
- Smith, M.R.; Saad, F.; Coleman, R.; Shore, N.; Fizazi, K.; Tombal, B.; Miller, K.; Sieber, P.; Karsh, L.; Damião, R.; et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebo-controlled trial. Lancet 2012, 379, 39–46. [Google Scholar]
- Nilsson, S.; Franzén, L.; Parker, C.; Tyrrell, C.; Blom, R.; Tennvall, J.; Lennernäs, B.; Petersson, U.; Johannessen, D.C.; Sokal, M.; et al. Bone-targeted radium-223 in symptomatic, hormonerefractory prostate cancer: A randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 2007, 8, 587–589. [Google Scholar]
- Xofigo full prescribing information. Available online: http://labeling.bayerhealthcare.com/html/products/pi/Xofigo_PI.pdf (on accessed 23 May 2013).
- Lassmann, M.; Nosske, D. Dosimetry of 223Ra-chloride: Dose to normal organs and tissues. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 207–212. [Google Scholar]
- Vogelzang, N.J.; Helle, S.I.; Johannessen, D.C.; O’Sullivan, J.M.; Garcia-Vargas, J.E.; O’Bryan-Tear, C.G.; Shan, M.; Parker, C. Efficacy and safety of radium-233 dichloride (Ra-233) in castration-resistant prostate cancer (CRPC) patients with bone metastases who did or did not receive prior docetaxel (D) in the phase III ALSYMPCA trial. J. Clin. Oncol. 2013, 31 supplementary 5. abstract 5068. [Google Scholar]
- Vasaitis, T.; Belosay, A.; Schayowitz, A.; Khandelwal, A.; Chopra, P.; Gediya, K.Z.; Guo, L.; Fang, H.B.; Njar, V.C.O.; Brodie, A.M.H. Androgen receptor inactivation contributes to antitumor efficacy of 17α-hydroxylase/17,20-lyase inhibitor 3β-hydroxy-17-(1H-benzimidazole-1-yl)-androsta- 5,16-diene in prostate cancer. Mol. Cancer Ther 2008, 7, 2348–2357. [Google Scholar]
- American Association for Cancer Research Web Page. Early Clinical Data Show Galeterone Safe, Effective against Prostate Cancer. Available online: http://www.aacr.org/home/public--media/aacr-press-releases.aspx?d=2769 (on accessed 6 May 2013).
- Eisner, J.R.; Abbott, D.H.; Bird, I.M.; Rafferty, S.W.; Moore, W.R.; Schotzinger, R.J. Assessment of steroid hormones upstream of P450c17 (CYP17) in chemically castrate male rhesus monkeys following treatment with the CYP17 inhibitors VT-464 and abiraterone acetate (AA). Endocr. Rev. 2012, 33(03_MeetingAbstracts), SAT-266. [Google Scholar]
- Abbott, D.H.; Eisner, J.R.; Bird, I.M.; Rafferty, S.W.; Moore, W.R.; Schotzinger, R.J. Plasma steroid concentrations in male rhesus monkeys following treatment with the P450c17 (CYP17) inhibitors VT-464 and abiraterone acetate: A comparison to human 17,20-Lyase (lyase) and combined lyase/17α-hydroxylase (hydroxylase) deficiencies. Endocr. Rev. 2012, 33(03_MeetingAbstracts), SAT-256. [Google Scholar]
- Liu, Q.; Sun, J.D.; Wang, J.; Ahluwalia, D.; Baker, A.F.; Cranmer, L.D.; Ferraro, D.; Wang, Y.; Duan, J.X.; Ammons, W.S.; et al. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: Optimization of dosing regimens and schedules. Cancer Chemother. Pharmacol 2012, 69, 1487–1498. [Google Scholar]
- Gulley, J.L.; Madan, R.A.; Stein, W.D.; Wilkerson, J.; Dahut, W.L.; Heery, C.R.; Schlom, J.; Wilding, G.; DiPaola, R.S. Effect of PSA-tricom, a pox-viral vaccine in prostate cancer (PCa), on tumor growth rates within 80 days after initiation in nonmetastatic PCa. J. Clin. Oncol. 2013, 31 supplementary 6. abstract 57. [Google Scholar]
- Drake, C.G.; Jaffee, E.; Pardoll, D.M. Mechanisms of immune evasion by tumors. Adv. Immunol 2006, 90, 51–81. [Google Scholar]
- Madan, R.A.; Mohebtash, M.; Arlen, P.M.; Vergati, M.; Rauckhorst, M.; Steinberg, S.M.; Tsang, K.Y.; Poole, D.J.; Parnes, H.L.; Wright, J.J.; et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol 2012, 13, 501–508. [Google Scholar]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med 2012, 366, 2443–2454. [Google Scholar]
- Agus, D.B.; Stadler, W.M.; Shevrin, D.H.; Hart, L.; MacVicar, G.R.; Hamid, O.; Hainsworth, J.D.; Gross, M.E.; Wang, J.; Webb, I.J.; et al. Safety, efficacy, and pharmacodynamics of the investigational agent orteronel (TAK-700) in metastatic castration-resistant prostate cancer (mCRPC): Updated data from a phase I/II study. J. Clin. Oncol. 2012, 30 supplementary 5. abstract 98. [Google Scholar]
- Clegg, N.J.; Wongvipat, J.; Joseph, J.D.; Tran, C.; Ouk, S.; Dilhas, A.; Chen, Y.; Grillot, K.; Bischoff, E.D.; Cai, L.; et al. ARN-509: A novel antiandrogen for prostate cancer treatment. Cancer Res 2012, 72, 1494–1503. [Google Scholar]
- Massard, C.; James, N.; Culine, S.; Jones, R.; Vuorela, A.; Mustonen, M.; Fizazi, K. ARADES Trial: A First-in-Man, Open-Label, Phase I/II Safety, Pharmacokinetic, and Proof-of-Concept Study of ODM-201 in Patients (pts) with Progressive Metastatic Castration-Resistant Prostate Cancer (mCRPC). Proceedings of the 2012 ESMO Congress, Vienna, Austria, 28 September–2 October 2012. Abstract LBA25_PR.
- Zhang, Y.; Castaneda, S.; Dumble, M.; Wang, M.; Mileski, M.; Qu, Z.; Kim, S.; Shi, V.; Kraft, P.; Gao, Y.; et al. Reduced expression of the androgen receptor by third generation of antisense shows antitumor activity in models of prostate cancer. Mol. Cancer Ther. 2011, 10, 2309–2319. [Google Scholar]
- Chi, K.; Yu, E.Y.; Ellard, S.; Hotte, S.J.; Gingerich, J.R.; Joshua, A.M.; Gleave, M.E. A Randomized Phase II Study of OGX-427 plus Prednisone (P) vs. P alone in Patients (pts) with Metastatic Castration Resistance Prostate Cancer (CRPC). Proceedings of the 2012 ESMO Congress, Vienna, Austria, 28 September–2 October 2012. Abstract 900PD.
- Smith, D.C.; Smith, M.R.; Sweeney, C.; Elfiky, A.A.; Logothetis, C.; Corn, P.G.; Vogelzang, N.J.; Small, E.J.; Harzstark, A.L.; Gordon, M.S.; et al. Cabozantinib in patients with advanced prostate cancer: Results of a phase II randomized discontinuation trial. J. Clin. Oncol 2013, 31, 412–419. [Google Scholar]
- Araujo, J.C.; Mathew, P.; Armstrong, A.J.; Braud, E.L.; Posadas, E.; Lonberg, M.; Gallick, G.E.; Trudel, G.C.; Paliwal, P.; Agrawal, S.; et al. Dasatinib combined with docetaxel for castration-resistant prostate cancer: Results from a phase 1–2 study. Cancer 2012, 118, 63–71. [Google Scholar]
- Dahut, W.L.; Madan, R.A.; Karakunnel, J.J.; Adelberg, D.; Gulley, J.L.; Turkbey, I.B.; Chau, C.H.; Spencer, S.D.; Mulquin, M.; Wright, J.; et al. Phase II clinical trial of cediranib in patients with metastatic castration-resistant prostate cancer. BJU Int. 2013. [Google Scholar] [CrossRef]
- Mardjuadi, F.; Medioni, J.; Kerger, J.; D’Hondt, L.; Canon, J.L.; Duck, L.; Musuamba, F.; Oudard, S.; Clausse, M.; Moxhon, A.; et al. Phase I study of sorafenib in combination with docetaxel and prednisone in chemo-naïve patients with metastatic castration-resistant prostate cancer. Cancer Chemother. Pharmacol 2012, 70, 293–303. [Google Scholar]
- Nabhan, C.; Villines, D.; Valdez, T.V.; Tolzien, K.; Lestingi, T.M.; Bitran, J.D.; Christner, S.M.; Egorin, M.J.; Beumer, J.H. Phase I study investigating the safety and feasibility of combining imatinib mesylate (Gleevec) with sorafenib in patients with refractory castration-resistant prostate cancer. Br. J. Cancer 2012, 107, 592–597. [Google Scholar]
- Saylor, P.J.; Mahmood, U.; Kunawudhi, A.; Smith, M.R.; Palmer, E.L.; Michaelson, M.D. Multitargeted tyrosine kinase inhibition produces discordant changes between 99mTc-MDP bone scans and other disease biomarkers: analysis of a phase II study of sunitinib for metastatic castration-resistant prostate cancer. J. Nucl. Med 2012, 53, 1670–1675. [Google Scholar]
- Kentepozidis, N.; Soultati, A.; Giassas, S.; Vardakis, N.; Kalykaki, A.; Kotsakis, A.; Papadimitraki, E.; Pantazopoulos, N.; Bozionellou, V.; Georgoulias, V. Paclitaxel in combination with carboplatin as salvage treatment in patients with castration-resistant prostate cancer: A Hellenic oncology research group multicenter phase II study. Cancer Chemother. Pharmacol 2012, 70, 161–168. [Google Scholar]
- Gasent, B.J.M.; Giner, M.V.; Giner-Bosch, V.; Cerezuela, F.P.; Alberola, C.V. Phase II trial of oxaliplatin and capecitabine after progression to first-line chemotherapy in androgen-independent prostate cancer patients. Am. J. Clin. Oncol 2011, 34, 155–159. [Google Scholar]
- Cetnar, J.; Wilding, G.; McNeel, D.; Loconte, N.K.; McFarland, T.A.; Eickhoff, J.; Liu, G. A phase 1/1b study of satraplatin (JM-216) in combination with docetaxel in patients with advanced solid tumors and metastatic castrate-resistant prostate cancer. Urol. Oncol 2013, 31, 436–441. [Google Scholar]
- Kelly, W.K.; Halabi, S.; Carducci, M.; George, D.; Mahoney, J.F.; Stadler, W.M.; Morris, M.; Kantoff, P.; Monk, J.P.; Kaplan, E.; et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J. Clin. Oncol 2012, 30, 1534–1540. [Google Scholar]
- Saad, F.; Hotte, S.; North, S.; Eigl, B.; Chi, K.; Czaykowski, P.; Wood, L.; Pollak, M.; Berry, S.; Lattouf, J.B.; et al. Randomized phase II trial of Custirsen (OGX-011) in combination with docetaxel or mitoxantrone as second-line therapy in patients with metastatic castrate-resistant prostate cancer progressing after first-line docetaxel: CUOG trial P-06c. Clin. Cancer Res 2011, 17, 5765–5773. [Google Scholar]
- Fizazi, K.S.; Higano, C.S.; Nelson, J.B.; Gleave, M.; Miller, K.; Morris, T.; Nathan, F.E.; McIntosh, S.; Pemberton, K.; Moul, J.W. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol 2013, 31, 1740–1747. [Google Scholar]
- Armstrong, A.J.; Creel, P.; Turnbull, J.; Moore, C.; Jaffe, T.A.; Haley, S.; Petros, W.; Yenser, S.; Gockerman, J.P.; Sleep, D.; et al. A phase I–II study of docetaxel and atrasentan in men with castration-resistant metastatic prostate cancer. Clin. Cancer Res 2008, 14, 6270–6276. [Google Scholar]
- Pili, R.; Häggman, M.; Stadler, W.M.; Gingrich, J.R.; Assikis, V.J.; Björk, A.; Nordle, O.; Forsberg, G.; Carducci, M.A.; Armstrong, A.J. Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J. Clin. Oncol 2011, 29, 4022–4028. [Google Scholar]
- Zhao, X.Y.; Malloy, P.J.; Krishnan, A.V.; Swami, S.; Navone, N.M.; Peehl, D.M.; Feldman, D. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat. Med 2000, 6, 703–706. [Google Scholar]
- Hu, Q.; Jagusch, C.; Hille, U.E.; Haupenthal, J.; Hartmann, R.W. Replacement of imidazolyl by pyridyl in biphenyl methylenes results in selective CYP17 and dual CYP17/CYP11B1 inhibitors for the treatment of prostate cancer. J. Med. Chem 2010, 53, 5749–5758. [Google Scholar]
- Efstathiou, J.A.; Bae, K.; Shipley, W.U.; Hanks, G.E.; Pilepich, M.V.; Sandler, H.M.; Smith, M.R. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J. Clin. Oncol 2008, 27, 92–99. [Google Scholar]
- Hu, Q.; Pinto-Bazurco Mendieta, M.A.E.; Hartmann, R.W. Highly potent and selective non-steroidal dual Inhibitors of CYP17/CYP11B2 for the treatment of prostate cancer in reducing cardiovascular complications. J. Med. Chem. 2013, in press. [Google Scholar]
- Mostaghel, E.A.; Marck, B.T.; Plymate, S.R.; Vessella, R.L.; Balk, S.; Matsumoto, A.M.; Nelson, P.S.; Montgomery, R.B. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin. Cancer Res 2011, 17, 5913–5925. [Google Scholar]
- Li, Y.; Chan, S.C.; Brand, L.J.; Hwang, T.H.; Silverstein, K.A.; Dehm, S.M. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res 2013, 73, 483–489. [Google Scholar]


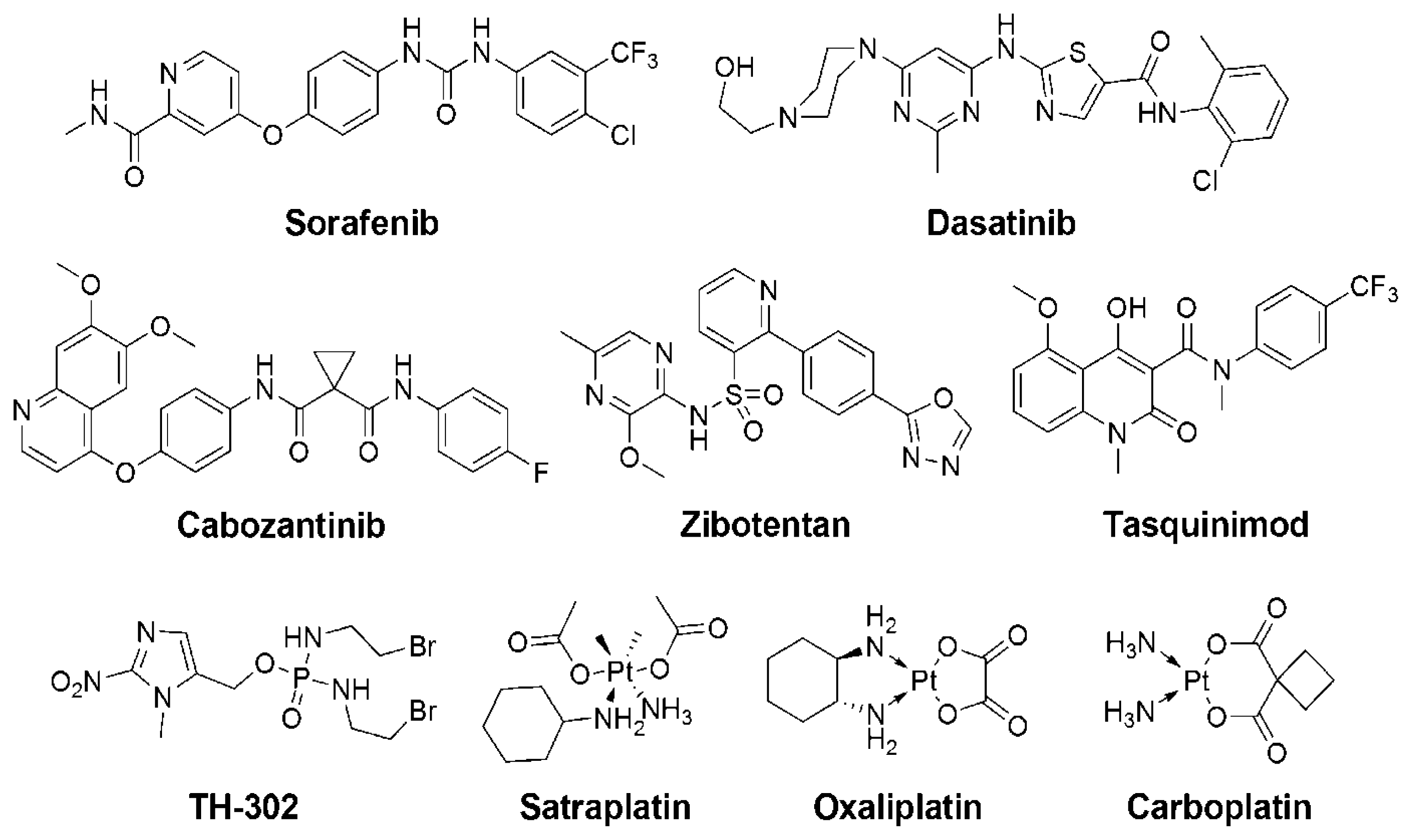
| Entity a | Category b | Mechanism c | Clinical Trials d |
|---|---|---|---|
| Orteronel [84] (TAK700) | SM | CYP17 inhibitor | phase I/II (NCT01666314, NCT01549951[P], NCT00569153, NCT01816048, NCT01658527[B], NCT01046916, NCT01084655[DP]) Phase III (NCT01707966, NCT01193257[P], NCT01809691[G]; NCT01546987[BG]) |
| Galeterone [75,76] (TOK-001) | SM | CYP17 inhibitor with AR antagonism | phase I/II (NCT00959959#, NCT01709734) |
| VT-464 [77,78] | SM | selective CYP17 C17-20 lyase inhibitor | phase I/II (2011-004103-20) e |
| CFG920 | SM | CYP17 inhibitor | phase I/II (NCT01647789) |
| ARN-509 [85] | SM | AR antagonist | phase I/II (NCT01822041, NCT01171898, NCT01792687[AP], NCT01790126/[G]) |
| ODM-201 [86] | SM | AR antagonist | phase I/II (NCT01429064, NCT01784757, NCT01317641) |
| AZD-3514 | SM | AR mRNA antagonist | Phase I (NCT01337518) |
| EZN-4176 [87] | AO | down-regulation of AR mRNA | Phase I (NCT01337518) |
| OGX-427 [88] | AO | heat shock protein 27 inhibitor | Phase I/II (NCT00487786/[D], NCT01120470[P], NCT01681433[AP]) |
| Cabozantinib (XL184) [89] | SM | dual inhibitor of VEGFR/MET Angiogenesis inhibition | Phase I/II (NCT01428219, NCT01834651, NCT01599793, NCT01703065, NCT01630590[G], NCT01812668, NCT01683994[DP], NCT01347788, NCT01574937[A], NCT00940225) Phase III (NCT01605227, NCT01522443) |
| Dasatinib [90] | SM | Src tyrosine kinase inhibitor | Phase I/II (NCT00570700, NCT00918385, NCT01260688[C], NCT01685125[AP], NCT00385580, NCT00385580#, NCT00936975, NCT01254864[AP], NCT00439270[D]#, NCT01826838[R]) |
| Cediranib [91] (AZD2171, Recentin) | SM | VEGFR tyrosine kinase inhibitor | Phase I/II (NCT01260688/[Da], NCT00527124 [DP], NCT00502164#, NCT00436956) |
| Sorafenib [92] (Nexavar, BAY43-9006) | SM | inhibitor of VEGFR, PDGFR and Raf kinase | Phase I/II (NCT00466752#, NCT00090545#, NCT00405210[D]#, NCT00619996[D]#, NCT00424385[I]#, NCT00093457#, NCT00430235[B]#, NCT00414388#, NCT00703638[PeCp]#) |
| Imatinib [93] (Gleevec, Glivec, STI571) | SM | BCR-Abl inhibitor | Phase I/II (NCT00080678[D]#, NCT00251225[D]#, NCT00038194[D]#, NCT00084825[D]#, NCT00427999#, NCT01316458#, NCT00171912#) |
| Sunitinib [94] (SU11248) | SM | inhibitor of VEGFR / PDGFR | Phase I/II (NCT00879619[DP], NCT00299741#, NCT00137436[DP]#, NCT00790595#, NCT00672594, NCT00631527[GR]#, NCT00879619[DP], NCT00748358, NCT00734851[DR], NCT00795171[D], NCT01254864[AP], NCT00550810, NCT00599313) |
| Aflibercept (AVE0005) | P | VEGF trap, anti-angiogensis | Phase III (NCT00519285[D]#) |
| TH-302 [79] | SM | hypoxia activated cytotoxicity | Phase I/II (NCT00743379) |
| Carboplatin [95] | PtC | cytotoxicity; inhibiting DNA synthesis | Phase I/II (NCT01051570[EP], NCT00973882[Ep]#, NCT00049257[Pt]#, NCT01505868[Ct], NCT00017576[Ca]#, NCT00514540[D]#, NCT00134706[D]#, CT00005627 [DEs]#, NCT00183924[DEs]#, NCT00016913, NCT00193193[EsPt]#, NCT01558492[Pt], NCT00003690[Fp]#, NCT00675545[D]#, NCT00005810[DEsFg]#) |
| Oxaliplatin [96] | PtC | cytotoxicity; inhibiting DNA synthesis | Phase I/II (NCT01338792[Pe]#, NCT00260611[D]#, NCT01487720[Gm]) |
| Satraplatin [97] | PtC | cytotoxicity; inhibiting DNA synthesis | Phase II (NCT00499694[Bv], NCT00634647[P]) Phase III (NCT00069745[P]#) |
| Bevacizumab [98] | AB | angiogenesis inhibitor | Phase II (NCT00349557[GR]#, NCT00478413, NCT00776594[BG], NCT00658697[BDG], NCT00027599[S]#, NCT00942578[DLP]#, NCT00499694[Sp], NCT00089609[DPT], NCT00574769[DEl], NCT00321646[D]#, NCT00348998[BGR]) Phase III (NCT00110214[DP]#, NCT00942331[CpGm]) |
| PROSTVAC-VF [80] (Prostvac) | V | immunotherapy recombinant vaccinia virus expresses PSA | Phase I/II (NCT00450463[F], NCT00078585, NCT00108732, NCT00113984, NCT00096551#, NCT00001382#, NCT00045227#, NCT00020254#, NCT00003871#, NCT00004029#) Phase III (NCT01322490) |
| Ipilimumab [82] (MDX010, Yervoy, BMS-734016, anti-CTLA4) | AB | immunotherapy anti-cytotoxic T lymphocyte-associated receptor 4 antibody | Phase I/II (NCT01530984, NCT01377389[G], NCT01194271[G], NCT01688492, NCT01498978, NCT00323882, NCT01832870[S], NCT00064129, NCT01804465, NCT00170157#, NCT00050596/[D]#, NCT00113984#) Phase III (NCT00861614, NCT01057810) |
| Nivolumab [83] (MDX1106, BMS-936558) | AB | immunotherapy anti programmed cell death protein 1 antibody | Phase I/II (NCT00441337#) |
| Custirsen [99] (OGX-011) | AO | clusterin inhibitor | Phase I/II (NCT00327340[D]#, NCT00054106[GF]#, NCT00258388[DP]#, NCT00471432[D]#) Phase III (NCT01578655[CtP], NCT01083615[Ct], NCT01188187[DP]#) |
| Zibotentan [100] (ZD4054) | SM | endothelin-A receptor antagonist | Phase I/II (NCT00090363#, NCT00055471#, NCT00314782[D]#, NCT01168141) Phase III (NCT00554229#, NCT00626548, NCT00617669[D]#) |
| Atrasentan [101] | SM | endothelin-A receptor antagonist | Phase I/II (NCT00181558[Z]#, NCT00038662#) Phase III (NCT00134056[DP], NCT00046943#, NCT00036556#, NCT00127478#) |
| Tasquinimod (ABR-215050) [102] | SM | anti-angiogenesis | Phase I/II (NCT01513733[CtP], NCT01732549, NCT00560482) Phase III (NCT01234311) |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yin, L.; Hu, Q.; Hartmann, R.W. Recent Progress in Pharmaceutical Therapies for Castration-Resistant Prostate Cancer. Int. J. Mol. Sci. 2013, 14, 13958-13978. https://doi.org/10.3390/ijms140713958
Yin L, Hu Q, Hartmann RW. Recent Progress in Pharmaceutical Therapies for Castration-Resistant Prostate Cancer. International Journal of Molecular Sciences. 2013; 14(7):13958-13978. https://doi.org/10.3390/ijms140713958
Chicago/Turabian StyleYin, Lina, Qingzhong Hu, and Rolf W. Hartmann. 2013. "Recent Progress in Pharmaceutical Therapies for Castration-Resistant Prostate Cancer" International Journal of Molecular Sciences 14, no. 7: 13958-13978. https://doi.org/10.3390/ijms140713958




