Facile Synthesis of FeCo/Fe3O4 Nanocomposite with High Wave-Absorbing Properties
Abstract
:1. Introduction
2. Results and Discussion
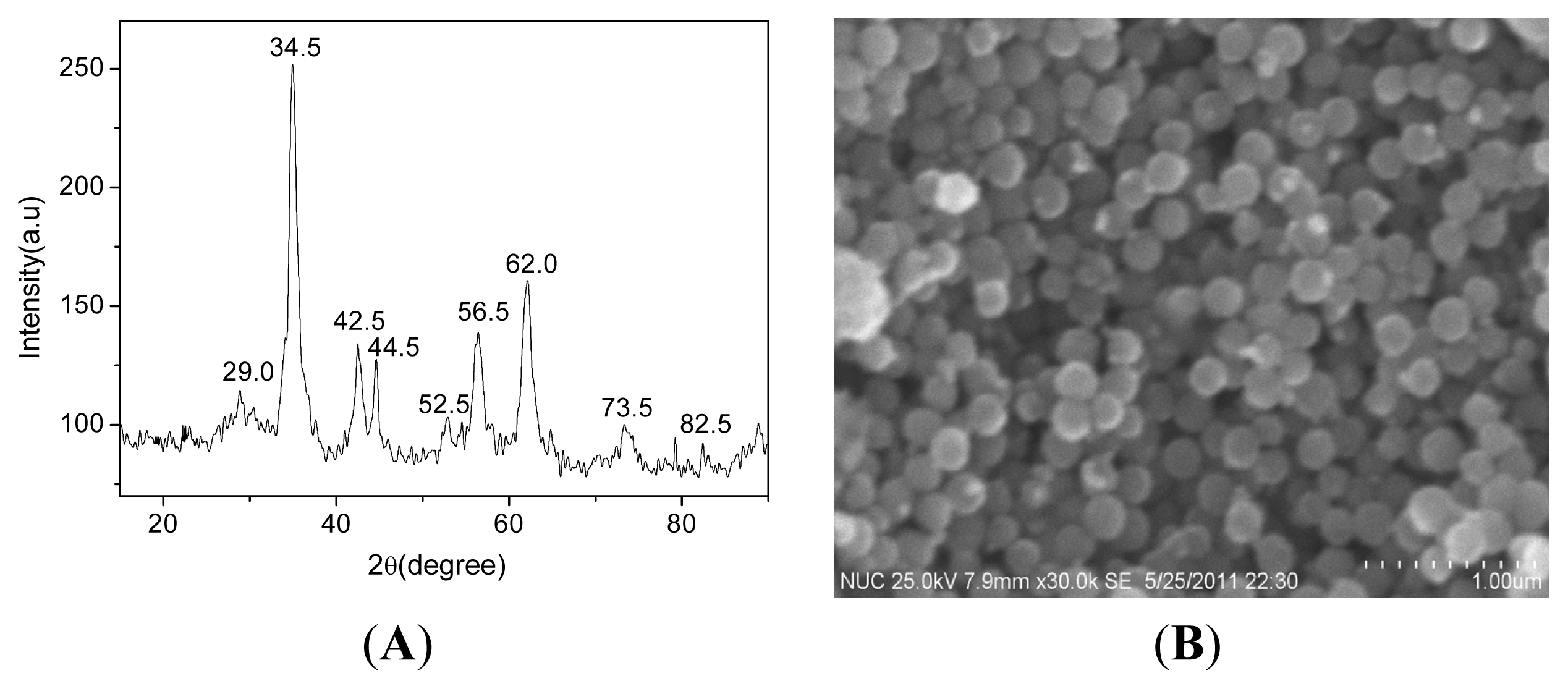
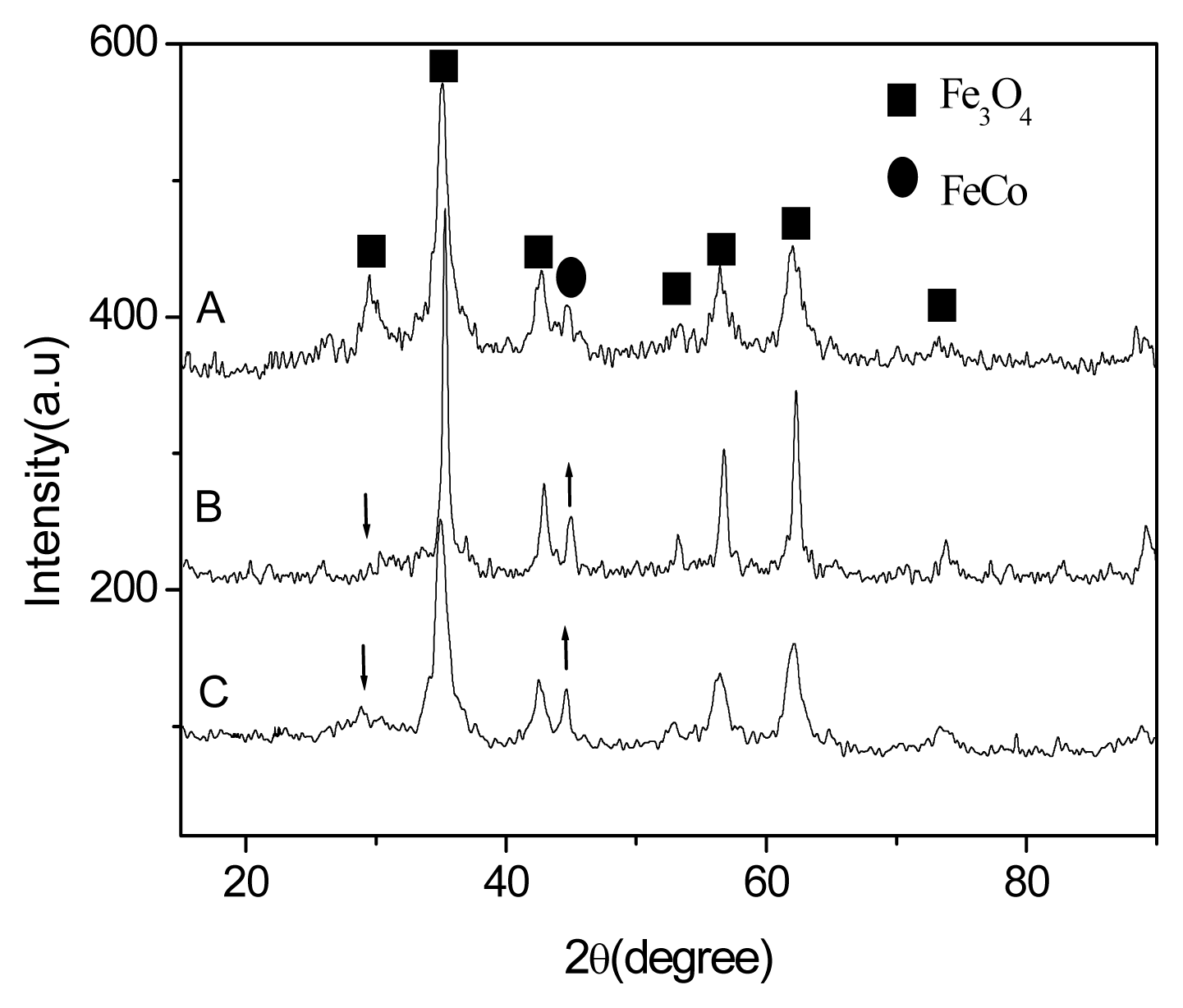
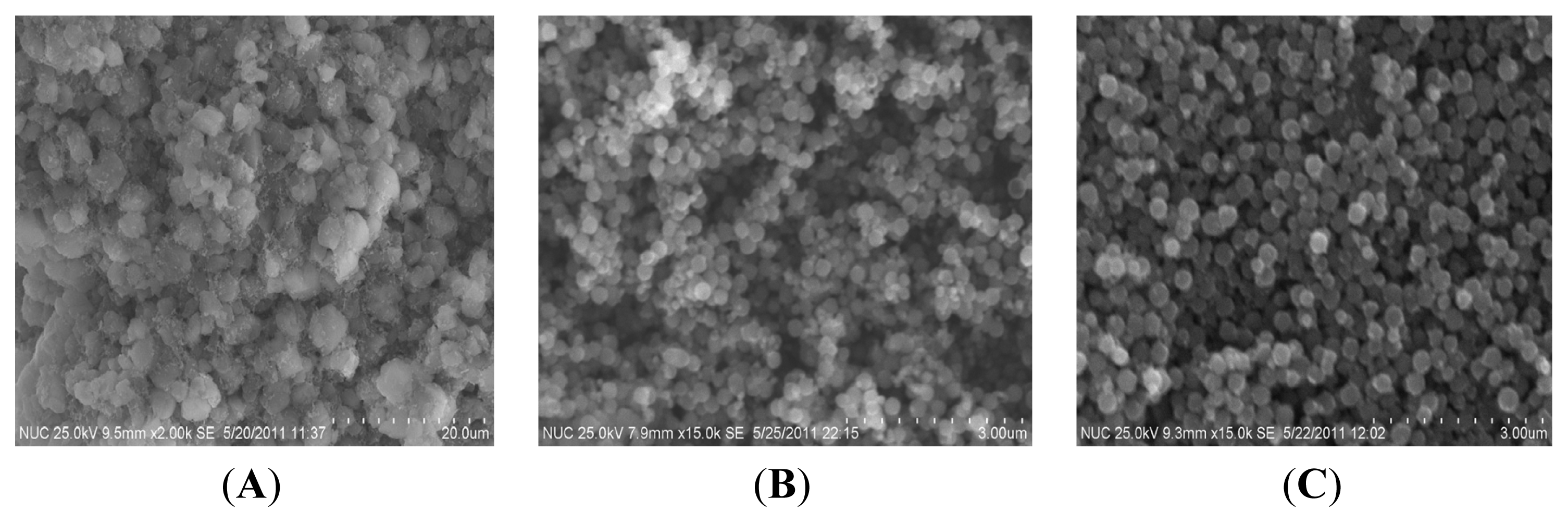
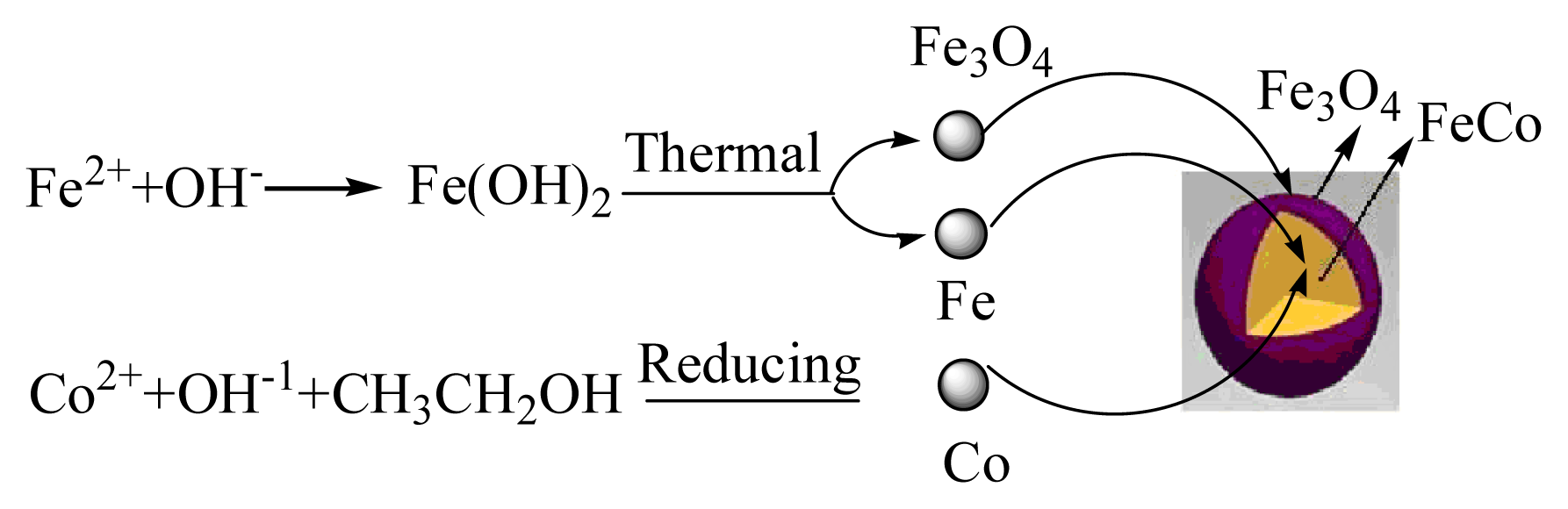
2.1. Preparation of the FeCo/Fe3O4 Nanocomposite
2.2. Properties of the FeCo/Fe3O4 Nanocomposite
3. Experimental Sections
3.1. Materials
3.2. Preparation of FeCo/Fe3O4 Nanocomposite
3.3. Characterization
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yan, S.J.; Zhen, L.; Xu, C.Y.; Jiang, J.T.; Shao, W.Z.; Tang, J.K. Synthesis, characterization and electromagnetic properties of Fe1−xCox alloy flower-like microparticles. J. Magnet. Magnet. Mater 2011, 323, 515–520. [Google Scholar]
- Zhu, J.J.; Wei, S.Y.; Haldolaarachchige, N.; Young, D.P.; Guo, Z.H. Electromagnetic field shielding polyurethane nanocomposites reinforced with core-shell Fe-Silica nanoparticles. J. Phys. Chem. C 2011, 115, 15304–15310. [Google Scholar]
- Han, M.G.; Lu, H.P.; Deng, L.J. Control of gigahertz permeability and permittivity dispersion by means of nanocrystallization in FeCo based nanocrystalline alloy. Appl. Phys. Lett 2010, 97, 192507, :1–192507:3.. [Google Scholar]
- Wei, X.W.; Zhu, G.X.; Liu, Y.J.; Ni, Y.H.; Song, Y.; Xu, Z. Large-scale controlled synthesis of FeCo nanocubes and microcages by wet chemistry. Chem. Mater 2008, 20, 6248–6253. [Google Scholar]
- Han, Z.; Li, D.; Wang, H.; Li, X.G.; Li, J.; Geng, D.Y.; Zhang, Z.D. Broadband electromagnetic wave absorption by FeCo/C nanocapsules. Appl. Phys. Lett 2009, 95, 209901, :1–209901:3.. [Google Scholar]
- Elena, H.; Ilana, P.; Aharon, G. Synthesis of air stable FeCo/C alloy nanoparticles by decomposing a mixture of the corresponding Metal-Acetyl Acetonates under their autogenic pressure. Inorg. Chem 2011, 50, 1288–1294. [Google Scholar]
- Bai, J.M.; Xu, Y.-H.; Thomas, J.; Wang, J.-P. (FeCo)3Si-SiOx core-shell nanoparticles fabricated in the gas phase. Nanotechnology 2007, 18, 065701–065705. [Google Scholar]
- Mendoza-Reséndez, R.; Pozas, R.; Morales, M.P.; Bonville, P.; Ocaña, M.; Serna, C.J. FeCo magnetic nanoneedles obtained by Co-coating haematite. Nanotechnology 2005, 16, 647–654. [Google Scholar]
- Srikanth, H.; Hajndl, R.; Chrinos, C.; Sanders, J.; Sampath, A.; Sudarshan, T.S. Magnetic studies of polymer-coated Fe nanoparticles synthesized by microwave plasma polymerization. Appl. Phys. Lett 2001, 79, 3503–3505. [Google Scholar]
- Chauvey, G.S.; Barcena, C.; Poudyal, N.; Rong, C.; Gao, J.; Sun, S.; Liu, J.P. Synthesis and stabilization of FeCo nanoparticles. J. Am. Chem. Soc 2007, 129, 7214–7215. [Google Scholar]
- Desvaux, C.; Amiens, C.; Fejes, P.; Respaud, P.M.; Lecante, P.; Snoeck, E.; Chaydret, B. Multimillimetre-large superlattices of air-stable iron-cobalt nanoparticles. Nat. Mater 2005, 4, 750–753. [Google Scholar]
- Turgut, Z.; Scott, J.H.; Huang, M.W.; Majetich, S.A.; MaHenry, M.E. Magnetic properties and ordering in C-coated Fex-Co1−x Alloy nanocrystals. J. Appl. Phys 1998, 83, 6468–6470. [Google Scholar]
- Li, Y.; Kim, Y.J.; Kim, A.Y.; Lee, K.; Jung, M.H.; Hur, N.H.; Park, K.H.; Seo, W.S. Highly stable and magnetically recyclable mesoporous silica spheres embedded with FeCo/graphitic shell nanocrystals for supported catalysts. Chem. Mater 2011, 23, 5398–5403. [Google Scholar]
- Wei, S.Y.; Wang, Q.; Zhu, J.H.; Sun, L.Y.; Line, H.F.; Guo, Z.H. Multifunctional composite core-shell nanoparticles. Nanoscale 2011, 3, 4474–4502. [Google Scholar]
- Sun, Y.Y.; Tian, Y.; He, M.H.; Zhao, Q.; Chen, C.; Hu, C.S.; Liu, Y.Q. Controlled synthesis of Fe3O4/Ag core-shell composite nanoparticles with high electrical conductivity. J. Electron. Mater 2012, 41, 519–523. [Google Scholar]
- Sun, Y.Y.; Zhou, X.; Liu, Y.Q.; Zhao, G.Z.; Jiang, Y. Effect of magnetic nanoparticles on the properties of magnetic rubber. Mater. Res. Bull 2010, 45, 878–881. [Google Scholar]
- Rafique, M.Y.; Pan, L.Q.; Iqbal, M.Z.; Javed, Q.U.A.; Qiu, H.M.; Rafi-ud-din Farooq, M.H.; Guo, Z.G. 3-D flower like FeCo alloy nanostructures assembled with nanotriangular prism: Facile synthesis, magnetic properties, and effect of NaOH on its formation. J. Alloys Comp. 2013, 550, 423–430. [Google Scholar]
- Cho, U.R.; Wang, K.; Kim, G.W.; Koo, B.H. Effects of Ag seed on synthesis of FeCo nano-particles prepared via the polyol method. J. Mater. Sci. Technol 2010, 26, 660–664. [Google Scholar]
- Hayashi, N.; Toriyama, T.; Yamashiro, M.; Moriwaki, T.; Oguri, Y.; Fukuda, K.; Sakamoto, I.; Hasegaw, M. Synthesis of nanosized FeCo granular alloys by ion implantation and TMR effect. Nuclear Instrum. Methods Phys. Res. B 2007, 257, 25–29. [Google Scholar]
- Yang, Y.; Xu, C.L.; Xia, Y.X.; Wang, T.; Li, S. Synthesis and microwave absorption properties of FeCo nanoplates. J. Alloys Comp 2010, 493, 549–552. [Google Scholar]
- Liu, H.L.; Sonn, C.H.; Wu, J.H.; Lee, K.M.; Kim, Y.K. Synthesis of streptavidin-FITC conjugated core-shell Fe3O4-Au nanocrystals and their application for the purification of CD4+ lymphocytes. Biomaterials 2008, 9, 4003–4011. [Google Scholar]
- Liu, H.L.; Hou, P.; Zhang, W.X.; Wu, J.H. Synthesis of monosized core–shell Fe3O4/Au multifunctional nanoparticles by PVP-assisted nanoemulsion process. Colloids Surf. A 2010, 356, 21–27. [Google Scholar]
- Qi, Z.; Ian, B.; Virginia, M.; Yan, Z.C. Soft ferromagnetism in nanostructured mechanical alloying FeCo-based powders. J. Magnet. Magnet. Mater 2007, 318, 28–38. [Google Scholar]
- Lu, X.Y.; Niu, M.; Qiao, R.R.; Gao, M.Y. Superdispersible PVP-coated Fe3O4 nanocrystals prepared by a “one-pot” reaction. J. Phys. Chem. B 2008, 112, 14390–14394. [Google Scholar]
- Kolhatkar, A.G.; Nekrashevich, I.; Litvinov, D.; Willson, R.C.; Lee, T.R. Cubic silica-coated and amine-functionalized FeCo nanoparticles with high saturation magnetization. Chem. Mater 2013, 25, 1092–1097. [Google Scholar]
- Soo Ja, S.; Kim, Y.H.; Kim, C.W.; Cha, H.G.; Kim, Y.J.; Kang, Y.S. Preparation of magnetic FeCo nanoparticles by coprecipitation route. Curr. Appl. Phys 2007, 7, 404–408. [Google Scholar]
- Mohanty, S.R.; Lee, P.; Tan, T.L.; Springham, S.V.; Patran, A.; Ramanujan, R.V.; Rawat, R.S. Effect of deposition parameters on morphology and size of FeCo nanoparticles synthesized by pulsed laser ablation deposition. Appl. Surface Sci 2006, 252, 2806–2816. [Google Scholar]
- Azizi, A.; Sadrnezhaad, S.K.; Hasani, A. Morphology and magnetic properties of FeCo nanocrystalline powder produced by modified mechanochemical procedure. J. Magnet. Magnet. Mater 2010, 322, 3551–3554. [Google Scholar]
- Viau, G.F.; Et-Vincent, F.; Fihet, F. Nucleation and growth of bimetallic CoNi and FeNi monodisperse particles prepared in polyols. Solid State Tonics 1996, 84, 259–270. [Google Scholar]
- Liu, X.G.; Geng, D.Y.; Zhang, Z.D. Microwave-absorption properties of FeCo microspheres self-assembled by Al2O3-coated FeCo nanocapsules. Appl. Phys. Lett 2008, 92, 243110, :1–243110:3.. [Google Scholar]
- Liu, X.G.; Geng, D.Y.; Ma, S.; Meng, H.; Tong, M.; Kang, D.J.; Zhang, Z.D. Electromagnetic wave absorption properties of FeCo nanocapsules and coral-like aggregates self-assembled by the nanocapsules. J. Appl. Phys 2008, 104, 064319, :1–064319:5.. [Google Scholar]
- Han, Z.; Li, D.; Wang, X.W.; Zhang, Z.D. Microwave response of FeCo/carbon nanotubes composites. J. Appl. Phys 2011, 109, 07, A301:1–07A301:3.. [Google Scholar]
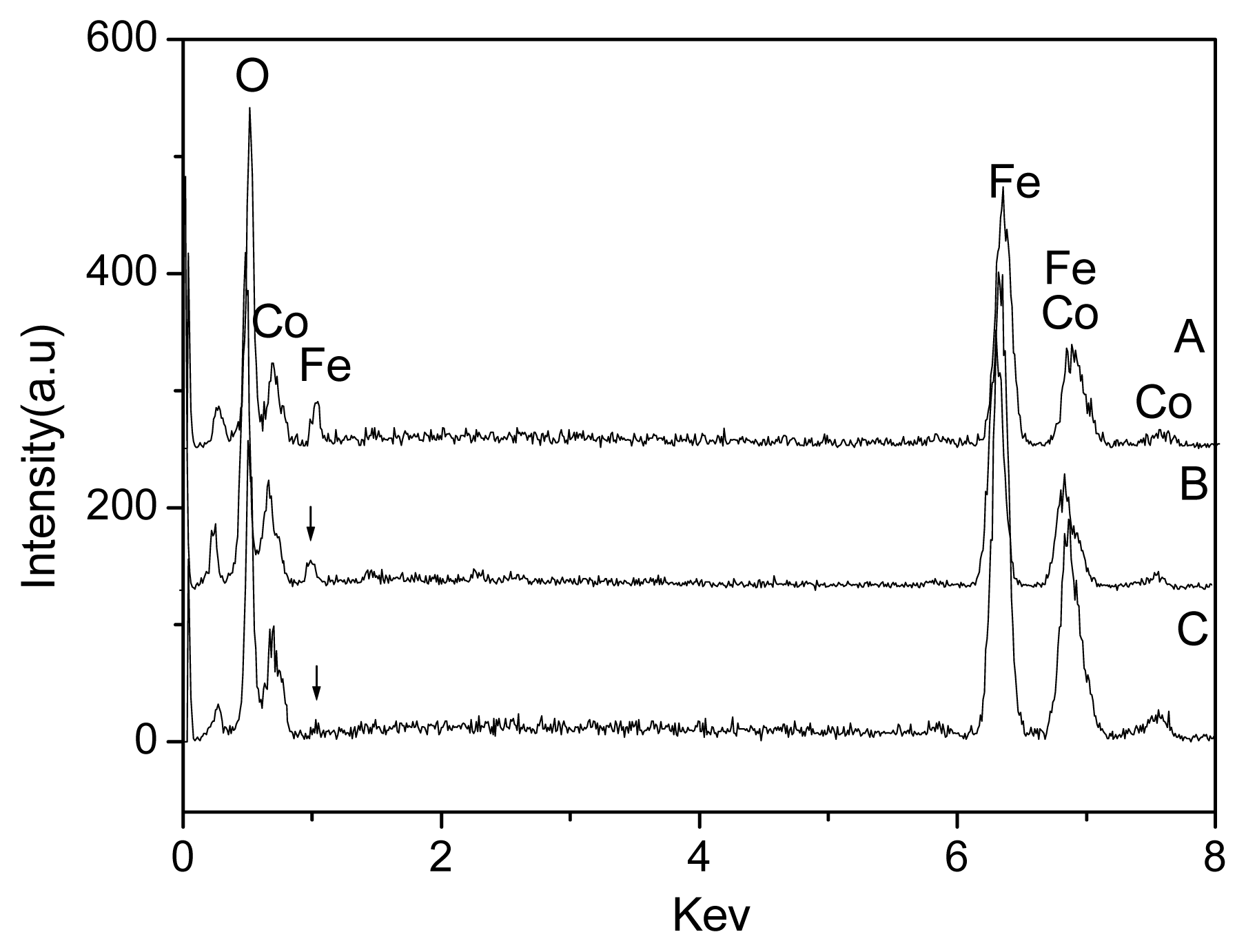
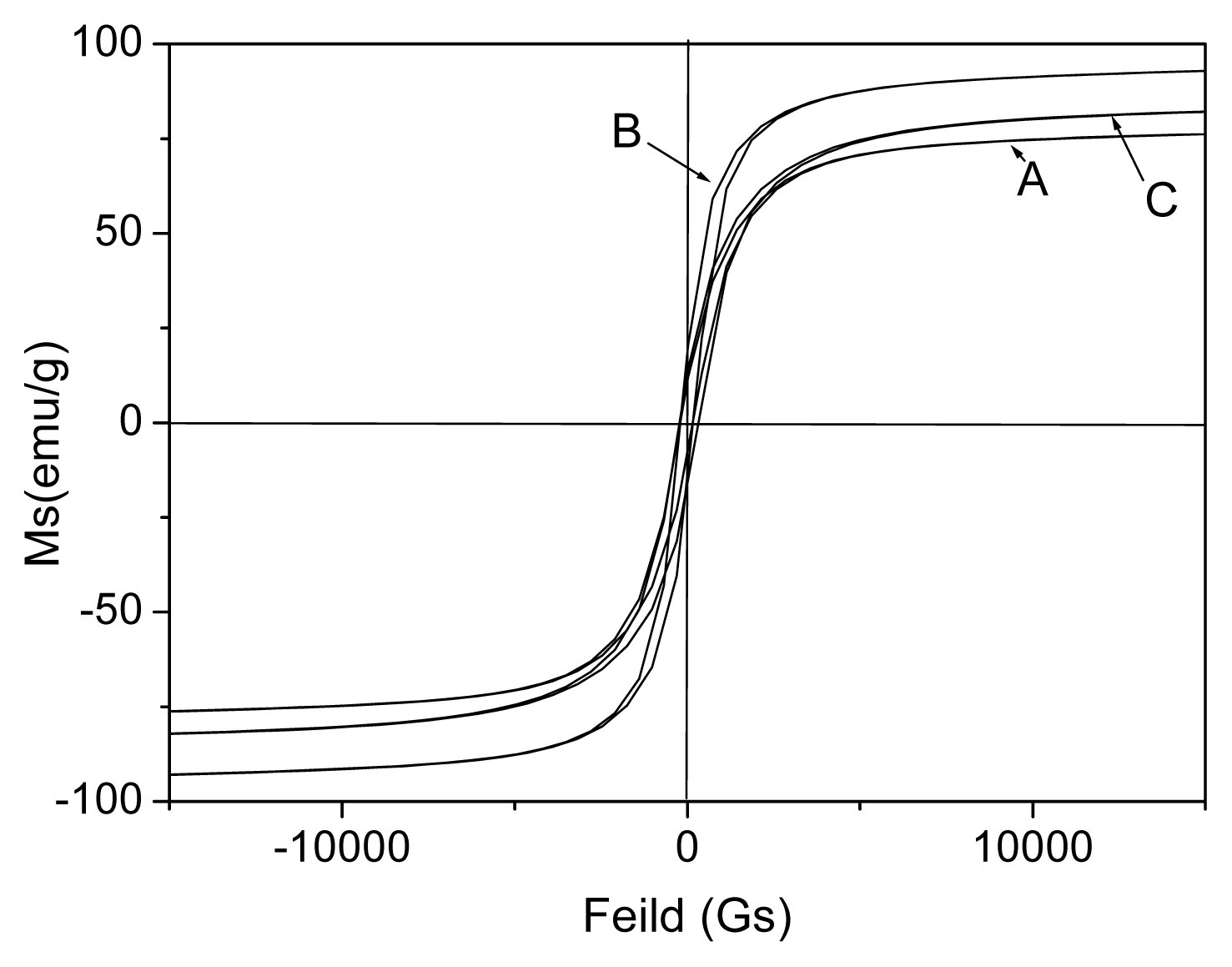

| Samples | A | B | C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Element | Co | Fe | O | Co | Fe | O | Co | Fe | O |
| Mass Weight (%) | 30.1 | 51.2 | 8.5 | 36.3 | 51.3 | 6.3 | 39.9 | 49.6 | 4.3 |
| Mass ratio | 1.9 | 3.1 | 4.8 | ||||||
| Reaction conditions | 130 °C | 150 °C | 170 °C |
|---|---|---|---|
| Ms (emu/g) | 88.6 | 92.4 | 112.8 |
| Hc (Gs) | 148.5 | 148.0 | 148.2 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gu, Y.; Cao, Y.; Chi, H.; Liang, Q.; Zhang, Y.; Sun, Y. Facile Synthesis of FeCo/Fe3O4 Nanocomposite with High Wave-Absorbing Properties. Int. J. Mol. Sci. 2013, 14, 14204-14213. https://doi.org/10.3390/ijms140714204
Gu Y, Cao Y, Chi H, Liang Q, Zhang Y, Sun Y. Facile Synthesis of FeCo/Fe3O4 Nanocomposite with High Wave-Absorbing Properties. International Journal of Molecular Sciences. 2013; 14(7):14204-14213. https://doi.org/10.3390/ijms140714204
Chicago/Turabian StyleGu, Yu, Yang Cao, Huijuan Chi, Qing Liang, Yongji Zhang, and Youyi Sun. 2013. "Facile Synthesis of FeCo/Fe3O4 Nanocomposite with High Wave-Absorbing Properties" International Journal of Molecular Sciences 14, no. 7: 14204-14213. https://doi.org/10.3390/ijms140714204




