Molecular Weight Dependent Glucose Lowering Effect of Low Molecular Weight Chitosan Oligosaccharide (GO2KA1) on Postprandial Blood Glucose Level in SD Rats Model
Abstract
:1. Introduction
2. Results and Discussion
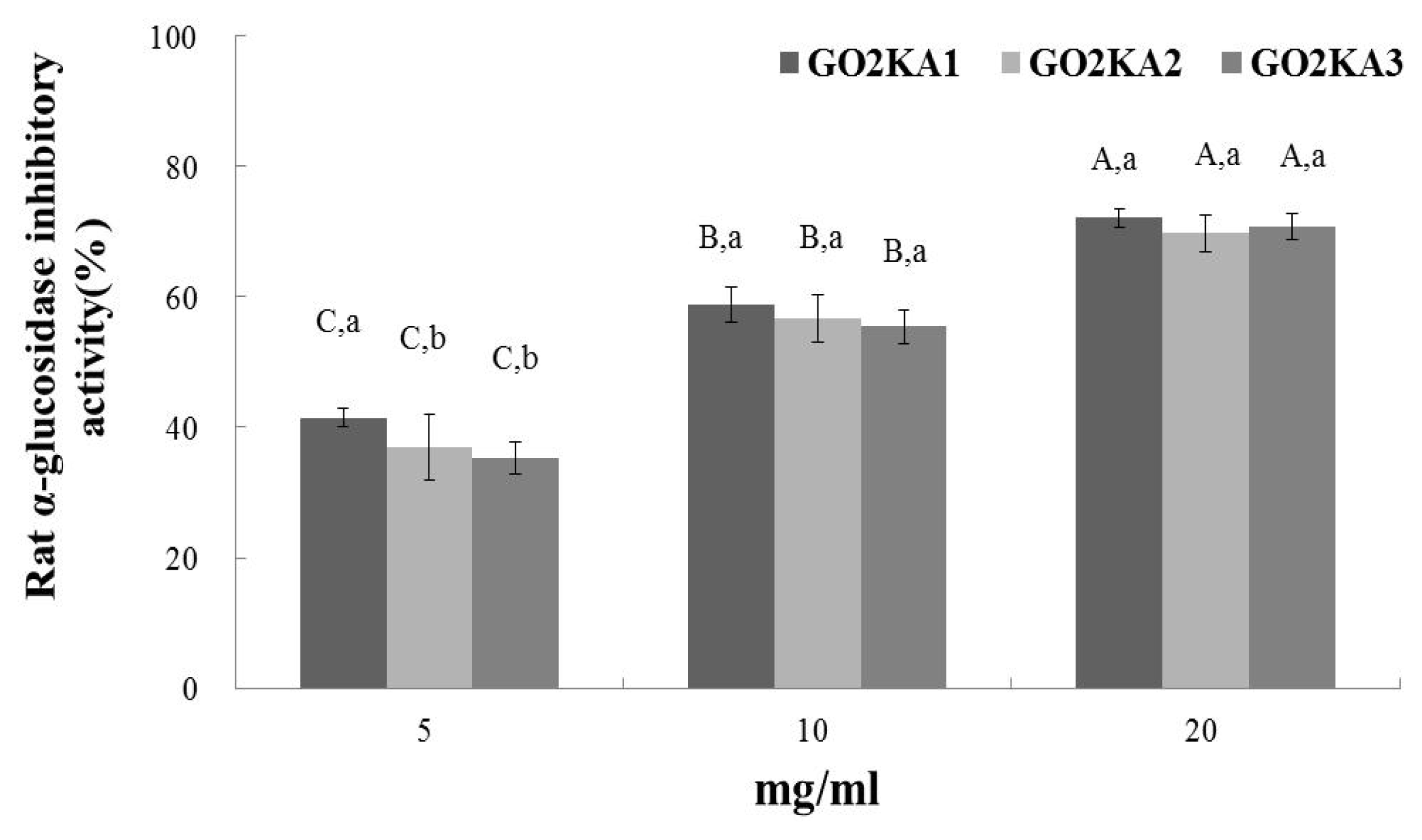
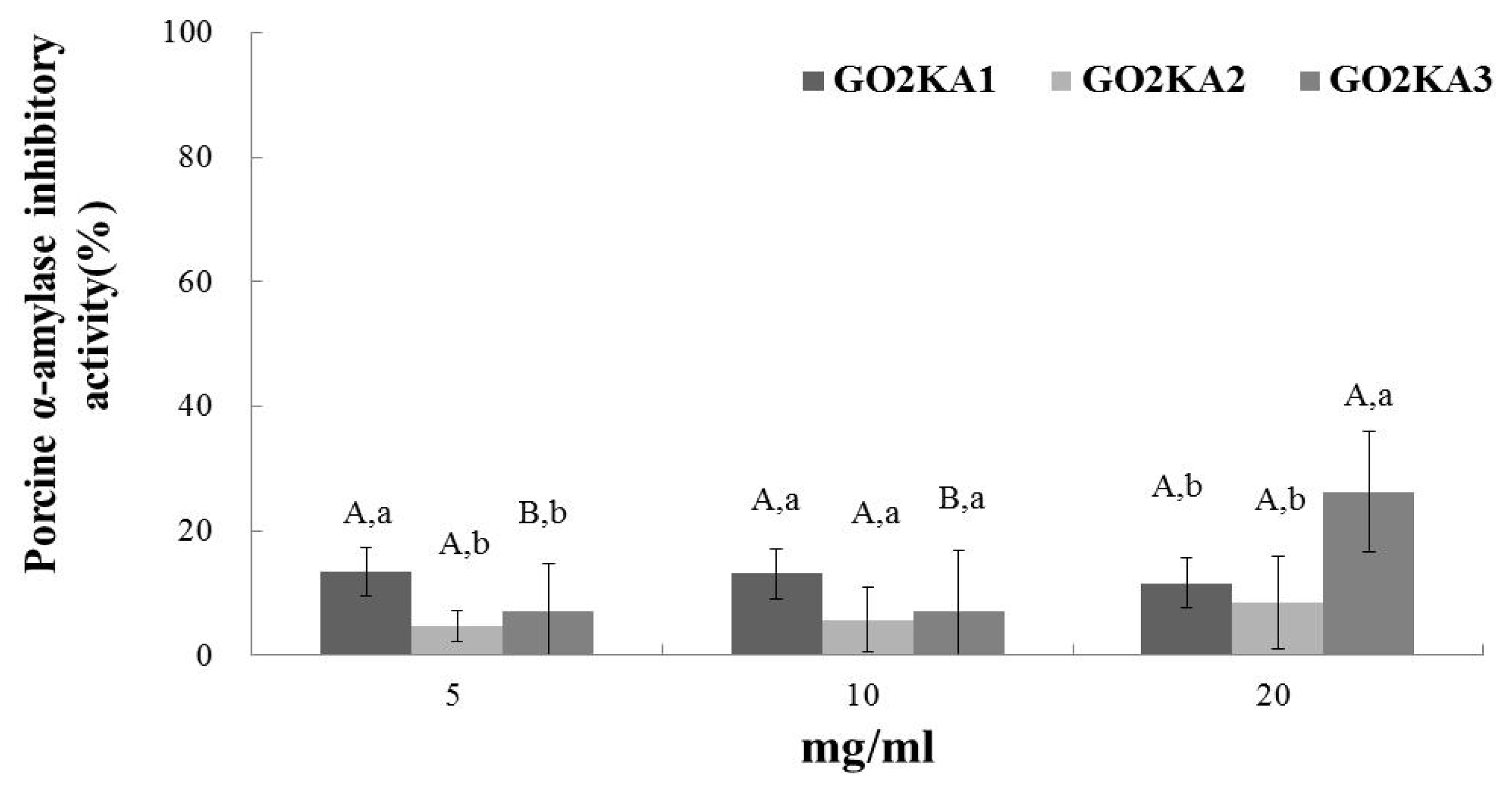
2.1. Rat α-Glucosidase and Porcine α-Amylase Assay
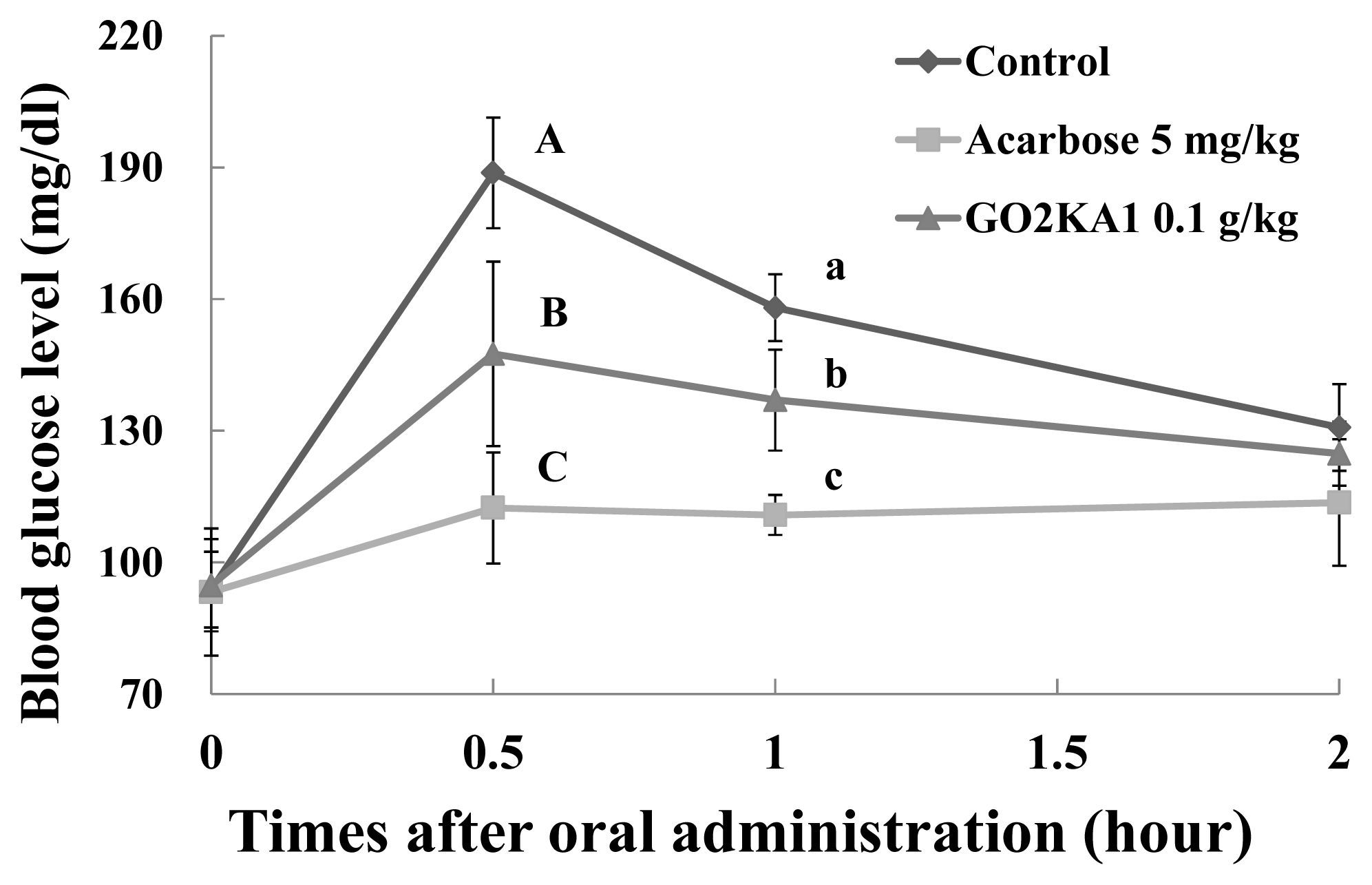
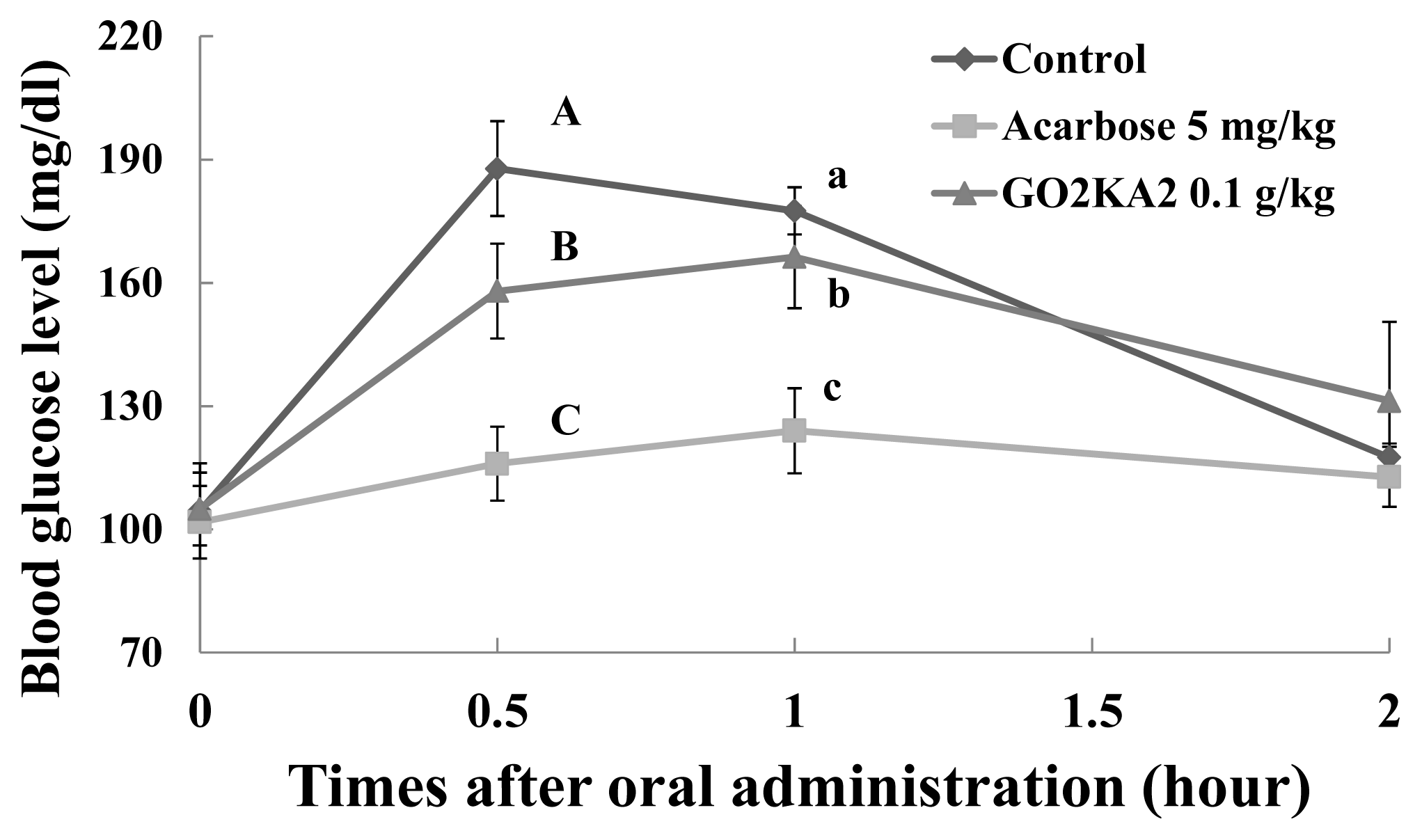
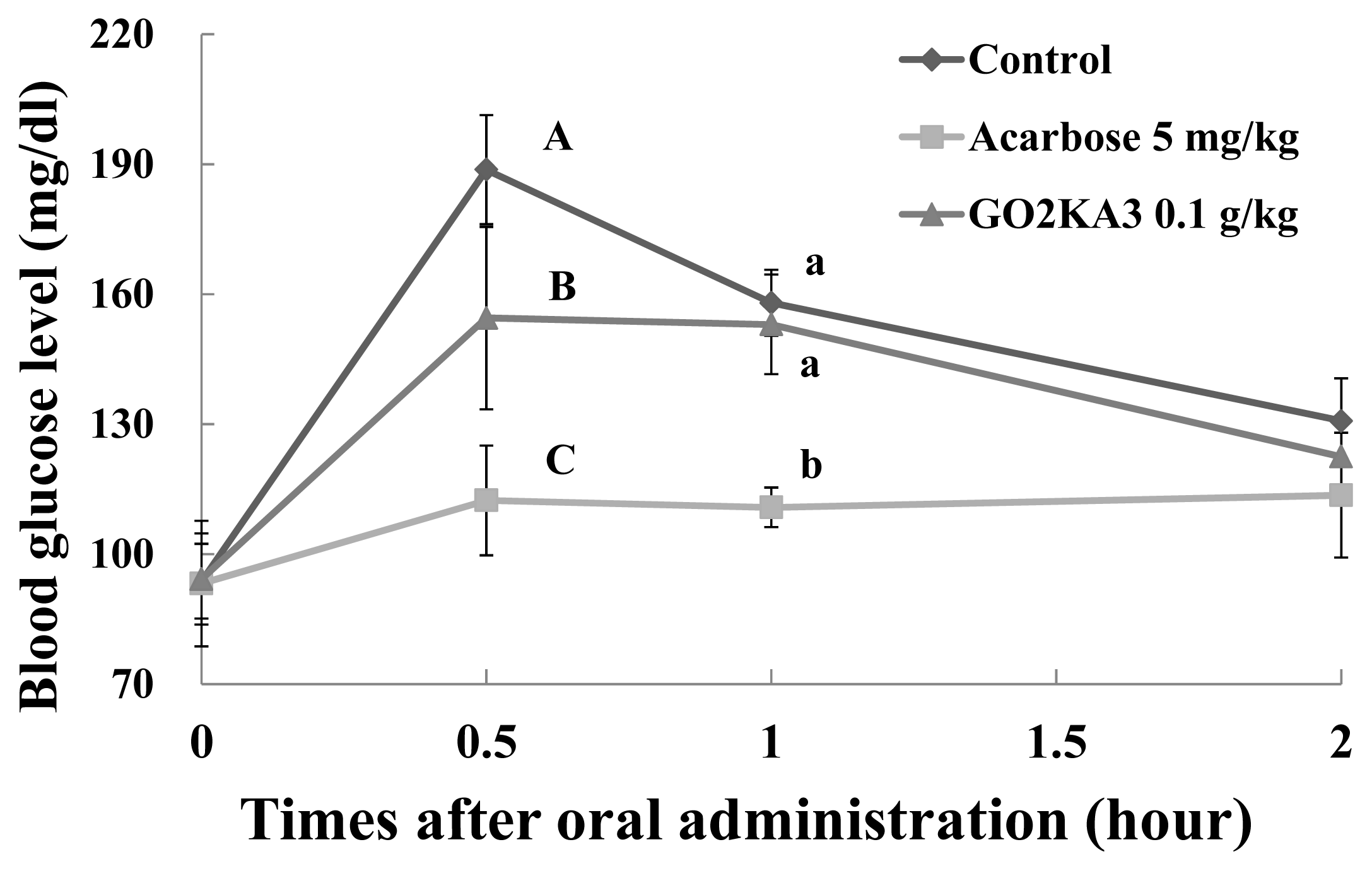
2.2. Sucrose Loading Test in SD Rat Model
3. Experimental Section
3.1. Materials
3.2. α-Glucosidase Inhibition Assay
3.3. a-Amylase Inhibition Assay
3.4. Sugar Loading Test
3.5. Statistical Analysis
4. Conclusions
Conflict of Interest
References
- Center for Disease Control. Available online: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007pdf (on accessed 15 May 2011).
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabet. Care 1997, 20, 1183–1197.
- Meigs, J.B.; Nathan, D.M.; D’Agostino, R.B., Sr; Wilson, P.W. Framingham offspring study. Fasting and postchallenge glycemia and cardiovascular disease risk: The framingham offspring study. Diabet. Care 2002, 10, 1845–1850. [Google Scholar]
- Smith, N.L.; Barzilay, J.I.; Shaffer, D.; Savage, P.J.; Heckbert, S.R.; Kuller, L.H.; Kronmal, R.A.; Resnick, H.E.; Psaty, B.M. Fasting and 2 hour postchallenge serum glucose measures and risk of incident cardiovascular events in the elderly: The Cardiovascular Health Study. Arch. Int. Med 2002, 162, 209–216. [Google Scholar]
- Coutinho, M.; Gerstein, H.C.; Wang, Y.; Yusuf, S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabet. Care 1999, 22, 233–240. [Google Scholar]
- World Health Organization. Available online: http://www.who.int/mediacentre/factsheets/fs312/en/index.html (on accessed 6 April 2013).
- Elsenhans, B.; Caspary, W.F. Absorption of Carbohydrates. In Amsterdam Excerptia Medica; Caspary, W.F., Ed.; Structure and Function of the Small Intestine: Amsterdam, Netherlands, 1987; pp. 139–159. [Google Scholar]
- Krentz, A.J.; Bailey, C.J. Oral antidiabetic agents: Current role in type 2 diabetes mellitus. Drugs 2005, 65, 385–411. [Google Scholar]
- Andlauer, W.; Furst, P. Special characteristics of non-nutrient food constituents of plants-phytochemicals. Introductory lecture. Int. J. Vitam. Nutr. Res 2003, 73, 55–62. [Google Scholar]
- Apostolidis, E.; Kwon, Y.-I.; Shetty, K. Potential of cranberry-based herbal synergies for diabetes and hypertension management. Asia Pac. J. Clin. Nutr 2006, 15, 433–441. [Google Scholar]
- Kwon, Y.I.; Vattem, D.A.; Shetty, K. Clonal herbs of Lamiaceae species against diabetes and hypertension. Asia Pac. J. Clin. Nutr 2006, 15, 107–118. [Google Scholar]
- Kwon, Y.I.; Apostolidis, E.; Kim, Y.C.; Shetty, K. Health benefits of traditional corn, beans and pumpkin: In vitro studies for hyperglycemia and hypertension management. J. Med. Foods 2007, 10, 266–275. [Google Scholar]
- Huang, R.; Mendis, E.; Kim, S.K. Improvement of ACE inhibitory activity of chitosanoligosacchiarides (COS) by carboxyl modificatrion. Bioorg. Med. Chem 2005, 23, 3649–3655. [Google Scholar]
- Maezaki, Y.; Tsuji, K.; Nakagawa, N.; Kawai, Y.; Akimoto, M.; Tsugita, T.; Takekawa, W.; Terada, A.; Hara, H.; Mitsoka, T. Hypocholestromic effect of chitoan in adult males. Biosci. Biotech. Biochem 1993, 57, 1439–1446. [Google Scholar]
- Okamoto, Y.; Inoue, A.; Miyatake, K.; Ogihara, K.; Sigemasa, Y.; Minami, S. Effect of chitin/chitosan and their oligomers/monomers on migration of macrophages. Macromol. Immunol 2003, 33, 357–367. [Google Scholar]
- Kim, S.K.; Rajapaske, N.; Shahidi, F. Production of Bioactive Chitosan Oligosaccharides and Their Potential Uses as Nutraceuticals. In Marine Nutraceuticals and Functional Foods; Barrow, C., Shahidi, F., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 183–196. [Google Scholar]
- Lim, S.H.; Hudson, S.M. Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemicals. J. Macromol. Sci 2003, C43, 223–269. [Google Scholar]
- Hall, G.; Lund, L.; Lamb, J.R.; Jarman, E.R. Kinetics and mode of peptide delivery via respiratory mucosa determine the outcome of activation versus TH2 immunity in allergic inflammation of the airways. J. Allerg. Clin. Immunol 2002, 110, 883–890. [Google Scholar]
- Kim, M.S.; Sung, M.J.; Seo, S.B.; Yoo, S.J.; Lim, W.K.; Kim, H.M. Water-soluble chitosan inhibits the production of pro-inflammatory cytokine in human astrocytoma cells activated by amyloid beta peptide and interleukin-1beta. Neurosci. Lett 2002, 321, 105–109. [Google Scholar]
- Kim, M.S.; You, H.J.; You, M.K.; Kim, M.S.; Shim, B.S.; Kim, H.M. Inhibitory effect of water-soluble chitosan on TNF-alpha and IL-8 secretion from HMC-1 cells. Immunopharmacol. Immunotoxicol 2004, 26, 401–409. [Google Scholar]
- Theocharides, T.C.; Cochrane, D.E. Critical role of mast cells in inflammatory disease and the effect of acute stress. J. Neuroimmunol. 2004, 146, 1–12. [Google Scholar]
- Nishimoto, N.; Kishimoto, T. Inhibition of IL-6 for the treatment of inflammatory diseases. Curr. Opin. Pharmacol 2004, 4, 386–391. [Google Scholar]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its derivatives. Critic. Rev. Food Sci. Nutr 2003, 43, 145–171. [Google Scholar]
- Kondo, Y.; Nakatani, A.; Hayashi, K.; Ito, M. Low molecular weight chitosan prevents the progression of low dose streptozotocin-induced diabetic mice. Biol. Pharm. Bull 2000, 23, 1458–1464. [Google Scholar]
- Kim, Y.C.; Kim, S.H.; Yoon, S.P.; Kim, J.W. Reducing effect of chitosan oligosaccharide on postprandrial blood glucose level in Koreans. J. Chitin Chitos 2009, 14, 107–111. [Google Scholar]
- Bischoff, H.; Puls, W.; Krause, H.P.; Schutt, H.; Thomas, G. Pharmacological properties of the novel glucosidase inhibitors BAY m 1099 (miglitol) and BAY o 1248. Diabetes Res. Clin. Pract 1985, 1, 53–62. [Google Scholar]
- Horii, S.; Fukasse, K.; Matrua, T.; Kameda, K.; Asano, N.; Masui, Y. Synthesis and α-d-glucosidase inhibitory activity of N-substituted valiolamine derivatives as potent oral antidiabetic agents. J. Med. Chem 1987, 29, 1038–1046. [Google Scholar]
- Jang, M.K.; Kang, S.K.; Nah, J.W. Absorption behavior in the body of chitosan oligosaccharide according to molecular weight; An in vitro and in vivo study. Food Sci. Biotechnol 2006, 15, 937–941. [Google Scholar]
- Chae, S.Y.; Jang, M.K.; Nah, J.W. Influence of molecular weight on oral absorption of water soluble chitosans. J. Control. Release 2005, 102, 383–394. [Google Scholar]
| Groups | PD parameters | |||
|---|---|---|---|---|
| AUClast (h·mg/dL) | Cmax (mg/dL) | Tmax (h) | ||
| Sucrose | Control | 305.1 ± 7.3 A | 193.0 ± 5.1 a | 0.5 ± 0.0 |
| Acarbose (5.0 mg/kg) | 214.7 ± 15.2 B | 118.8 ± 10.9 b | 1.2 ± 0.8 | |
| GO2KA1 (0.1 g/kg) | 262.4 ± 11.9 C | 152.0 ± 6.3 c | 0.9 ± 0.7 | |
| PD parameters | ||||
| AUClast(h·mg/dL) | Cmax(mg/dL) | Tmax(h) | ||
| Sucrose | Control | 306.8 ± 7.3 A | 192.0 ± 8.8 a | 0.6 ± 0.2 |
| Acarbose (5.0 mg/kg) | 226.1 ± 5.8 B | 125.2 ± 7.0 b | 0.9 ± 0.2 | |
| GO2KA2 (0.1 g/kg) | 289.1 ± 10.1 A | 167.0 ± 12.8 c | 0.9 ± 0.2 | |
| PD parameters | ||||
| AUClast(h·mg/dL) | Cmax(mg/dL) | Tmax(h) | ||
| Sucrose | Control | 305.1 ± 7.3 A | 193.0 ± 5.1 a | 0.5 ± 0.0 |
| Acarbose (5.0 mg/kg) | 222.5 ± 17.8 B | 116.4 ± 10.8 b | 1.2 ± 0.8 | |
| GO2KA3 (0.1 g/kg) | 268.3 ± 11.2 C | 154.0 ± 9.2 c | 0.7 ± 0.3 | |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jo, S.-H.; Ha, K.-S.; Moon, K.-S.; Kim, J.-G.; Oh, C.-G.; Kim, Y.-C.; Apostolidis, E.; Kwon, Y.-I. Molecular Weight Dependent Glucose Lowering Effect of Low Molecular Weight Chitosan Oligosaccharide (GO2KA1) on Postprandial Blood Glucose Level in SD Rats Model. Int. J. Mol. Sci. 2013, 14, 14214-14224. https://doi.org/10.3390/ijms140714214
Jo S-H, Ha K-S, Moon K-S, Kim J-G, Oh C-G, Kim Y-C, Apostolidis E, Kwon Y-I. Molecular Weight Dependent Glucose Lowering Effect of Low Molecular Weight Chitosan Oligosaccharide (GO2KA1) on Postprandial Blood Glucose Level in SD Rats Model. International Journal of Molecular Sciences. 2013; 14(7):14214-14224. https://doi.org/10.3390/ijms140714214
Chicago/Turabian StyleJo, Sung-Hoon, Kyoung-Soo Ha, Kyoung-Sik Moon, Jong-Gwan Kim, Chen-Gum Oh, Young-Cheul Kim, Emmanouil Apostolidis, and Young-In Kwon. 2013. "Molecular Weight Dependent Glucose Lowering Effect of Low Molecular Weight Chitosan Oligosaccharide (GO2KA1) on Postprandial Blood Glucose Level in SD Rats Model" International Journal of Molecular Sciences 14, no. 7: 14214-14224. https://doi.org/10.3390/ijms140714214





