Synergistic Effects of Nano-Sized Titanium Dioxide and Zinc on the Photosynthetic Capacity and Survival of Anabaena sp.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization and Sedimentation of Nano-TiO2 in Culture Medium
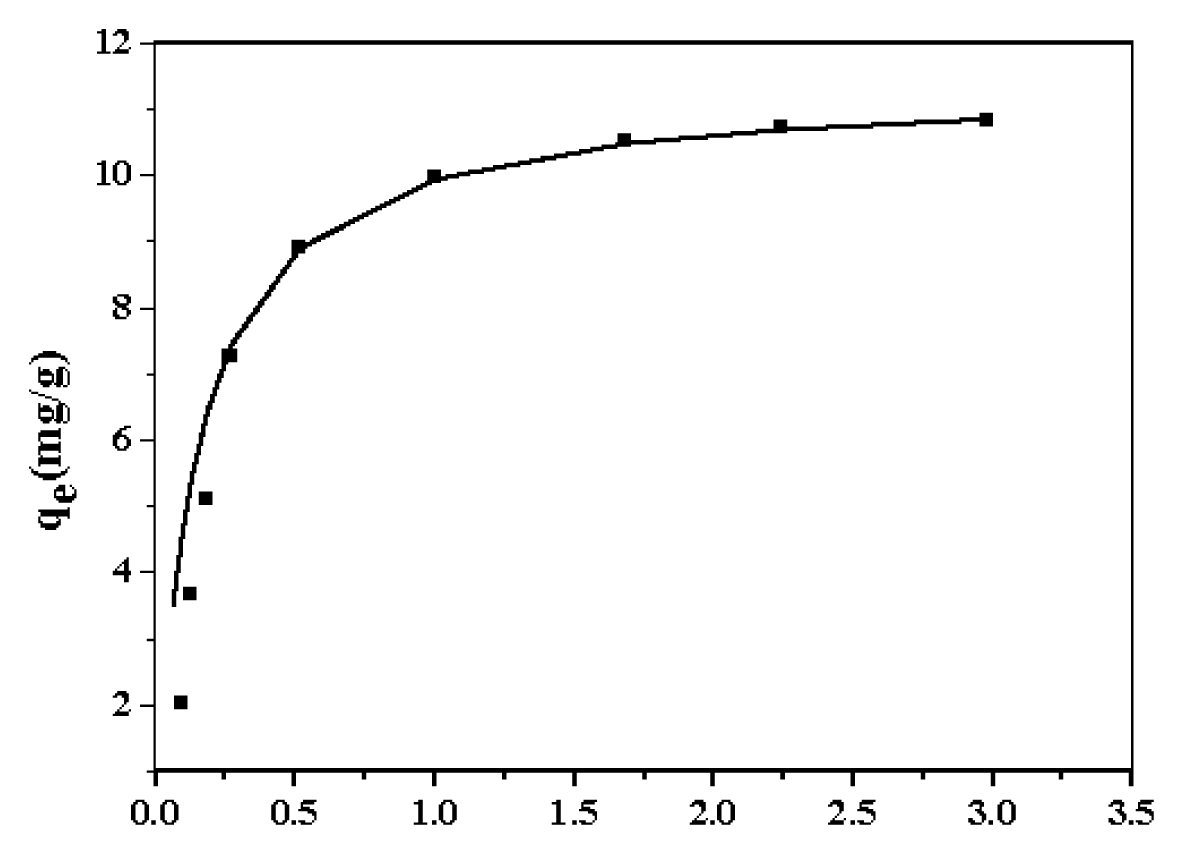
2.2. Sorption of Zn2+ onto Nano-TiO2
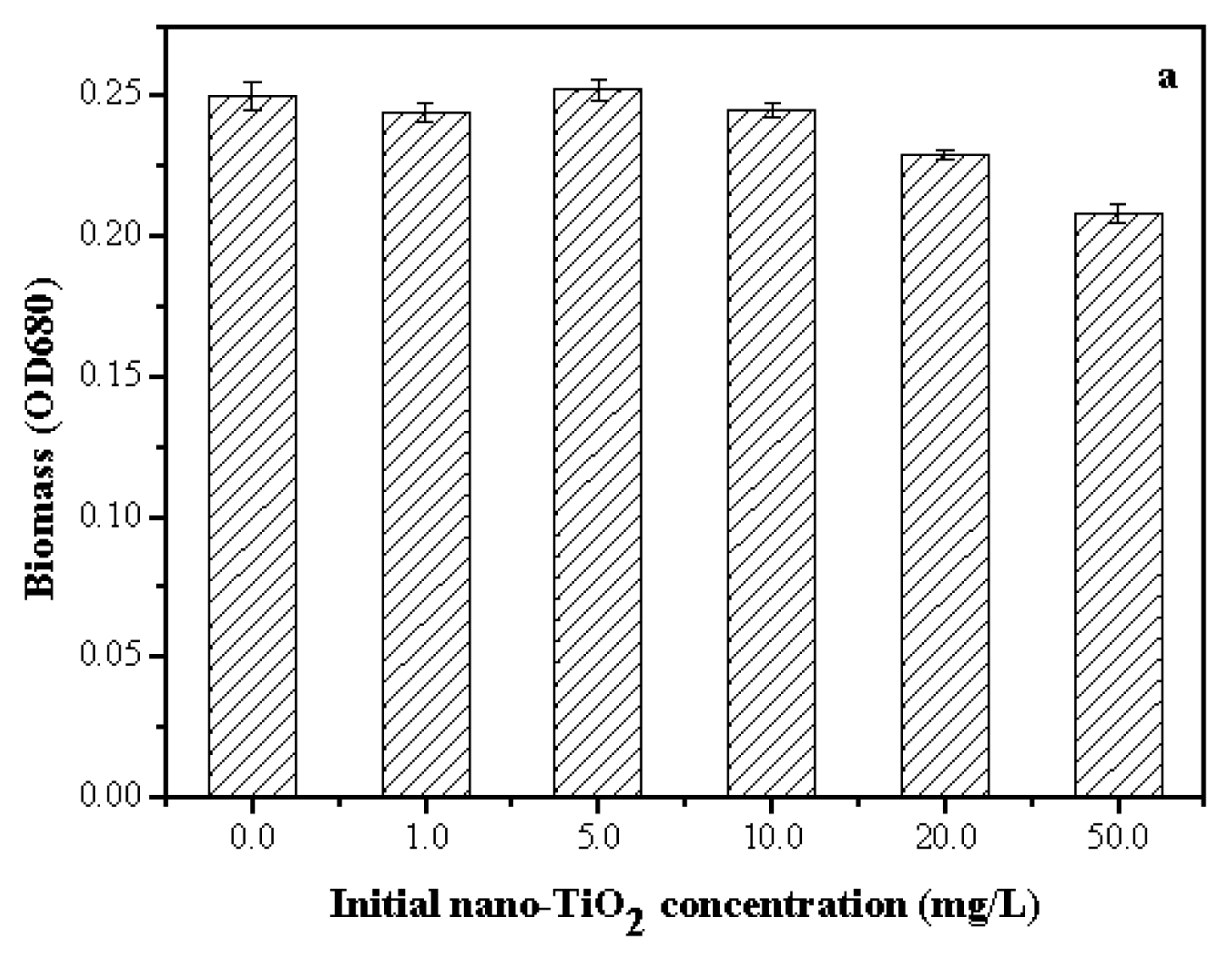
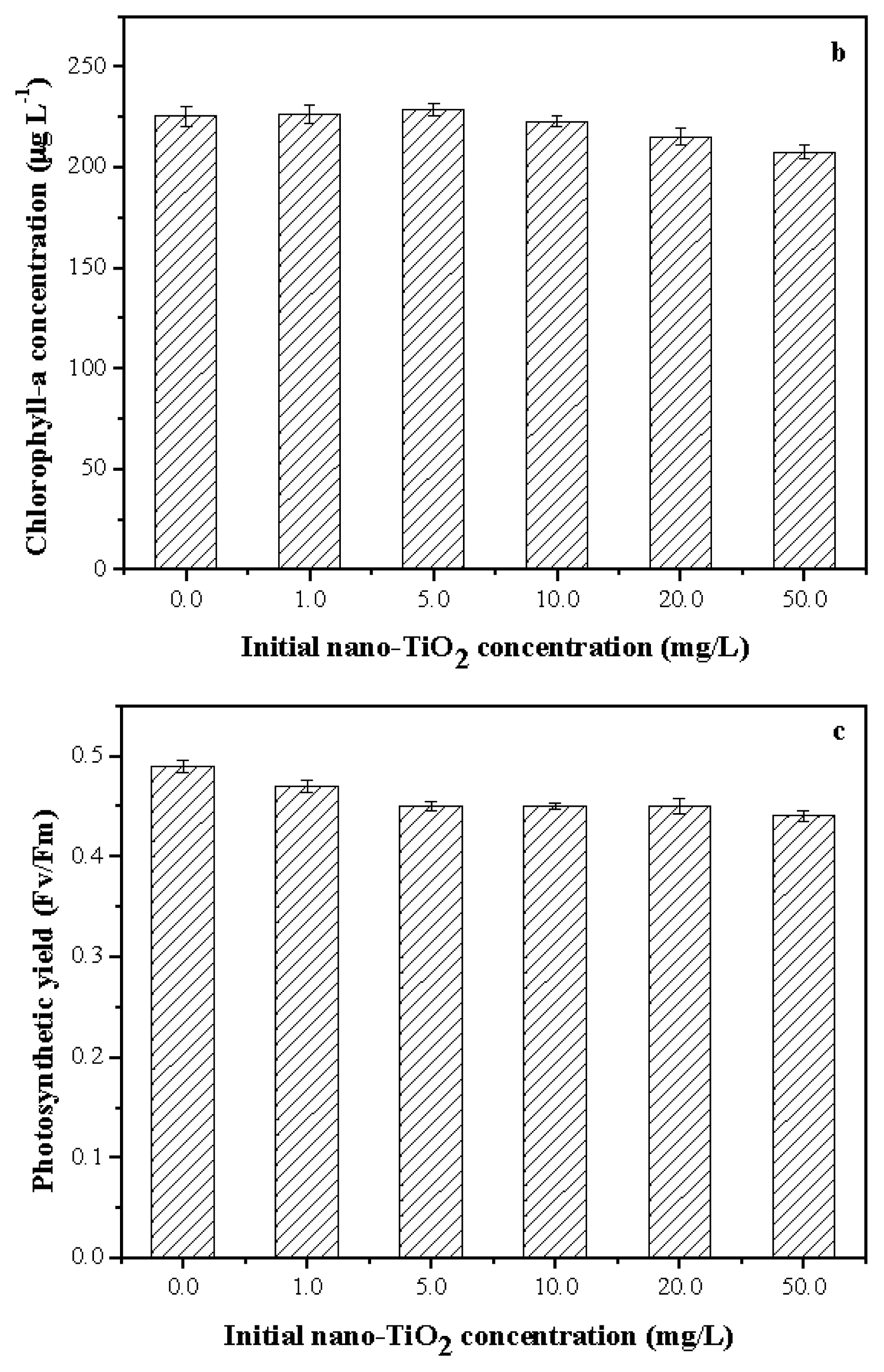
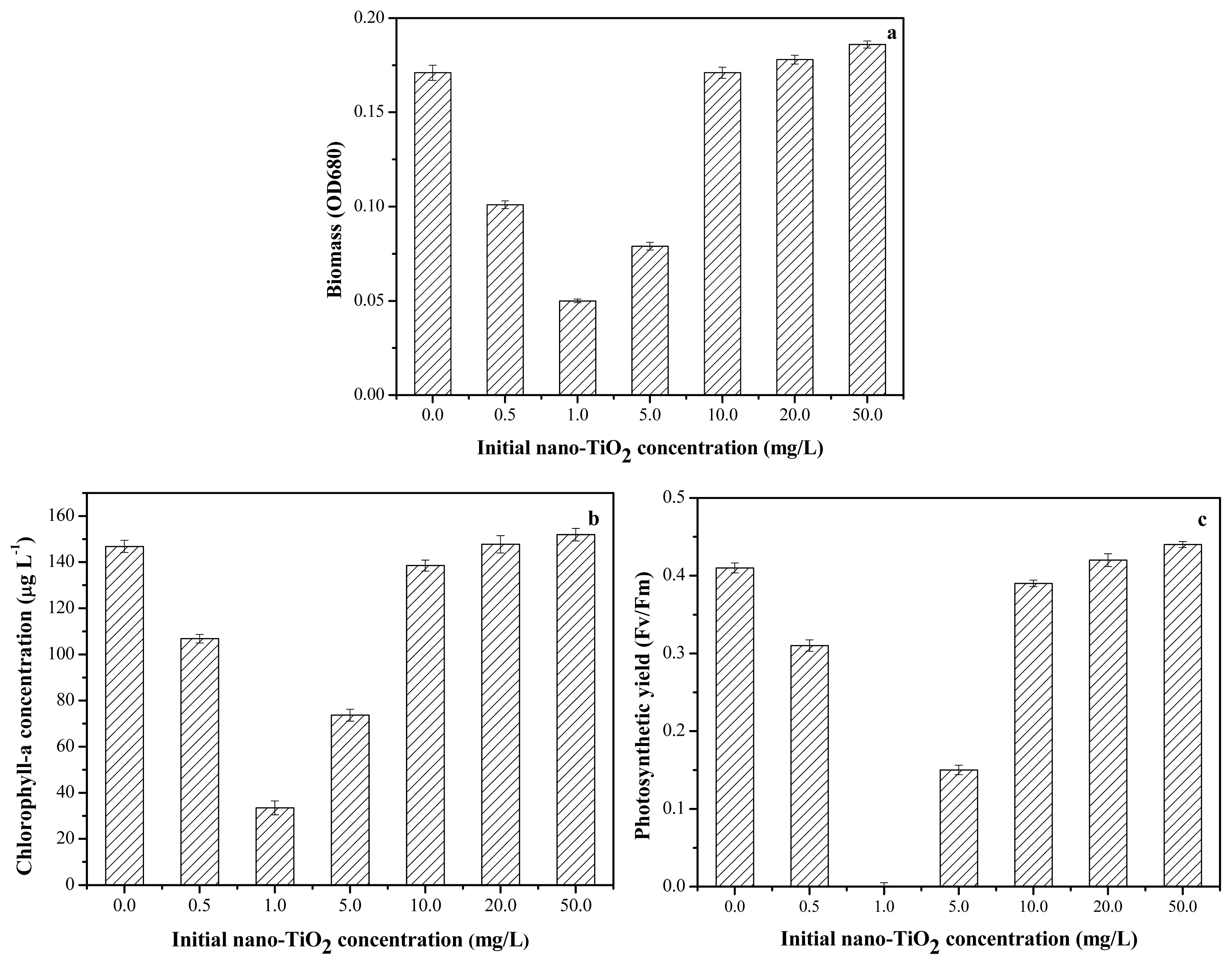
2.3. Toxicity of Nano-TiO2
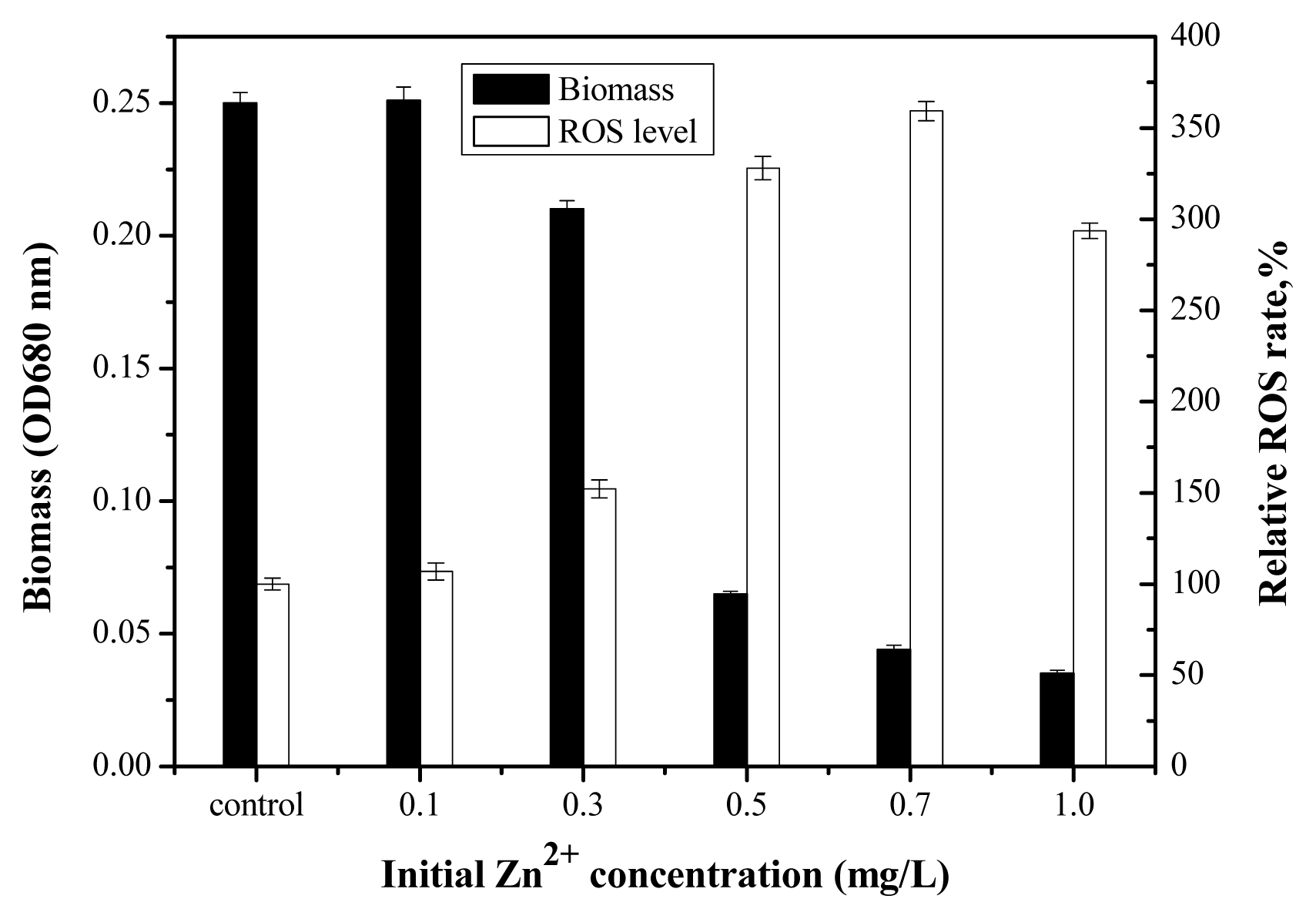
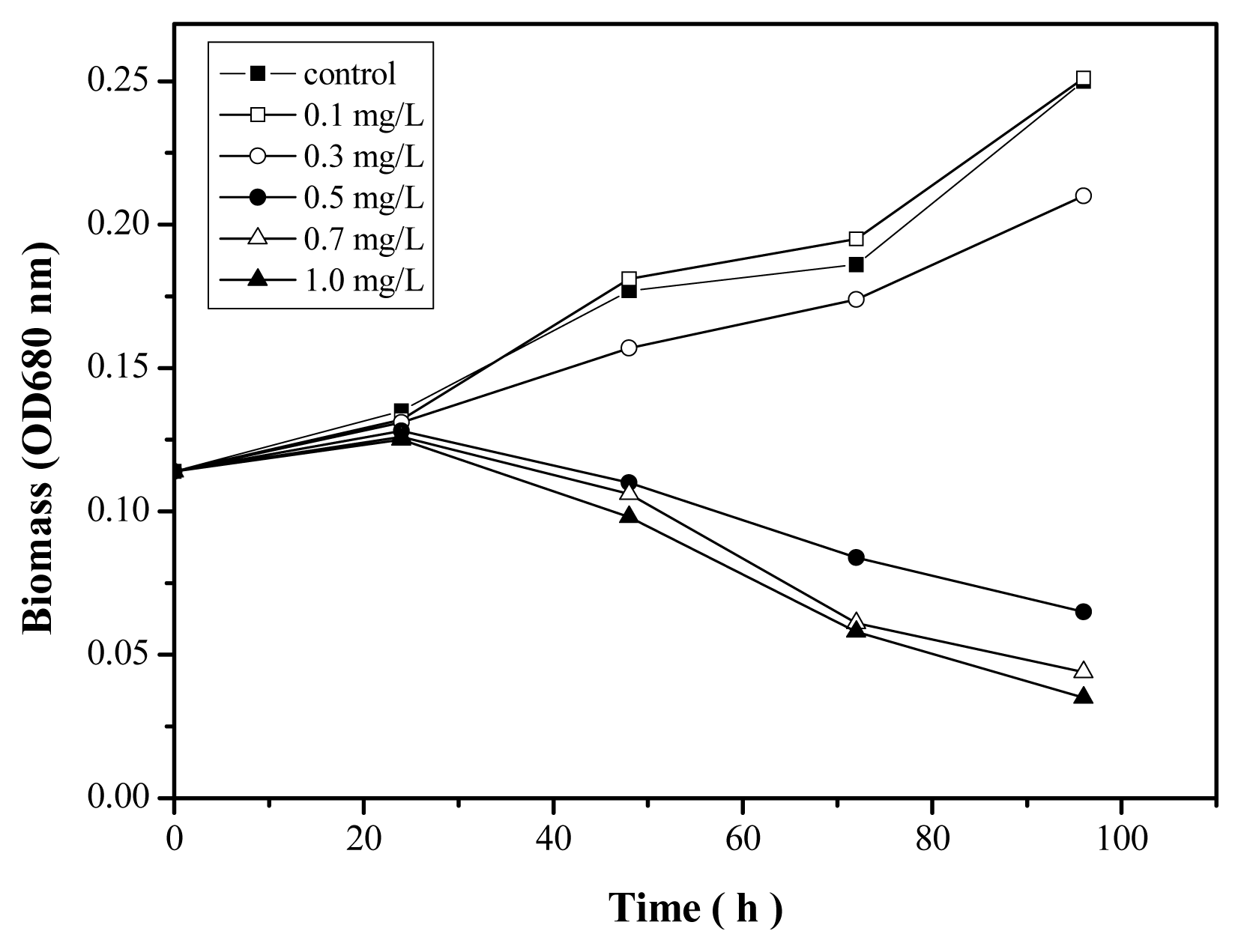
2.4. Toxicity of Zn2+ in the Absence and Presence of Nano-TiO2
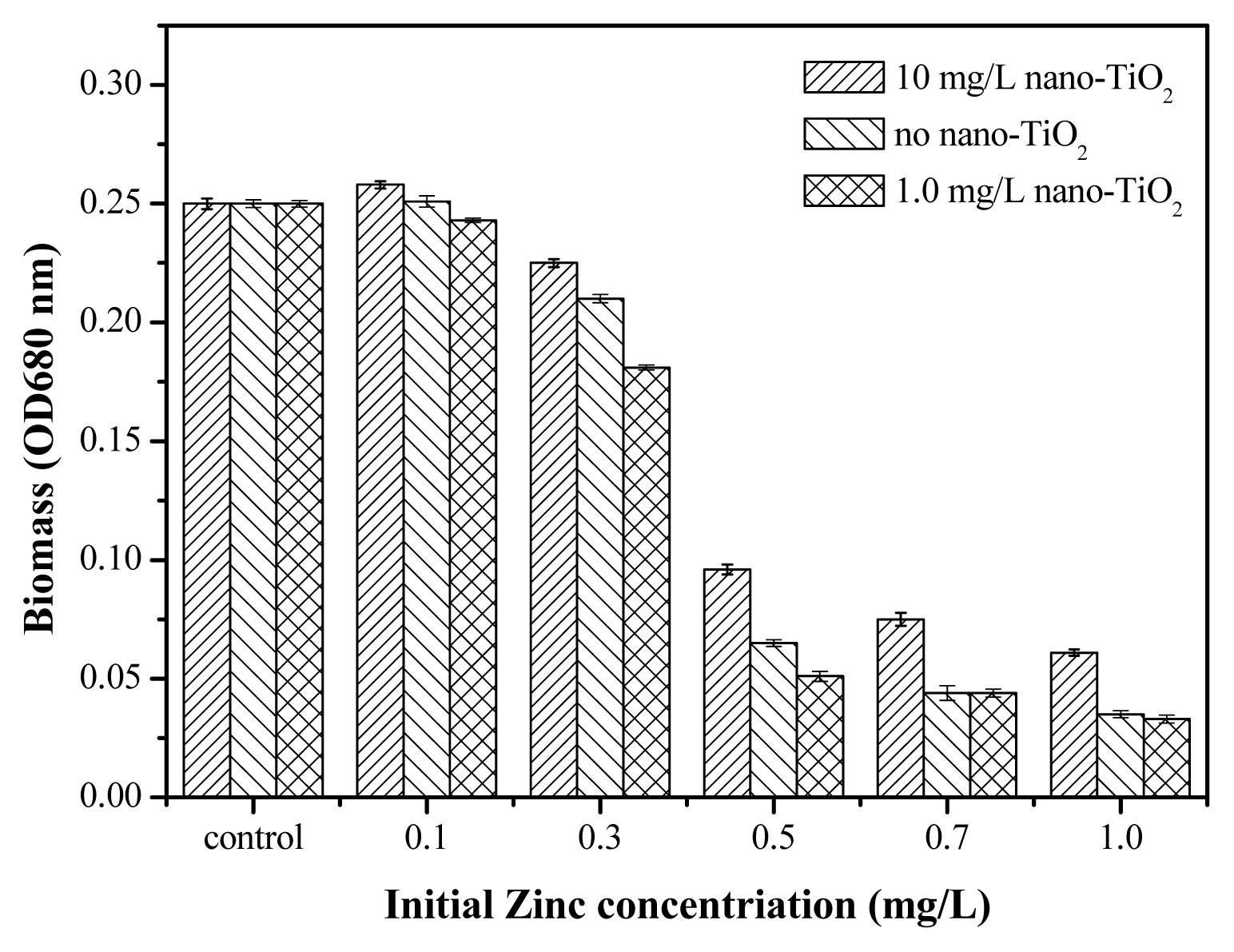
2.5. Toxicity of Nano-TiO2 in the Presence of Zn2+
3. Experimental Section
3.1. Culture of Anabaena sp
3.2. Characterization and Behavior of Nano-TiO2 in the Medium
3.3. Sorption of Zn2+ on Nano-TiO2
3.4. Toxicity Tests
3.5. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-14-14395-s001.pdfAcknowledgments
Conflict of Interest
References
- Bulgariu, D.; Bulgariu, L. Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresour. Technol 2012, 103, 489–493. [Google Scholar]
- Silva, A.; Figueiredo, S.A. Ecotoxicity tests using the green algae Chlorella vulgaris—A useful tool in hazardous effluents management. J. Hazard. Mater 2009, 167, 179–185. [Google Scholar]
- Ismael, R.P.; Coral, G.G. Metal toxicity to Chlorella pyrenoidosa assessed by a short-term continuous test. Toxicology 2009, 57, 477–487. [Google Scholar]
- Miao, A.J.; Wang, W.X. Toxicity and bioaccumulation of copper in three green microalgae species. Aquat. Toxicol 2006, 78, 114–126. [Google Scholar]
- Yon, J.-N.; Jamie, L.R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ 2008, 400, 396–414. [Google Scholar]
- Zhu, X.S.; Zhou, J.; Cai, Z.H. Suppression of chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Environ. Sci. Technol 2011, 45, 3753–3758. [Google Scholar]
- Hu, J.; Wang, D.M.; Wang, J.T.; Wang, J.M. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Environ. Pollut. 2012, 162, 216–222. [Google Scholar]
- Oukarroum, A.; Bras, S.; Perreault, F.; Popovic, R. Cadmium toxicity to two marine phytoplankton under different nutrient conditions. Ecotoxicol. Environ. Saf 2012, 78, 80–85. [Google Scholar]
- Ji, J.; Long, Z.F.; Lin, D.H. Znic acclimation and its effect on the Znic tolerance of Raphidocelis Subcapitata and Chlorella vulgaris in laboratory experiments. Chem. Eng. J 2011, 170, 525–530. [Google Scholar]
- Levonas, M.; Celine, C.; Louis, F. Evaluation of the role of the glutathione redox cycle in Cu (II) toxicity to green algae by a chiral perturbation approach. Environ. Toxicol. Chem 2011, 31, 108–114. [Google Scholar]
- Metzler, D.M.; Li, M.H. Heavy metal-induced oxidative stress in algae. Chem. Eng. J 2011, 170, 538–546. [Google Scholar]
- Sadiq, I.M.; Pakrashi, S.; Chandrasekaran, N.; Mukherjee, A. Effect of pH, EDTA, and anions on heavy metal toxicity toward a bioluminescent cyanobacterial bioreporter. J. Nanopart. Res 2011, 13, 3287–3299. [Google Scholar]
- Lin, D.H.; Ji, J.; Tian, X.L.; Liu, N.; Yang, K.; Zhu, L.Z.; Xing, B.S.; Wu, F.C.; Wang, Z.Y. Temperature and irradiance influences on cadmium and zinc uptake and toxicity in a freshwater cyanobacterium, Microcystis aeruginosa. Chin. Sci. Bull 2009, 54, 3590–3604. [Google Scholar]
- Yan, H.; Pan, G. Bioaccumulation of Fe2O3(magnetic) nanoparticles in Ceriodaphnia dubia. Chemosphere 2002, 49, 471–476. [Google Scholar]
- Lin, K.C.; Lee, Y.L.; Chen, C.Y. Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. J. Hazard. Mater 2007, 142, 236–241. [Google Scholar]
- Zeng, J.; Yang, L.Y.; Wang, W.X. TiO2 nanoparticles in the marine environment: Impact on the toxicity of tributyltin to abalone (Haliotis diversicolor supertexta) embryos. Aquat. Toxicol 2009, 93, 1–10. [Google Scholar]
- Bajguz, A. Toxicity of oxide nanoparticles to the green algae Chlorella sp. Arch. Environ. Contam. Toxicol 2011, 60, 406–416. [Google Scholar]
- Alicja, P.N.; Andrzej, B. Toxicity of copper oxide nanoparticle suspensions to aquatic biota. Plant Physiol. Biochem 2012, 52, 52–65. [Google Scholar]
- Muyssen, B.T.A.; Janssen, C.R. Responses of algae to photocatalytic nano-TiO2 particles with an emphasis on the effect of particle size. Chemosphere 2001, 45, 407–415. [Google Scholar]
- Pinto, E. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J. Phycol 2003, 39, 1008–1018. [Google Scholar]
- Chen, H.; Chen, J.; Guo, Y.N. Evaluation of the role of the glutathione redox cycle in Cu(II) toxicity to green algae by a chiral perturbation approach. Aquat. Toxicol. 2012, 120–121, 9–26. [Google Scholar]
- Yang, W.W.; Miao, A.J.; Yang, L.Y. Cd2+ toxicity to a Green Alga Chlamydomonas reinhardtii as influenced by its adsorption on TiO2 engineered nanoparticles. PLoS One 2012, 7, e32300. [Google Scholar]
- Yang, W.W.; Li, Y.; Miao, A.J.; Yang, L.Y. Cd2+ toxicity as affected by bare TiO2 nanoparticle sand their bulk counterpart. Ecotoxicol. Environ. Saf 2012, 85, 44–51. [Google Scholar]
- Hartmann, N.B.; Kammer, F.V.; Hofmann, T.; Baalousha, M.; Ottofuelling, S.; Baun, A. Algal testing of titanium dioxide nanoparticles-Testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology 2010, 269, 190–197. [Google Scholar]
- Baumann, A.; Morrison, L.; Stengel, D.B. Metal accumulation and toxicity measured by PAM-Chlorophyll fluorescence in seven species of marine macroalgae. Ecotoxicol. Environ. Saf 2009, 72, 1063–1075. [Google Scholar]
- Rodea, P.I.; Gonzalo, S.; Santiago, M.J.; Leganes, F.; Garcia, C.E.; Rosal, R.; Fernandez, P.F. An insight into the mechanisms of nanoceria toxicity in aquatic photosynthetic organisms. Aquat. Toxicol. 2012, 122–123, 133–143. [Google Scholar]
- Juhel, G.; Batisse, E.; Hugues, Q.; Daly, D. Alumina nanoparticles enhance growth of Lemna minor. Aquat. Toxicol 2011, 105, 328–336. [Google Scholar]
- Dewez, D.; Didur, O.; Vincent, H.; Popovic, R. Validation of photo synthetic-fluorescence parameters as biomarkers for isoproturon toxic effect on alga Scenedesmus obliquus. Environ. Pollut 2008, 151, 93–100. [Google Scholar]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol 2009, 43, 1354–1359. [Google Scholar]
- Keller, A.A.; Wang, H.T.; Zhou, D.X.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z.X. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol 2010, 44, 1962–1967. [Google Scholar]
- Aruoja, V.; Dubourguier, H.-C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ 2009, 407, 1461–1468. [Google Scholar]
- Huang, C.P. Responses of algae to photocatalytic nano-TiO2 particles with an emphasis on the effect of particle size. Chem. Eng. J 2011, 170, 538–546. [Google Scholar]
- Wiesner, M.R.; Loway, G.V. Accessing the Risk of Manufactured Nanomaterials; Environmental Science and Technology: St. Louis, MO, USA, 2006; pp. 4337–4345. [Google Scholar]
- Kuwabara, J.S.; Davis, J.A.; Chang, C.C.Y. Algal growth response to particle-bound orthophosphate and zinc. Limnol. Oceanogr 1986, 31, 503–511. [Google Scholar]
- Toumi, A.; Belkoura, M.; Benabdallah, S.; Alami, M.; Idrissi, L.; Nejmeddine, A. Effect and bioaccumulation of heavy metals (Zn, Cd) on Micractinium pusillum Alga. Environ. Technol 2007, 28, 19–23. [Google Scholar]
- Choudhary, M.; Jetley, U.K.; Khan, M.A. Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotoxicol. Environ. Saf 2007, 66, 204–209. [Google Scholar]
- Zhu, X.S.; Tian, S.Y.; Cai, Z.H. Toxicity assessment of iron oxide nanoparticles in zebrafish early life stages. PLoS One 2012, 7, e46286. [Google Scholar]
- Wang, D.M.; Hu, J.; Irons, D.R. Synergistic toxic effect of nano-TiO2 and As(V) on Ceriodaphnia dubia. Sci. Total Environ 2011, 409, 1351–1356. [Google Scholar]
- Qian, H.F.; Li, J.J.; Sun, L.W. Combined effect of copper and cadmium on Chlorella vulgaris growth and photosynthesis-related gene transcription. Aquat. Toxicol 2009, 49, 56–61. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tang, Y.; Li, S.; Qiao, J.; Wang, H.; Li, L. Synergistic Effects of Nano-Sized Titanium Dioxide and Zinc on the Photosynthetic Capacity and Survival of Anabaena sp. Int. J. Mol. Sci. 2013, 14, 14395-14407. https://doi.org/10.3390/ijms140714395
Tang Y, Li S, Qiao J, Wang H, Li L. Synergistic Effects of Nano-Sized Titanium Dioxide and Zinc on the Photosynthetic Capacity and Survival of Anabaena sp. International Journal of Molecular Sciences. 2013; 14(7):14395-14407. https://doi.org/10.3390/ijms140714395
Chicago/Turabian StyleTang, Yulin, Shuyan Li, Junlian Qiao, Hongtao Wang, and Lei Li. 2013. "Synergistic Effects of Nano-Sized Titanium Dioxide and Zinc on the Photosynthetic Capacity and Survival of Anabaena sp." International Journal of Molecular Sciences 14, no. 7: 14395-14407. https://doi.org/10.3390/ijms140714395



