Biohybrid Nanostructured Iron Oxide Nanoparticles and Satureja hortensis to Prevent Fungal Biofilm Development
Abstract
:1. Introduction
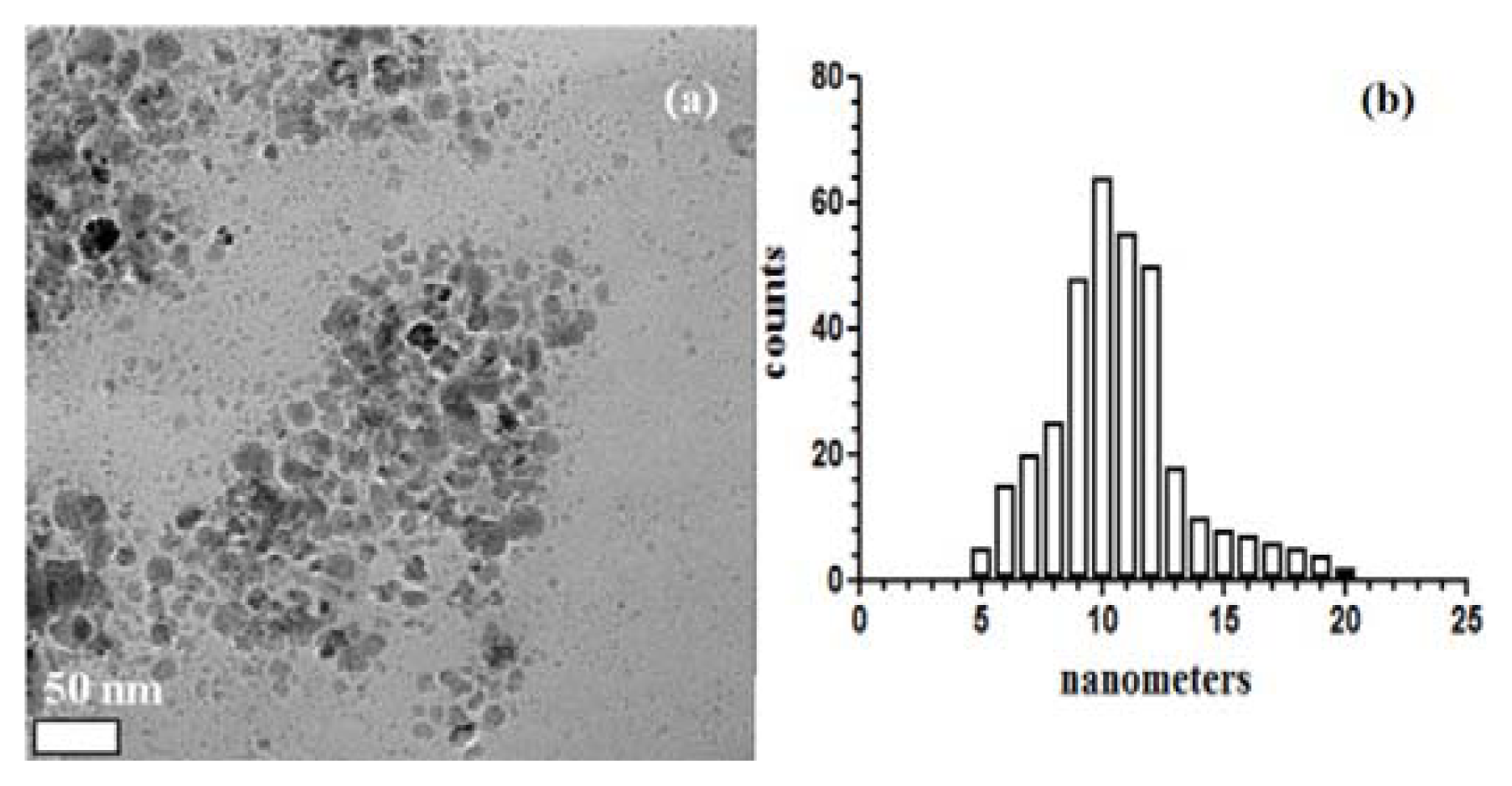
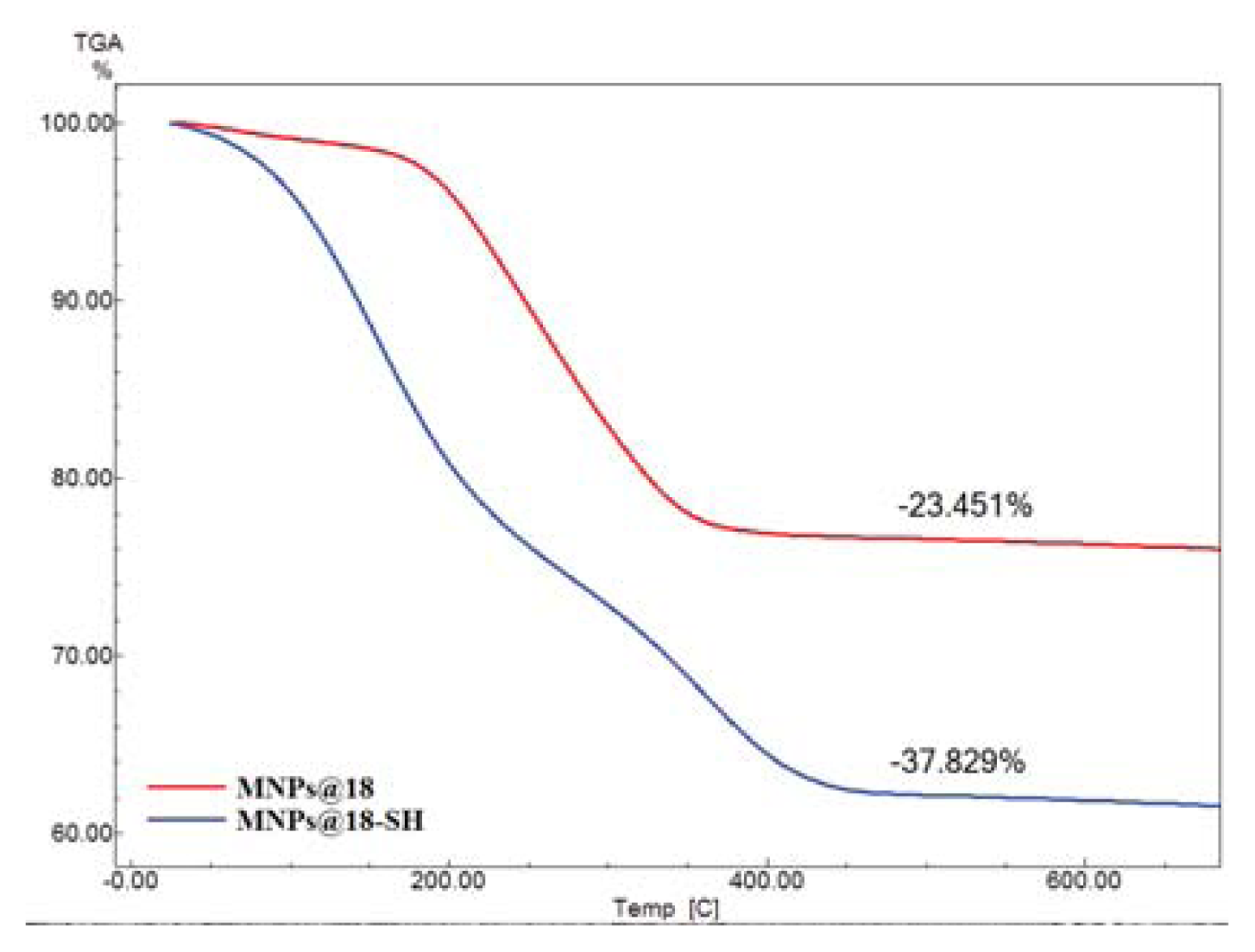
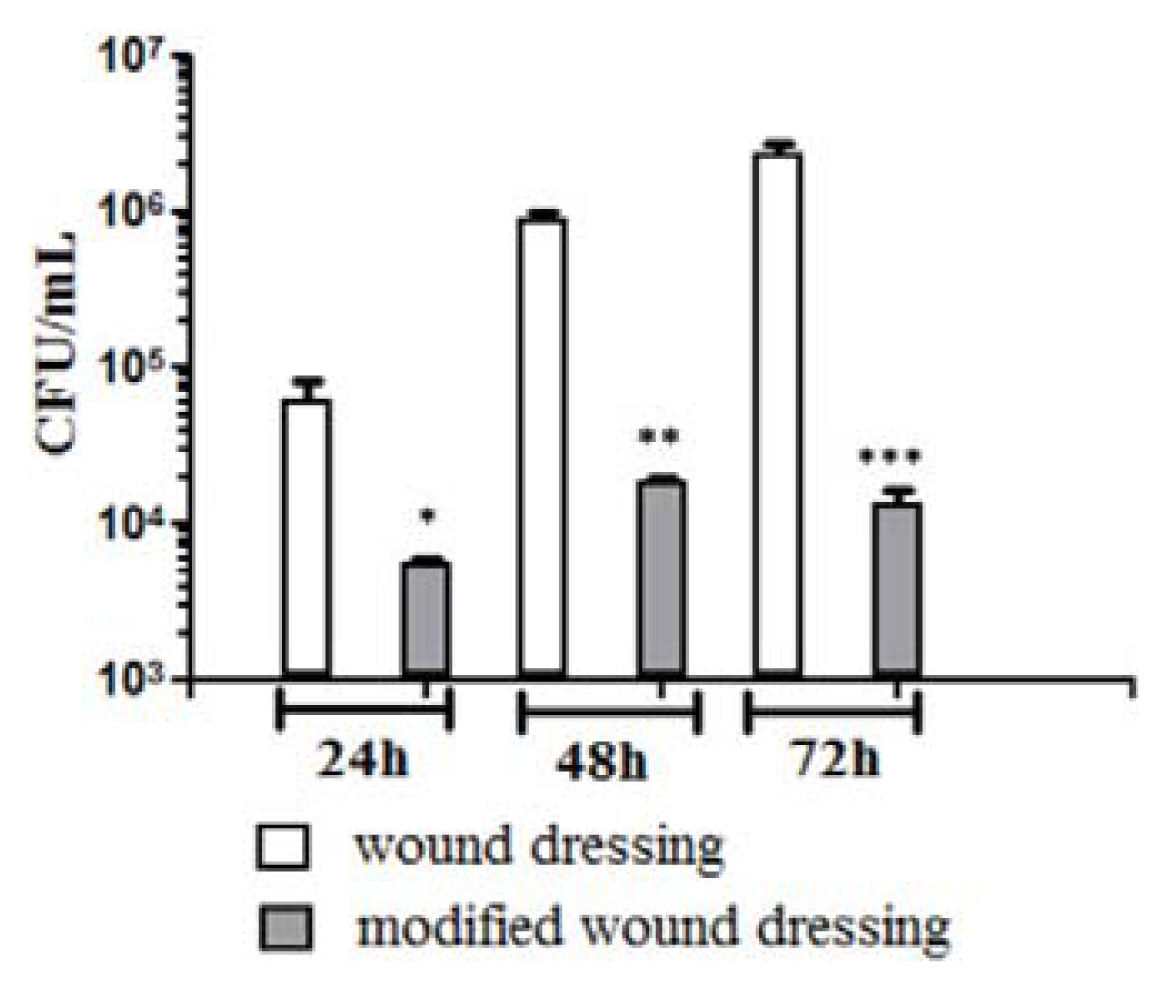
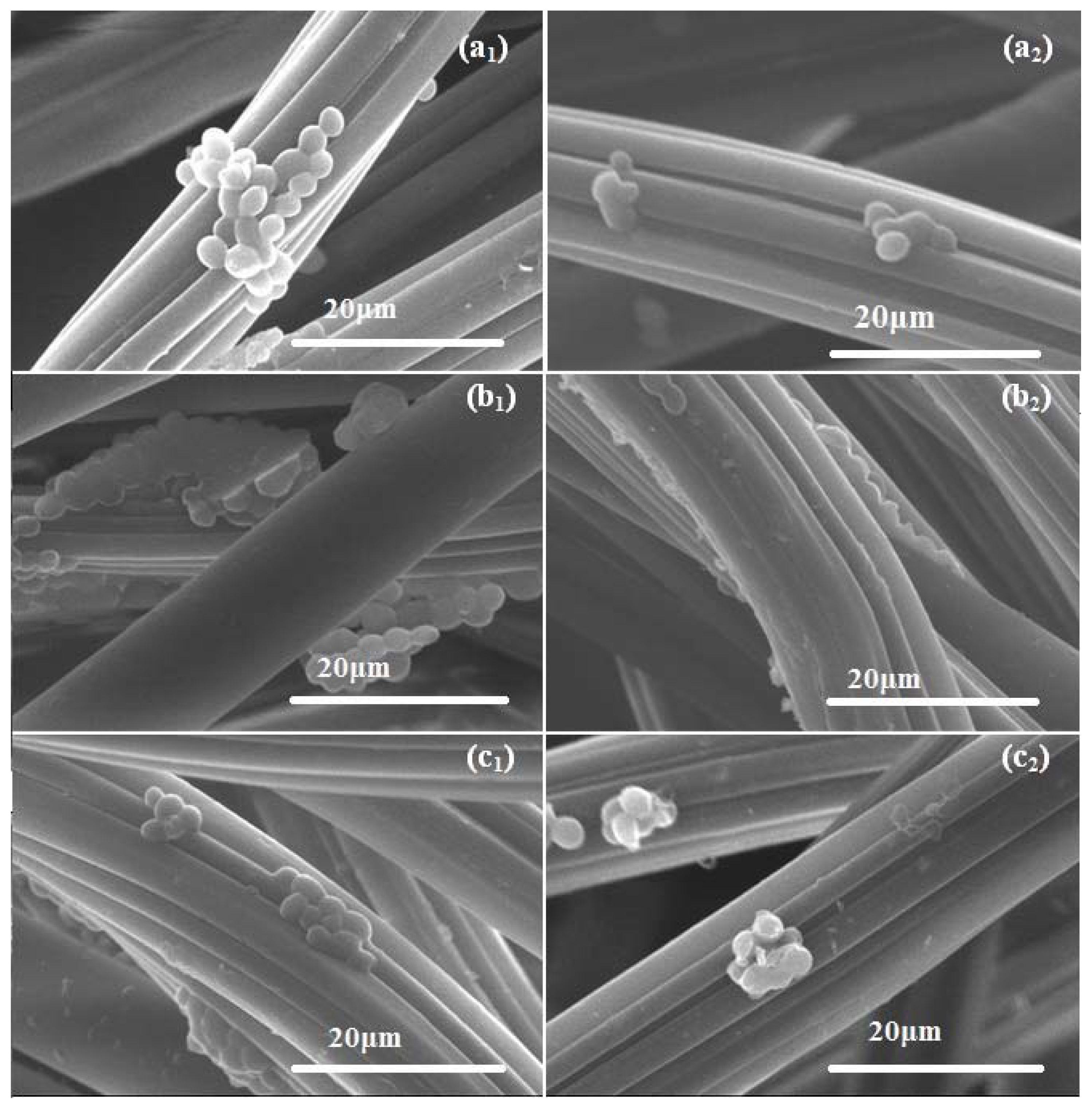
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Synthesis of Functionalized Magnetite Nanostructure
3.3. Extraction and Analysis of Satureja hortensis (SH) Essential Oil
3.4. Fabrication of Functionalized Magnetite Biohybrid Nanostructure
3.5. Fabrication of Modified Wound Dressing
3.6. Characterization
3.6.1. TEM
3.6.2. XRD
3.6.3. FT-IR
3.6.4. TG analysis
3.6.5. SEM
3.7. Strains and Culture Conditions
3.8. In Vitro Fungal Biofilm Development
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Anghel, I.; Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Anghel, A.G.; Maganu, M.; Lazar, V.; Chifiriuc, M.C. Modified wound dressing with phyto-nanostructured coating to prevent staphylococcal and pseudomonal biofilms development. Nanoscale Res. Lett 2012, 7, 690. [Google Scholar]
- Capoor, M.R.; Sarabahi, S.; Tiwari, V.K.; Narayanan, R.P. Fungal infections in burns: Diagnosis and management. Indian J. Plast. Surg 2010, 43, S37–S42. [Google Scholar]
- Okesola, A.O.; Kehinde, A.O. Bacteriology of non-surgical wound infections in Ibadan, Nigeria. Afr. J. Med. Med. Sci 2008, 37, 261–264. [Google Scholar]
- Toniolo, A.; Endimiani, A.; Luzzaro, F. Microbiology of postoperative infections. Surg. Infect. (Larchmt. ) 2006, 7, S13–S16. [Google Scholar]
- Anghel, I.; Grumezescu, V.; Andronescu, E.; Anghel, G.A.; Grumezescu, A.M.; Mihaiescu, D.E.; Chifiriuc, M.C. Protective effect of magnetite nanoparticle/Salvia officinalis essential oil hybrid nanobiosystem against fungal colonization on the Provox® voice section prosthesis. Dig. J. Nanomater. Biostruct 2012, 7, 1205–1212. [Google Scholar]
- Saviuc, C.; Holban, A.M.; Grumezescu, A.M.; Bleotu, C.; Banu, O.; Lazar, V.; Mihaiescu, D.E.; Chifiriuc, M.C. Testing antifungal activity of some essential oils using flow cytometry. Lett. Appl. NanoBioSci 2012, 1, 67–71. [Google Scholar]
- Nett, J.; Lincoln, L.; Marchillo, K.; Massey, R.; Holoyda, K.; Hoff, B.; VanHandel, M.; Andes, D. Putative role of β-1,3glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother 2007, 51, 510–520. [Google Scholar]
- Holban, A.M.; Saviuc, C.; Grumezescu, A.M.; Chifiriuc, M.C.; Banu, O.; Lazar, V. Phenotypic investigation of virulence profiles in some Candida spp. Strains isolated from different clinical specimens. Lett. Appl. Nanobiosci 2012, 1, 72–76. [Google Scholar]
- Masoudi, A.; Hosseini, H.R.M.; Reyhani, S.M.S.; Shokrgozar, M.A.; Oghabian, M.A.; Ahmadi, R. Long-term investigation on the phase stability, magnetic behavior, toxicity, and MRI characteristics of superparamagnetic Fe/Fe-oxide core/shell nanoparticles. Int. J. Pharm 2012, 439, 28–40. [Google Scholar]
- Azhar, S.L.; Lotfipour, F. Magnetic nanoparticles for antimicrobial drug delivery. Pharmazie 2012, 67, 817–821. [Google Scholar]
- Jansch, M.P.; Graf, S.C.; Rühl, E.; Müller, R.H. Adsorption kinetics of plasma proteins on ultrasmallsuperparamagnetic iron oxide (USPIO) nanoparticles. Int. J. Pharm 2012, 428, 125–133. [Google Scholar]
- Huang, C.; Tang, Z.; Zhou, Y.; Zhou, X.; Jin, Y.; Li, D.; Yang, Y.; Zhou, S. Magnetic micelles as a potential platform for dual targeted drug delivery in cancer therapy. Int. J. Pharm 2012, 429, 113–122. [Google Scholar]
- Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Ficai, D.; Huang, K.S.; Gheorghe, I.; Chifiriuc, M.C. Water soluble magnetic biocomposite with pontetial applications for the antimicrobial therapy. Biointerface Res. Appl. Chem 2012, 2, 469–475. [Google Scholar]
- Andronescu, E.; Grumezescu, A.M.; Ficai, A.; Gheorghe, I.; Chifiriuc, M.C.; Mihaiescu, D.E.; Lazar, V. In vitro efficacy of antibiotic magnetic dextran microspheres complexes against Staphylococcus aureus and Pseudomonas aeruginosa strains. Biointerface Res. Appl. Chem 2012, 2, 332–338. [Google Scholar]
- Anghel, I.; Grumezescu, A.M.; Andronescu, E.; Anghel, A.G.; Ficai, A.; Saviuc, C.; Grumezescu, V.; Vasile, B.S.; Chifiriuc, M.C. Magnetite nanoparticles for functionalized textile dressing to prevent fungal biofilms development. Nanoscale Res. Lett 2012, 7, 501. [Google Scholar]
- Masoudi, A.; Hosseini, H.R.M.; Shokrgozar, M.A.; Ahmadi, R.; Oghabian, M.A. The effect of poly(ethylene glycol) coating on colloidal stability of superparamagnetic iron oxide nanoparticles as potential MRI contrast agent. Int. J. Pharm 2012, 433, 129–141. [Google Scholar]
- Alphandéry, E.; Guyot, F.; Chebbi, I. Preparation of chains of magnetosomes, isolated from Magnetospirillummagneticum strain AMB-1 magnetotactic bacteria, yielding efficient treatment of tumors using magnetic hyperthermia. Int. J. Pharm 2012, 434, 444–452. [Google Scholar]
- Al-Kattan, A.; Girod-Fullana, S.; Charvillat, C.; Ternet-Fontebasso, H.; Dufour, P.; Dexpert-Ghys, J.; Santran, V.; Bordère, J.; Pipy, B.; Bernad, J.; et al. Biomimetic nanocrystallineapatites: Emerging perspectives in cancer diagnosis and treatment. Int. J. Pharm 2012, 423, 26–36. [Google Scholar]
- Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Yang, C.H.; Huang, K.S.; Vasile, B.S.; Voicu, G.; Mihaiescu, D.E.; Bleotu, C. Magnetic nanofluid with antitumoral properties. Lett. Appl. NanoBioSci 2012, 1, 56–60. [Google Scholar]
- Grumezescu, A.M.; Chifiriuc, M.C.; Saviuc, C.; Grumezescu, V.; Hristu, R.; Mihaiescu, D.; Stanciu, G.A.; Andronescu, E. Hybrid nanomaterial for stabilizing the antibiofilm activity of Eugenia carryophyllata essential oil. IEEE Trans. Nanobiosci 2012, 11, 360–365. [Google Scholar]
- Saviuc, C.; Grumezescu, A.M.; Chifiriuc, M.C.; Bleotu, C.; Stanciu, G.; Hristu, R.; Mihaiescu, D.; Lazăr, V. In vitro methods for the study of microbial biofilms. Biointerface Res. Appl. Chem 2011, 1, 31–40. [Google Scholar]
- Mihaiescu, D.E.; Horja, M.; Gheorghe, I.; Ficai, A.; Grumezescu, A.M.; Bleotu, C.; Chifiriuc, M.C. Water soluble magnetite nanoparticles for antimicrobial drugs delivery. Lett. Appl. NanoBioSci 2012, 1, 45–49. [Google Scholar]
- Zhang, L.; Pornpattananangku, D.; Hu, C.M.; Huang, C.M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem 2010, 17, 585–594. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol 2004, 94, 223–253. [Google Scholar]
- Kavanaugh, N.L.; Ribbeck, K. Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilms. Appl. Environ. Microbiol 2012, 78, 4057–4061. [Google Scholar]
- Sefidkon, F.; Abbasi, K.; Khaniki, G.B. Influence of drying and extraction methods on yield and chemical composition of the essential oil of Satureja hortensis. Food Chem 2006, 99, 19–23. [Google Scholar]
- Khajeh, M. Optimization of process variables for essential oil components from Satureja hortensis by supercritical fluid extraction using Box-Behnken experimental design. J. Supercrit. Fluids 2011, 55, 944–948. [Google Scholar]
- Skocibusic, M.; Bezic, N.; Dunkic, V. Phytochemical composition and antimicrobial activities of the essential oils from Satureja subspicata V. is. growing in Croatia. Food Chem 2006, 96, 20–28. [Google Scholar]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Protoc 2000, 19, 603–608. [Google Scholar]
- Chifiriuc, C.; Grumezescu, V.; Grumezescu, A.M.; Saviuc, C.; Lazăr, V.; Andronescu, E. Hybrid magnetite nanoparticles/Rosmarinus officinalis essential oil nanobiosystem with antibiofilm activity. Nanoscale Res. Lett 2012, 7, 209. [Google Scholar]
- Grumezescu, A.M.; Saviuc, C.; Chifiriuc, M.C.; Hristu, R.; Mihaiescu, D.E.; Balaure, P.; Stanciu, G.; Lazar, V. Inhibitory activity of Fe3O4/oleic acid/usnic acid-core/shell/extra-shell nanofluid on S. aureus biofilm development. IEEE Trans. Nanobiosci 2011, 10, 269–274. [Google Scholar]
- Hadian, J.; Ebrahimi, S.N.; Salehi, P. Variability of morphological and phytochemical characteristics among Satureja hortensis L. accessions of Iran. Ind. Crops Prod 2010, 32, 62–69. [Google Scholar]
- Tozlu, E.; Cakir, A.; Kordali, S.; Tozlu, G.; Ozer, H.; Akcin, T.A. Chemical compositions and insecticidal effects of essential oils isolated from Achillea gypsicola, Satureja hortensis, Origanum acutidens and Hypericum scabrum against broad bean weevil (Bruchus dentipes). Sci. Hortic 2011, 130, 9–17. [Google Scholar]
- Giri, S.K.; Das, N.N.; Pradhan, G.C. Synthesis and characterization of magnetite nanoparticles using waste iron ore tailings for adsorptive removal of dyes from aqueous solution. Colloids Surf. A 2011, 389, 43–49. [Google Scholar]
- Maity, D.; Agrawal, D.C. Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J. Magn. Magn. Mater 2007, 308, 46–55. [Google Scholar]
- Klein, G.; Rüben, C.; Upmann, M. Antimicrobial activity of essential oil components against potential food spoilage microorganisms. Curr. Microbiol 2013, 67, 200–208. [Google Scholar]
- Sahin, F.; Karaman, I.; Güllüce, M.; Oğütçü, H.; Sengül, M.; Adigüzel, A.; Oztürk, S.; Kotan, R. Evaluation of antimicrobial activities of Satureja hortensis L. J. Ethnopharmacol 2003, 87, 61–65. [Google Scholar]
- Saharkhiz, M.J.; Zomorodian, K.; Rezaei, M.R.; Saadat, F.; Rahimi, M.J. Influence of growth phase on the essential oil composition and antimicrobial activities of Satureja hortensis. Nat. Prod. Commun 2011, 6, 1173–1178. [Google Scholar]
- Mahboubi, M.; Kazempour, N. Chemical composition and antimicrobial activity of Satureja hortensis and Trachyspermum copticum essential oil. Iran. J. Microbiol 2011, 3, 194–200. [Google Scholar]
- Sabzghabaee, A.M.; Davoodi, N.; Ebadian, B.; Aslani, A.; Ghannadi, A. Clinical evaluation of the essential oil of “Satureja Hortensis” for the treatment of denture stomatitis. Dent. Res. J. (Isfahan) 2012, 9, 198–202. [Google Scholar]
- Marcos-Arias, C.; Eraso, E.; Madariaga, L.; Quindós, G. In vitro activities of natural products against oral Candida isolates from denture wearers. BMC Complement. Altern. Med 2011, 11, 119. [Google Scholar]
- Boyraz, N.; Ozcan, M. Inhibition of phytopathogenic fungi by essential oil, hydrosol, ground material and extract of summer savory (Satureja hortensis L.) growing wild in Turkey. Int. J. Food Microbiol 2006, 107, 238–242. [Google Scholar]
- Delgado, B.; Fernandez, P.S.; Palop, A.; Periago, P.M. Effect of thymol and cymene on Bacillus cereus vegetative cells evaluated through the use of frequency distributions. Food Microbiol 2004, 21, 327–334. [Google Scholar]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol 2001, 91, 453–462. [Google Scholar]
- Saviuc, C.; Grumezescu, A.M.; Bleotu, C.; Holban, A.; Chifiriuc, C.; Balaure, P.; Lazar, V. Phenotipical studies for raw and nanosystem embedded Eugenia carryophyllata buds essential oil effect on Pseudomonas aeruginosa and Staphylococcus aureus strains. Biointerface Res. Appl. Chem 2011, 1, 111–118. [Google Scholar]
- Grumezescu, A.M.; Holban, A.M.; Andronescu, E.; Tomoiaga, M.; Ficai, A.; Bleotu, C.; Chifiriuc, M.C. Microbiological applications of a new water dispersible magnetic nanobiocomposite. Lett. Appl. NanoBioSci 2012, 1, 83–90. [Google Scholar]
- Grumezescu, A.M.; Andronescu, E.; Voicu, G.; Huang, K.S.; Ficai, A.; Yang, C.H.; Bleotu, C.; Chifiriuc, M.C. Antitumor activity of magnetite nanoparticles: Influence of hydrocarbonated chain of saturated aliphatic monocarboxylic acids. Curr. Org. Chem 2013, 17, 831–840. [Google Scholar]


| No. | Compound | Retention Index 1 | (%) |
|---|---|---|---|
| 1 | α-thujene | 927 | 1 |
| 2 | α-pinene | 940 | 0.9 |
| 3 | β-pinene | 984 | 0.7 |
| 4 | myrcene | 1000 | 1.3 |
| 5 | α-terpinene | 1019 | 3.6 |
| 6 | p-cymene | 1028 | 4.8 |
| 7 | γ-terpinene | 1057 | 38.7 |
| 8 | linalool | 1103 | 0.9 |
| 9 | carvacrol | 1301 | 46.9 |
| 10 | β-caryophyllene | 1413 | 0.1 |
| 11 | β-bisabolone | 1504 | 0.7 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Anghel, I.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Biohybrid Nanostructured Iron Oxide Nanoparticles and Satureja hortensis to Prevent Fungal Biofilm Development. Int. J. Mol. Sci. 2013, 14, 18110-18123. https://doi.org/10.3390/ijms140918110
Anghel I, Grumezescu AM, Holban AM, Ficai A, Anghel AG, Chifiriuc MC. Biohybrid Nanostructured Iron Oxide Nanoparticles and Satureja hortensis to Prevent Fungal Biofilm Development. International Journal of Molecular Sciences. 2013; 14(9):18110-18123. https://doi.org/10.3390/ijms140918110
Chicago/Turabian StyleAnghel, Ion, Alexandru Mihai Grumezescu, Alina Maria Holban, Anton Ficai, Alina Georgiana Anghel, and Mariana Carmen Chifiriuc. 2013. "Biohybrid Nanostructured Iron Oxide Nanoparticles and Satureja hortensis to Prevent Fungal Biofilm Development" International Journal of Molecular Sciences 14, no. 9: 18110-18123. https://doi.org/10.3390/ijms140918110






