Design of Phosphonium-Type Zwitterion as an Additive to Improve Saturated Water Content of Phase-Separated Ionic Liquid from Aqueous Phase toward Reversible Extraction of Proteins
Abstract
:1. Introduction
2. Results and Discussion
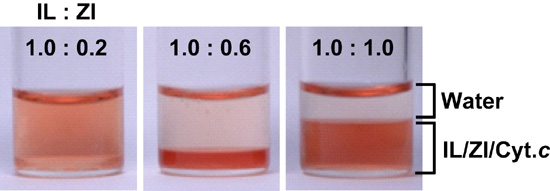
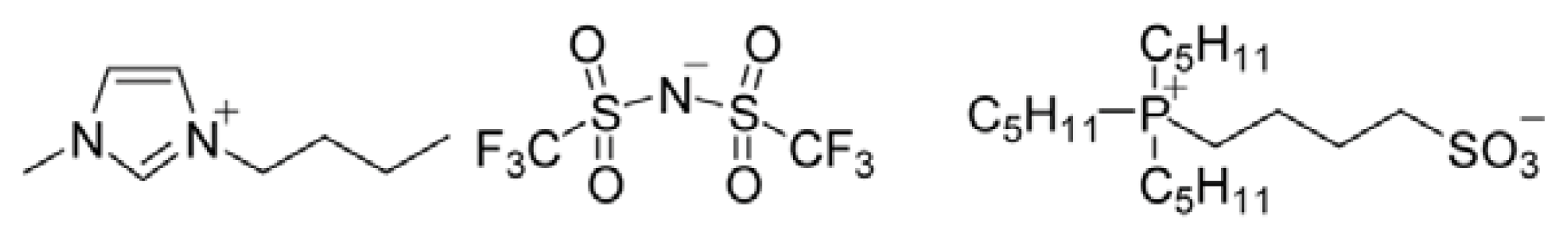
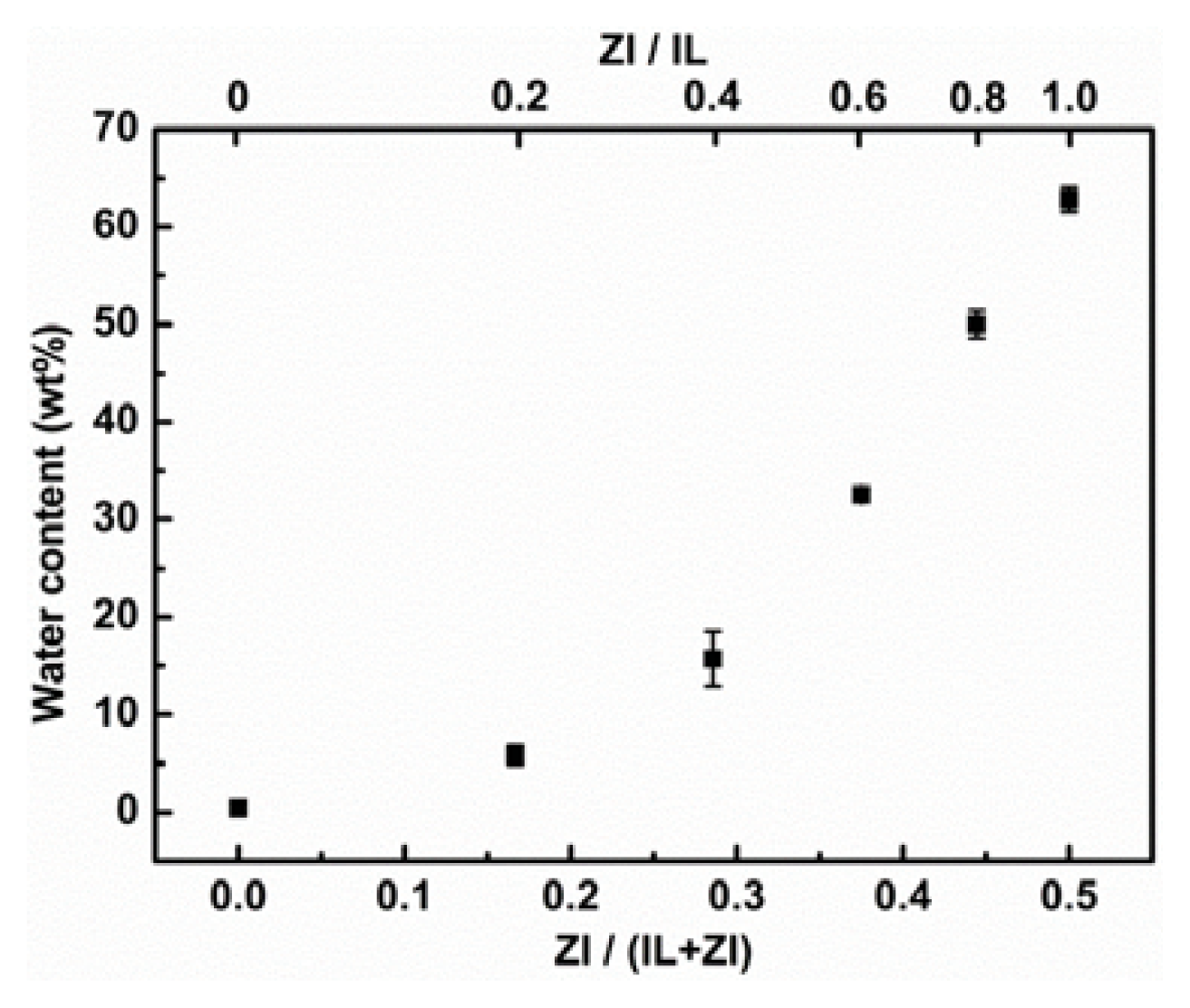
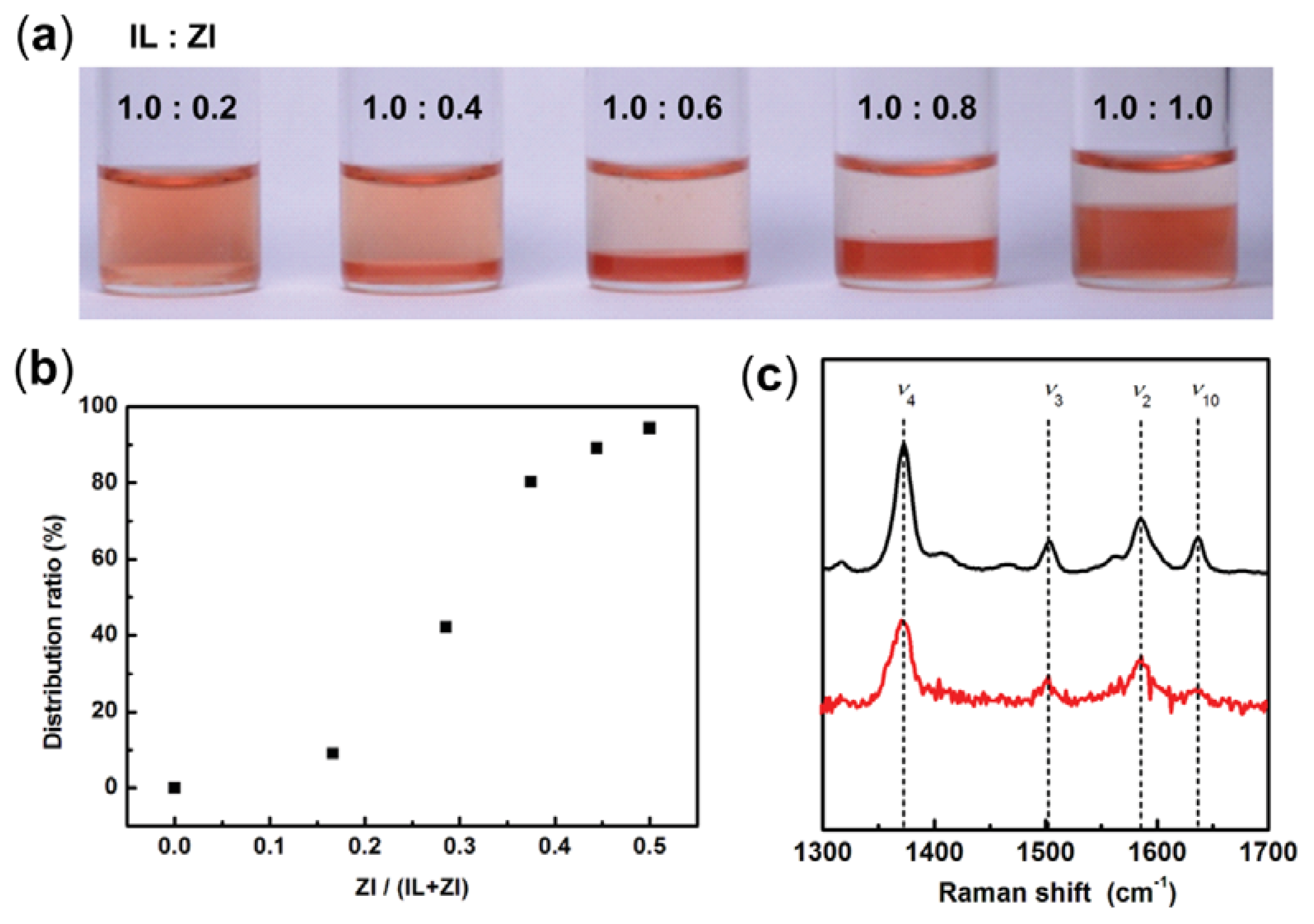
2.1. Control of Saturated Water Content of Separated IL Phase by Mixing Phosphonium-Type ZI
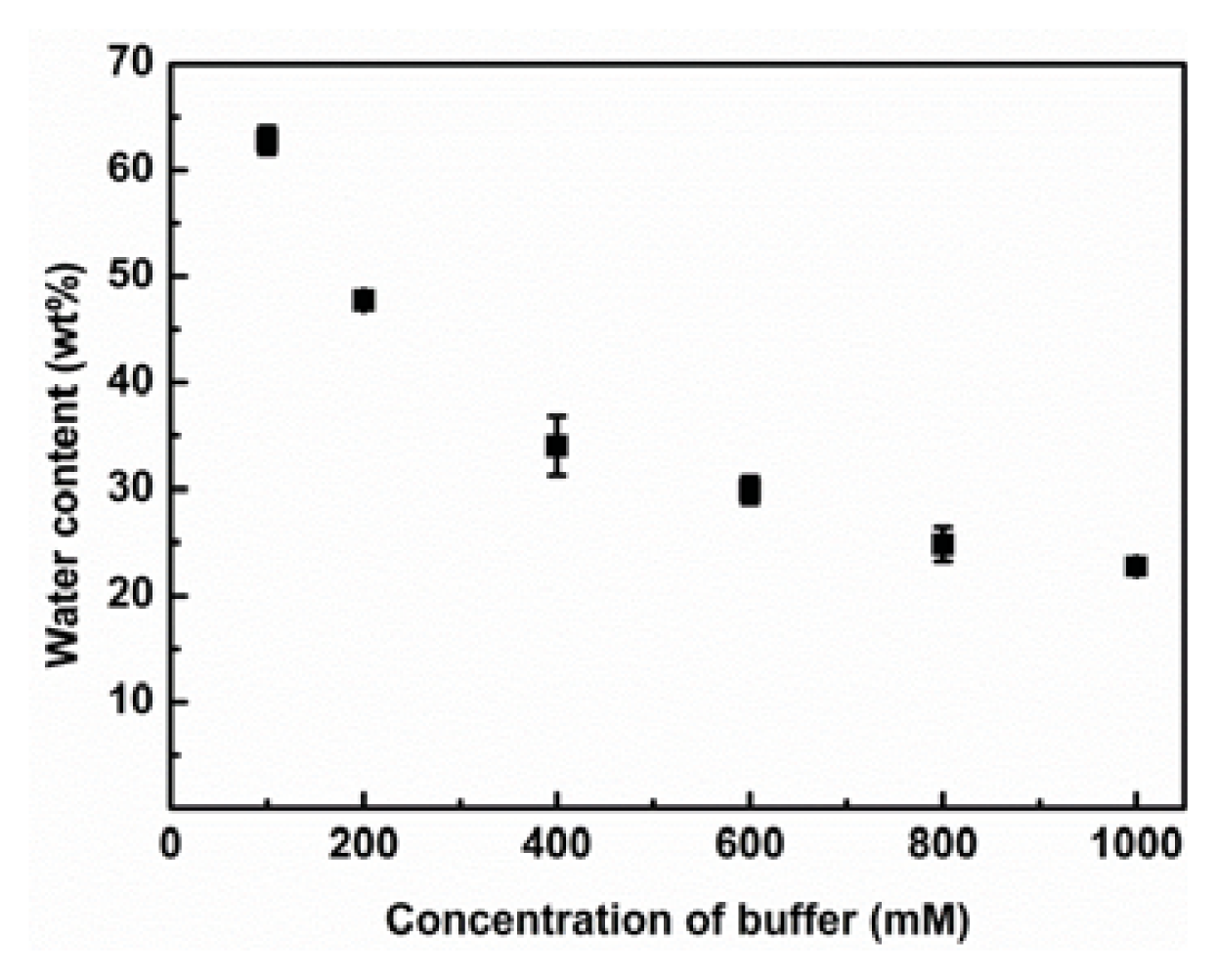
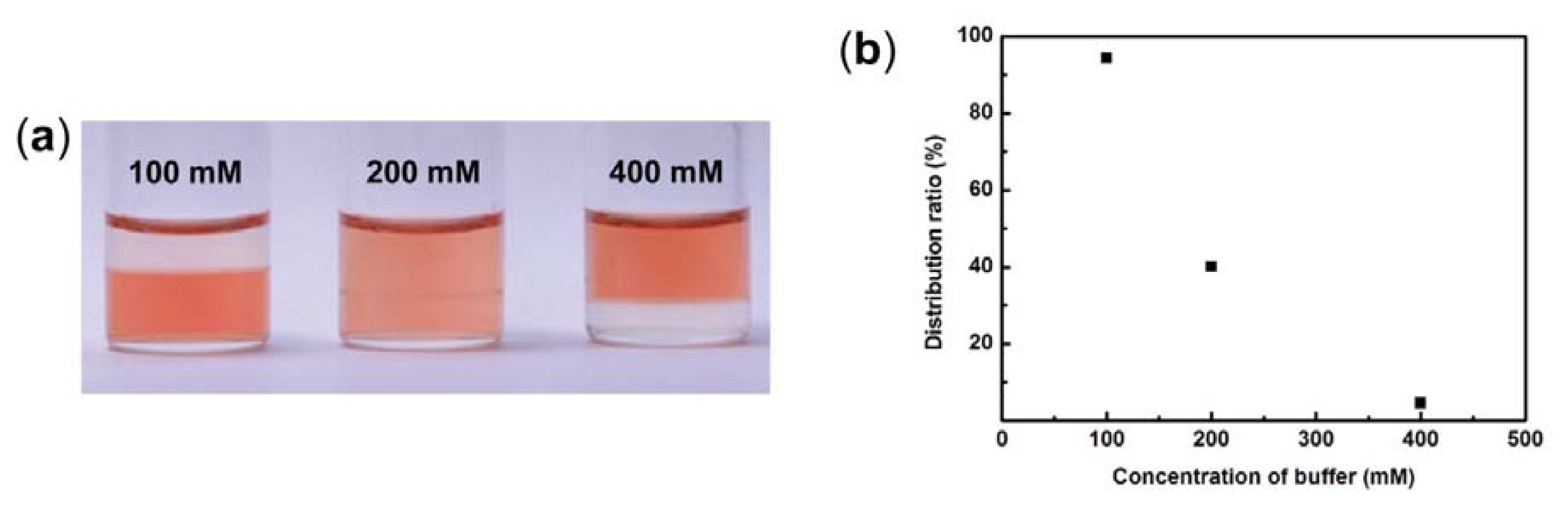
2.2. Effect of K2HPO4/KH2PO4 Concentration in Aqueous Phase on the Water Content of IL Phase
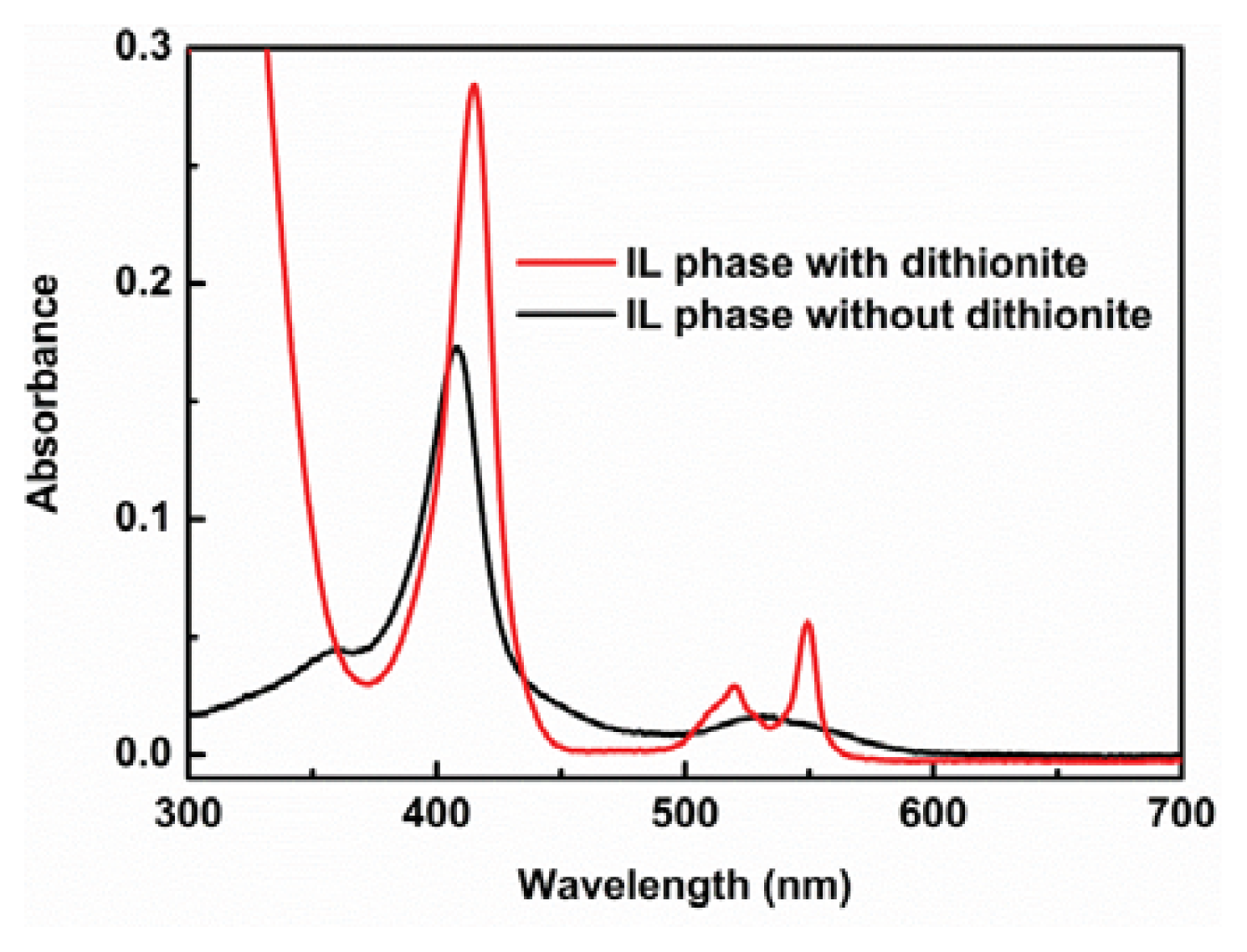
2.3. Extraction of Cytochrome c from Aqueous Phase to IL Phase by Mixing Phosphonium-Type ZI
2.4. Effect of K2HPO4/KH2PO4 Concentration in Aqueous Phase on the Distribution Ratio of cyt.c
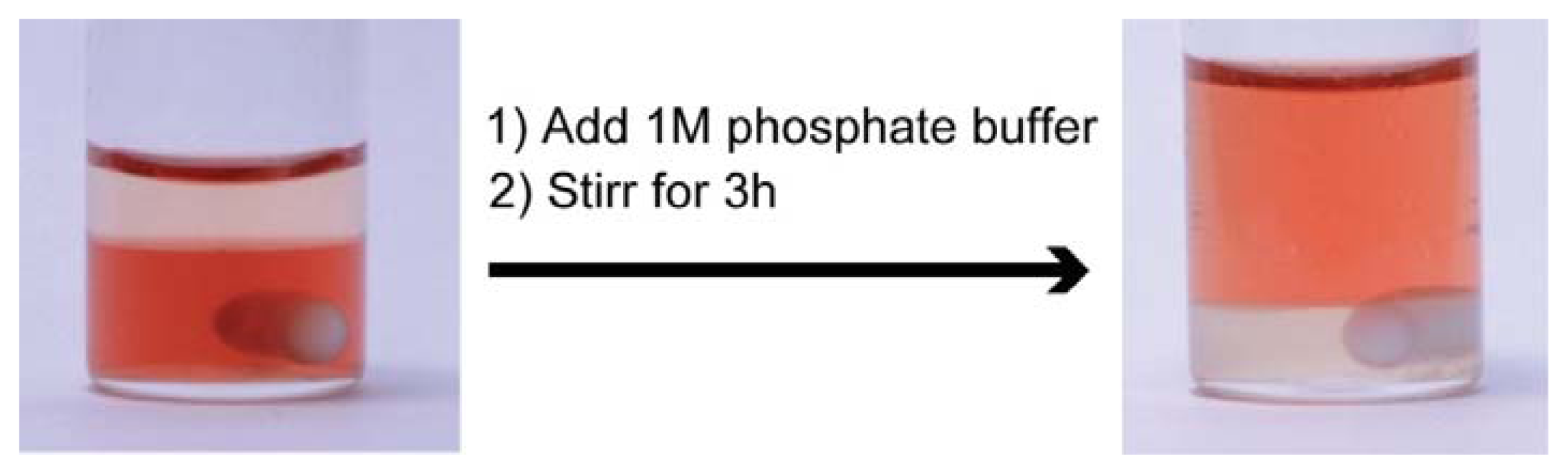
2.5. Construction of Reversible Extraction Process for cyt.c by [C4mim][Tf2N]/P555C4S/Buffer Mixture
3. Experimental Section
3.1. Materials
3.2. Preparation of ILs and ZI
3.2.1. 1-Butyl-3-Methylimidazolium Bromide ([C4mim]Br)
3.2.2. 1-Butyl-3-Methylimidazolium Bis(trifluoromethanesulfonyl)imide ([C4mim][Tf2N])
3.2.3. N,N,N-Tripentyl-4-Sulfonyl-1-Butanephosphonium (P555C4S)
3.3. Water Content of Hydrophobic IL Phase after Mixing with P555C4S
3.4. Control of Water Content of Hydrophobic IL Phase by Adding Phosphate Buffers
3.5. Distribution Ratio of cyt.c in Hydrophobic IL/Water Biphasic System
3.6. Extraction of cyt.c Dissolved in IL Phase to Aqueous Phase
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc. Chem. Commun 1992, 965–967. [Google Scholar]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev 1999, 99, 2071–2083. [Google Scholar]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, Germeny; p. 2003.
- Crowhurst, L.; Mawdsley, P.R.; Perez-Arlandis, J.M.; Salter, P.A.; Welton, T. Solvent–solute interactions in ionic liquids. Phys. Chem. Chem. Phys 2003, 5, 2790–2794. [Google Scholar]
- Bönhote, P.; Dias, A.P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg. Chem 1996, 35, 1168–1178. [Google Scholar]
- Seddon, K.R.; Stark, A.; Torres, M.J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem 2000, 72, 2275–2287. [Google Scholar]
- Freire, M.G.; M Cláudio, A.F.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev 2012, 41, 4966–4995. [Google Scholar]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the aqueous miscibility of ionic liquids: Aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J. Am. Chem. Soc 2003, 125, 6632–6633. [Google Scholar]
- Kawahara, N.V.; Ohno, H. Stability of poly(ethylene oxide)-modified myoglobin in ion conductive polymers at high temperatures. Solid State Ionics 1998, 114, 161–166. [Google Scholar]
- Nakashima, K.; Maruyama, T.; Kamiya, N.; Goto, M. Comb-shaped poly(ethylene glycol)-modified subtilisin carlsberg is soluble and highly acti ve in ioni c liquids. Chem. Commun. 2005, 4297–4299. [Google Scholar]
- Shimojo, K.; Nakagawa, H.; Kamiya, N.; Goto, M. Crown ether-mediated extraction and functional conversion of cytochrome c in ionic liquids. Biomacromolecules 2006, 7, 2–5. [Google Scholar]
- Moniruzzaman, M.; Kamiya, N.; Nakashima, K.; Goto, M. Water-in-ionic liquid microemulsions as a new medium for enzymatic reactions. Green Chem 2008, 10, 497–500. [Google Scholar]
- Fujita, K.; MacFarlane, D.R.; Forsyth, M. Protein solubilising and stabilising ionic liquids. Chem. Commun 2005, 4804–4806. [Google Scholar]
- Fujita, K.; Ohno, H. Enzymatic activity and thermal stability of metallo proteins in hydrated ionic liquids. Biopolymers 2010, 93, 1093–1099. [Google Scholar]
- Fujita, K.; MacFarlane, D.R.; Forsyth, M.; Yoshizawa-Fujita, M.; Murata, K.; Nakamura, N.; Ohno, H. Solubility and stability of cytochrome c in hydrated ionic liquids: Effect of oxo acid residues and kosmotropicity. Biomacromolecules 2007, 8, 2080–2086. [Google Scholar]
- Kohno, Y.; Ohno, H. Temperature-responsive ionic liquid/water interfaces: Relation between hydrophilicity of ions and dynamic phase change. Phys. Chem. Chem. Phys 2012, 14, 5063–5070. [Google Scholar]
- Kohno, Y.; Ohno, H. Ionic liquid/water mixtures: From hostility to conciliation. Chem. Commun 2012, 48, 7119–7130. [Google Scholar]
- Ito, Y.; Kohno, Y.; Nakamura, N.; Ohno, H. Addition of suitably-designed zwitterions improves the saturated water content of hydrophobic ionic liquids. Chem. Commun 2012, 48, 11220–11222. [Google Scholar]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem 2001, 3, 156–164. [Google Scholar]
- Freire, M.G.; Carvalho, P.J.; Silva, A.M.S.; Santos, L.M.N.B.F.; Rebelo, L.P.N.; Marrucho, I.M.; Coutinho, J.A.P. Ion specific effects on the mutual solubilities of water and hydrophobic ionic liquids. J. Phys. Chem. B 2009, 113, 202–211. [Google Scholar]
- Freire, M.G.; Neves, C.M.S.S.; Silva, A.M.S.; Santos, L.M.N.B.F.; Marrucho, I.M.; Rebelo, L.P.N.; Shah, J.K.; Maginn, E.J.; Coutinho, J.A.P. 1H NMR and molecular dynamics evidence for an unexpected interaction on the origin of salting-in/salting-out phenomena. J. Phys. Chem. B 2010, 114, 2004–2014. [Google Scholar]
- Shahriari, S.; Neves, C.M.S.S.; Freire, M.G.; Countinho, J.A.P. Role of the Hofmeister series in the formation of ionic-liquid-based aqueous biphasic systems. J. Phys. Chem. B 2012, 116, 7252–7258. [Google Scholar]
- Scott, R.A.; Mauk, A.G. Cytochrome c: A Multidisciplinary Approach; University Science Books: Sausalito, CA, USA; p. 1996.
- Ueda, S.; Kagimoto, J.; Ichikawa, T.; Kato, T.; Ohno, H. Anisotropic proton-conductive materials formed by the self-organization of phosphonium-type zwitterions. Adv. Mater 2011, 23, 3071–3074. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ito, Y.; Kohno, Y.; Nakamura, N.; Ohno, H. Design of Phosphonium-Type Zwitterion as an Additive to Improve Saturated Water Content of Phase-Separated Ionic Liquid from Aqueous Phase toward Reversible Extraction of Proteins. Int. J. Mol. Sci. 2013, 14, 18350-18361. https://doi.org/10.3390/ijms140918350
Ito Y, Kohno Y, Nakamura N, Ohno H. Design of Phosphonium-Type Zwitterion as an Additive to Improve Saturated Water Content of Phase-Separated Ionic Liquid from Aqueous Phase toward Reversible Extraction of Proteins. International Journal of Molecular Sciences. 2013; 14(9):18350-18361. https://doi.org/10.3390/ijms140918350
Chicago/Turabian StyleIto, Yoritsugu, Yuki Kohno, Nobuhumi Nakamura, and Hiroyuki Ohno. 2013. "Design of Phosphonium-Type Zwitterion as an Additive to Improve Saturated Water Content of Phase-Separated Ionic Liquid from Aqueous Phase toward Reversible Extraction of Proteins" International Journal of Molecular Sciences 14, no. 9: 18350-18361. https://doi.org/10.3390/ijms140918350