Microfluidic Biosensing Systems Using Magnetic Nanoparticles
Abstract
:1. Introduction
1.1. Superparamagnetism
1.2. Magnetic Nanoparticles
1.3. Magnetoresistance Sensors
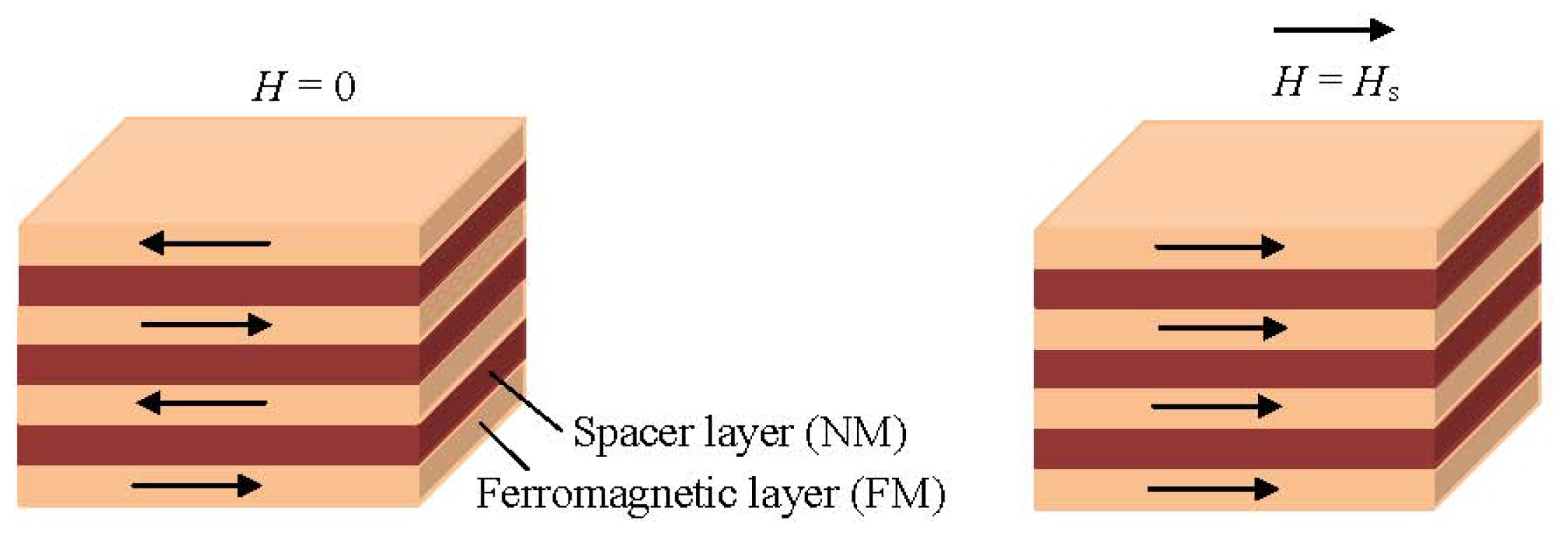
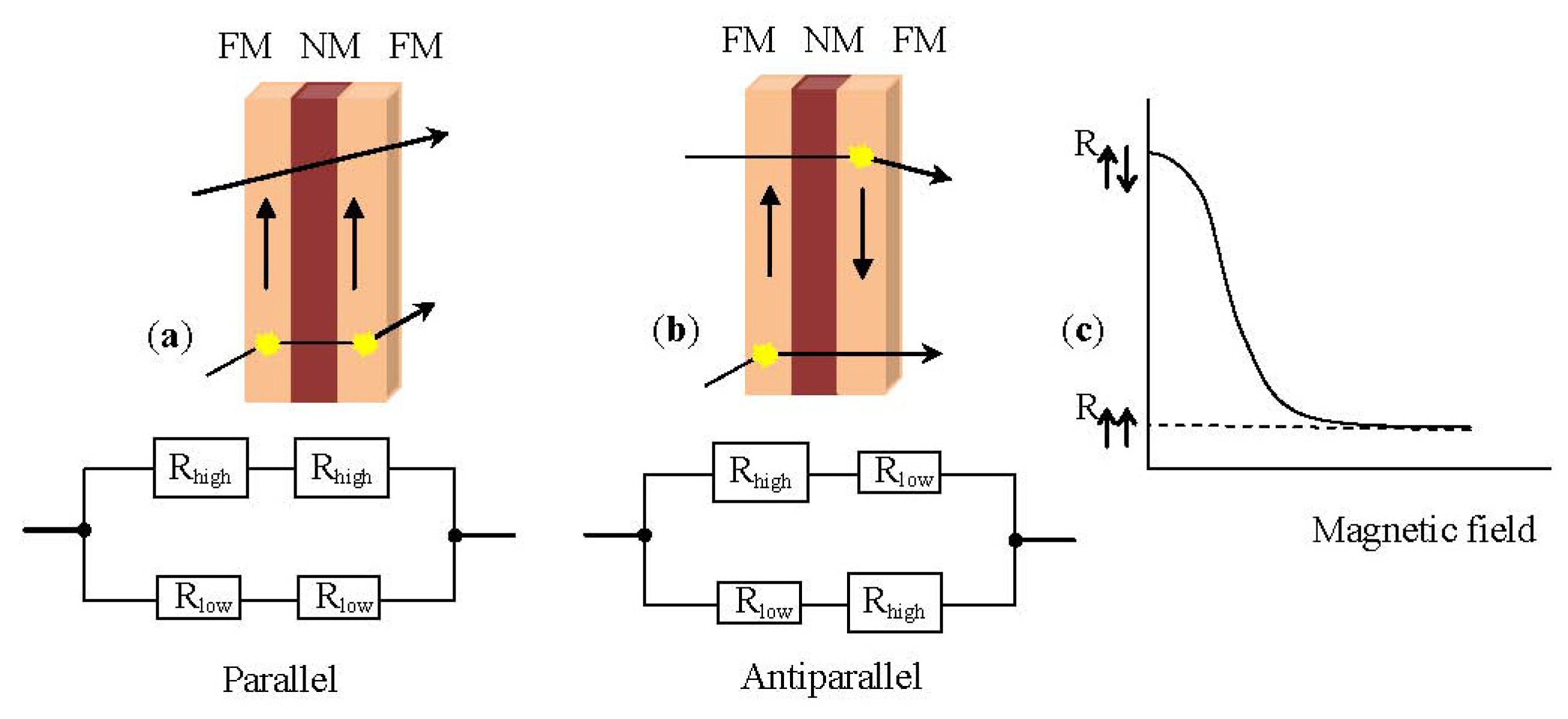
1.3.1. Giant Magnetoresistance Sensors
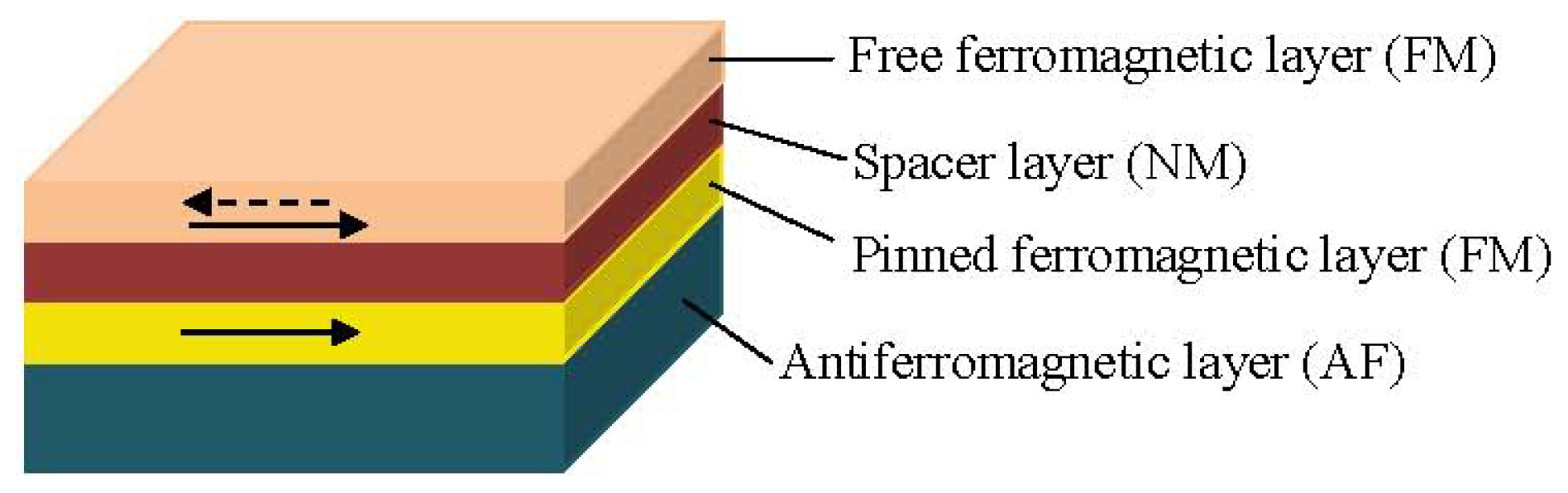
1.3.2. Spin Valve Sensors
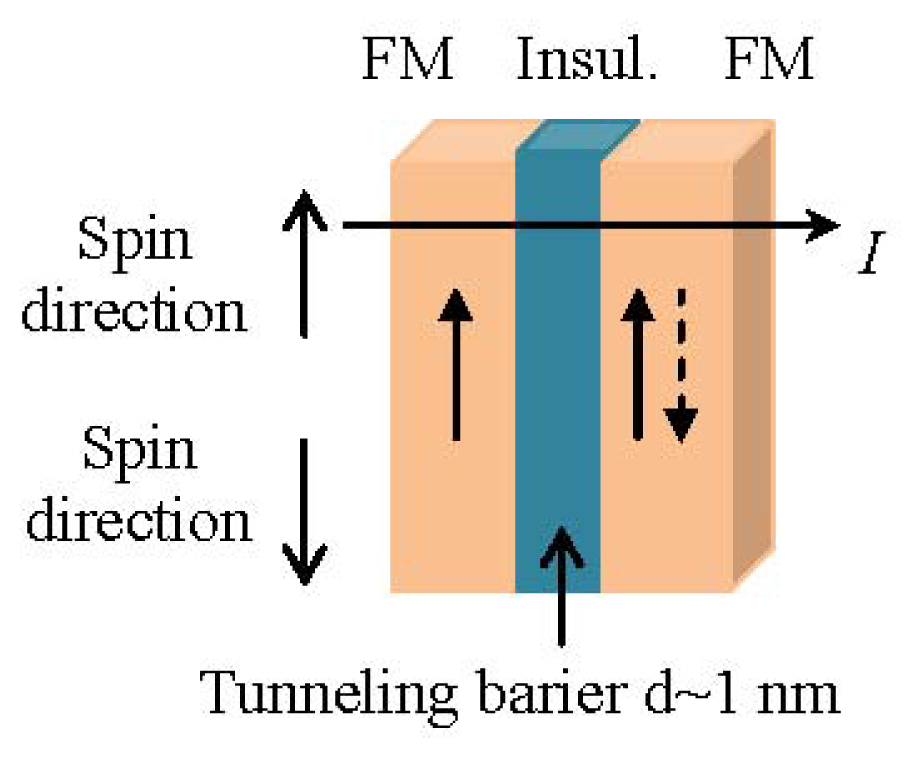
1.3.3. Tunneling Magnetoresistance Sensors
1.4. Microfluidics
2. Microfluidic Biosensing Systems: State of the Art
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Varadan, V.K.; Chen, L.; Xie, J. Nanomedicine: Design and Applications of Magnetic Nanomaterials, Nanosensors and Nanosystems; John Wiley & Sons, Ltd: West Sussex, UK; p. 2008.
- Guimaraes, A.P. Principles of Nanomagnetism; Springer: Berlin, Heidelberg, Germany; p. 2009.
- Li, G.X.; Sun, S.H.; Wilson, R.J.; White, R.L.; Pourmand, N.; Wang, S. Spin valve sensors for ultrasensitive detection of superparamagnetic nanoparticles for biological applications. Sens. Actuators A 2006, 126, 98–106. [Google Scholar]
- Samal, D.; Kumar, P.S.A. Giant magnetoresistance. Resonance 2008, 13, 343–354. [Google Scholar]
- Nickel, J. Magnetoresitance overview. HP Labs. 1995. Available online: http://www.hpl.hp.com/techreports/95/HPL-95-60.pdf (accessed on 26 July 2013).
- Moscelli, N.; van den Driesche, S.; Witarski, W.; Pastorekova, S.; Vellekoop, M. An imaging system for real-time monitoring of adherently grown cells. Sens. Actuators A 2008, 172, 175–180. [Google Scholar]
- Peham, J.R.; Vellekoop, M.; Nöhammer, C.; Wiesinger-Mayr, H. PCR product detector with LED-photodiode fluorescence sensing in a nanoliter flow-cell for the high-throughput detection of double-stranded DNA. Procedia Eng 2011, 25, 936–939. [Google Scholar]
- Rosenauer, M.; Buchegger, W.H.; Finoulst, I.; Verhaert, P.; Vellekoop, M. Miniaturized flow cytometer with 3d hydrodynamic particle focusing and integrated optical elements applying silicon photodiodes. Microfluid. Nanofluid 2011, 10, 761–771. [Google Scholar]
- Weber, E.; Keplinger, F.; Vellekoop, M. Detection of dissolved lactose employing an optofluidic micro-system. Diagnostics 2012, 2, 97–106. [Google Scholar]
- Weber, E.; Vellekoop, M. Optofluidic micro-sensors for the determination of liquid concentrations. Lab Chip 2012, 1219, 3754–3759. [Google Scholar]
- Giouroudi, I.; Kosel, J. Recent progress in biomedical applications of magnetic nanoparticles. Recent Pat. Nanotechnol 2010, 4, 111–118. [Google Scholar]
- Antipas, G.S.E.; Statharas, E.; Tserotas, P.; Papadopoulos, N.; Hristoforou, E. Experimental and first principles’ characterization of functionalized magnetic nanoparticles. ChemPhysChem 2013, 14, 1934. [Google Scholar]
- Bean, C.P.; Livingston, J.D. Superparamagnetism. J. Appl. Phys 1959, 30, 120–129. [Google Scholar]
- Indira, T.K.; Lakshmi, P.K. Magnetic nanoparticles—A review. Int. J. Pharm. Sci. Nanotechnol 2010, 3, 1035–1042. [Google Scholar]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem 2010, 20, 8760–8767. [Google Scholar]
- Hristoforou, E. Magnetic effects in physical sensor design. J. Opt. Adv. Mat 2002, 4, 245–260. [Google Scholar]
- Thorek, D.; Chen, A.; Czupryna, J.; Tsourkas, A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann. Biomed. Eng 2006, 34, 23–38. [Google Scholar]
- Gupta, A.; Curtis, A. Surface modified superparamagnetic nanoparticles for drug delivery: Interaction studies with human fibroblasts in culture. J. Mater. Sci. Mater. Med 2004, 15, 493–496. [Google Scholar]
- Melancon, M.; Lu, W.; Li, C. Gold-based magneto/optical nanostructures: Challenges for in vivo applications in cancer diagnostics and therapy. Mater. Res. Bull 2009, 34, 415–421. [Google Scholar]
- Bumb, A.; Brechbiel, M.W.; Choyke, P.L.; Fugger, L.; Eggeman, A.; Prabhakaran, D.; Hutchinson, J.; Dobson, P.J. Synthesis and characterization of ultra-small superparamagnetic iron oxide nanoparticles thinly coated with silica. Nanotechnology 2008, 19, 335601. [Google Scholar]
- Tartaj, P.; Morales, M.P.; Gonzalez-Carreno, T.; Veintemillas-Verdaguer, S.; Serna, C.J. Advances in magnetic nanoparticles for biotechnology applications. J. Magn. Magn. Mater. 2005, 290–291, 28–34. [Google Scholar]
- Iida, H.; Takayanagi, K.; Nakanishi, T.; Kume, A.; Muramatsu, K.; Kiyohara, Y.; Akiyama, Y.; Osaka, T. Preparation of human immune effector T cells containing iron-oxide nanoparticles. Biotechnol. Bioeng 2008, 101, 1123–1128. [Google Scholar]
- Karami, H. Synthesis and characterization of iron oxide nanoparticles by solid state chemical reaction method. J. Clust. Sci 2009, 21, 11–20. [Google Scholar]
- Koutzarova, T.; Kolev, S.; Ghelev, C.H.; Paneva, D.; Nedkov, I. Microstructural study and size control of iron oxide nanoparticles produced by microemulsion technique. Physica Status Solidi C 2006, 3, 1302–1307. [Google Scholar]
- Loo, A.L.; Pineda, M.G.; Saade, H.; Trevino, M.E.; Lopez, R.G. Synthesis of magnetic nanoparticles in bicontinuous microemulsions. Effect of surfactant concentration. J. Mater. Sci 2008, 43, 3649–3654. [Google Scholar]
- Lu, A.H.; Salabas, E.L.; Schueth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed 2007, 46, 1222–1244. [Google Scholar]
- Ripka, P. Magnetic Sensors and Magnetometers; Artech House Publishers: Boston, UK, 2001. [Google Scholar]
- Freitas, P.P.; Ferreira, R.; Cardoso, S.; Cardoso, F. Magnetoresistive sensors. J. Phys. Condens. Matter 2007, 19, 165221. [Google Scholar]
- Ennen, I.; Albon, C.; Weddemann, A.; Auge, A.; Hedwig, P.; Wittbracht, F.; Regtmeier, A.-K.; Akemeier, D. From magnetic nanoparticles to magnetoresistive biosensors. Acta Phys. Pol. A 2012, 121, 420–425. [Google Scholar]
- Gaster, R.S.; Xu, L.; Han, S.J.; Wilson, R.J.; Hall, D.A.; Osterfeld, S.J.; Yu, H.; Wang, S.X. Quantification of protein interactions and solution transport using high-density GMR sensor arrays. Nat. Nanotechnol 2011, 6, 314–320. [Google Scholar]
- Xu, L.; Yu, H.; Akhras, M.S.; Han, S.-J.; Osterfeld, S.; White, R.L.; Pourmand, N.; Wang, S.X. Giant magnetoresistive biochip for DNA detection and HPV genotyping. Biosens. Bioelectron 2008, 24, 99–103. [Google Scholar]
- Giouroudi, I.; van den Driesche, S.; Kosel, J.; Grössinger, R.; Vellekoop, M. On-chip bio-analyte detection utilizing the velocity of magnetic microparticles in a fluid. J. Appl. Phys 2011, 109, 07, B304.. [Google Scholar]
- Gooneratne, C.; Liang, C.; Giouroudi, I.; Kosel, J. An integrated micro-chip for rapid detection of magnetic particles. J. Appl. Phys 2012, 111, 07, B327.. [Google Scholar]
- Li, F.; Giouroudi, I.; Kosel, J. A biodetection method using magnetic particles and micro traps. J. Appl. Phys 2012, 111, 07, B328.. [Google Scholar]
- Manteca, A.; Mujika, M.; Arana, S. GMR sensors: Magnetoresistive behaviour optimization for biological detection by means of superparamagnetic nanoparticles. Biosens. Bioelectron 2011, 26, 3705–3709. [Google Scholar]
- Graham, D.L.; Ferreira, H.A.; Freitas, P.P. Magnetoresistive-based biosensors and biochips. Trends Biotechnol. 2004, 22. [Google Scholar] [CrossRef]
- Reig, C.; Cubells-Beltrán, M.D.; Ramírez Muñoz, D. Magnetic field sensors based on giant magnetoresistance (GMR) technology: Applications in electrical current sensing. Sensors 2009, 9, 7919–7942. [Google Scholar]
- Vovk, V.; Schmitz, G.; Hütten, A.; Heitmann, S. Mismatch-induced recrystallization of giant magneto-resistance (GMR) multilayer systems. Acta Mater 2007, 55, 3033–3047. [Google Scholar]
- Pelkner, M.; Neubauer, A.; Reimund, V.; Kreutzbruck, M.; Schütze, A. Routes for GMR-sensor design in non-destructive testing. Sensors 2012, 12, 12169–12183. [Google Scholar]
- Liu, S.; Huang, Q.; Li, Y.; Zhen, W. Experimental research on hysteresis effects in GMR sensors for analog measurement applications. Sens. Actuators A 2012, 182, 72–81. [Google Scholar]
- Jiles, D. Introduction to Magnetism and Magnetic Materials; Chapman & Hall: London, UK; p. 1991.
- Jansen, R. The spin-valve transistor: A review and outlook. J. Phys. D 2003, 36, 289–308. [Google Scholar]
- Lederman, M. Performance of metallic antiferromagnets for use in spin-valve read sensors. IEEE Trans. Magn 1999, 35, 794–799. [Google Scholar]
- Albon, C.; Weddemann, A.; Auge, A.; Rott, K.; Hütten, A. Tunneling magnetoresistance sensors for high resolutive particle detection. Appl. Phys. Lett 2009, 95, 023101. [Google Scholar]
- Han, S.-J.; Wang, S. Magnetic nanotechnology for biodetection. J. Lab. Autom 2010, 15, 93. [Google Scholar]
- Lee, Y.M.; Hayakawa, J.; Ikeda, S.; Matsukura, F.; Ohno, H. Effect of electrode composition on the tunnel magnetoresistance of pseudo-spin-valve magnetic tunnel junction with a MgO tunnel barrier. Appl. Phys. Lett 2007, 90, 212507. [Google Scholar]
- Reiss, G.; Brückl, H.; Hütten, A.; Schotter, J.; Brzeska, M.; Panhorst, M.; Sudfeld, D.; Becker, A.; Kamp, P.B.; Pühler, A.; et al. Magnetoresistive sensors and magnetic nanoparticles for biotechnology. Mater. Res. Soc 2005, 20, 3294–3302. [Google Scholar]
- Eijkel, J.C.T.; van den Berg, A. Nanofluidics: What is it and what can we expect from it. Microfluid. Nanofluid 2005, 1, 249–267. [Google Scholar]
- Gomez, F.A. Biological Applications of Microfluidics; John Wiley & Sons, Inc: Hoboken, NJ, USA; p. 2008.
- Prakash, S.; Piruska, A.; Gatimu, E.N.; Bohn, P.W.; Sweedler, J.V.; Shannon, M.A. Nanofluidics: Systems and applications. IEEE Sens. J. 2008, 8. [Google Scholar] [CrossRef]
- Gijs, M.A.M. Device physics: Will fluidic electronics take off? Nat. Nanotechnol 2007, 2, 268–270. [Google Scholar]
- Tay, F.E.H. Microfluidics and BioMEMS Applications; Kluwer Academic Publishers: Dordrecht, The Netherlands; p. 2002.
- Gijs, M.A.M.; Lacharme, F.; Lehmann, U. Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem. Rev 2010, 110, 1518–1563. [Google Scholar]
- Llandro, J.; Palfreyman, J.J.; Ionescu, A.; Barnes, C.H.W. Magnetic biosensor technologies for medical applications: A review. Med. Biol. Eng. Comput. 2010, 48, 977–998. [Google Scholar]
- Djamal, M. Biosensor based on giant magnetoresistance material. Int. J. E-Health Med. Commun 2010, 1, 3. [Google Scholar]
- Xu, W.-Z.; Li, Y.; Wu, Q.; Wang, M.; Wu, Z.-G. Brief communication: Comparison the diagnostic performance of four HBsAg ELISA kits. J. Clin. Lab. Anal 2003, 27, 294–296. [Google Scholar]
- Wormser, G.P.; Schriefer, M.; Aguero-Rosenfeld, M.E.; Levin, A.; Steere, A.C.; Nadelman, R.B.; Nowakowski, J.; Marques, A.; Johnson, B.J.; Dumler, J.S. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn. Microbiol. Infect. Dis 2013, 75, 9–15. [Google Scholar]
- Peeling, R.W.; Artsob, H.; Pelegrino, J.L.; Buchy, P.; Cardosa, M.J.; Devi, S.; Enria, D.A.; Farrar, J.; Gubler, D.J.; Guzman, M.G.; et al. Evaluation of diagnostic tests: Dengue. Nat. Rev. Microbiol. 2010. [Google Scholar] [CrossRef]
- Fagbemi, B.O.; Obarisiagbon, I.O. Comparative evaluation of the enzyme-linked immunosorbent assay (ELISA) in the diagnosis of natural Fasciola gigantica infection in cattle. Vet Q 1990, 12, 35–38. [Google Scholar]
- Megens, M.; Prins, M. Magnetic biochips: A new option for sensitive diagnostics. J. Magn. Magn. Mater 2005, 293, 702–708. [Google Scholar]
- Hall, D.A.; Gaster, R.S.; Lin, T.; Osterfeld, S.J.; Han, S.; Murmann, B.; Wang, S.X. GMR Biosensor arrays: A system perspective. Biosens. Bioelectron 2010, 25, 2051–2057. [Google Scholar]
- MagArray Homepage. Available online: http://magarray.com/ (accessed on 19 March 2013).
- Zhi, X.; Liu, Q.S.; Zhang, X.; Zhang, Y.X.; Feng, J.; Cui, D.X. Quick genotyping detection of HBV by giant magnetoresistive biochip combined with PCR and line probe assay. Lab Chip 2012, 12, 741–745. [Google Scholar]
- Feng, J.; Wang, Y.; Li, F.; Shi, H.; Chen, X. Detection of magnetic microbeads and ferrofluid with giant magnetoresistance sensors. J. Phys. Conf. Ser 2011, 263, 012002. [Google Scholar]
- Philips NewsCenter. Available online: http://www.newscenter.philips.com (accessed on 19 March 2013).
- Philips Business Sites. Available online: http://www.business-sites.philips.com (accessed on 19 March 2013).
- Imego Homepage. Available online: http://www.imego.com (accessed on 19 March 2013).
- Minchole, A.; Astalan, A.; Johansson, C.; Lagerwall, K.; Krozer, A. Method and arrangement for analyzing substances. Available online: http://www.imego.com/patents-and-publications/patents/method-and-arrangement-for-analyzing-substances.aspx (accessed on 20 March 2013).
- Krozer, A.; Johansson, C.; Blomgren, J.; Jonasson, C.; Pettersson, K.; Ilver, D.; Astalan, A. Detection device and method (dynamic magnetic susceptibility). Available online: http://www.imego.com/patents-and-publications/patents/detection-device-and-method.aspx (accessed on 20 March 2013).
- Wang, H.; Chen, Y.; Hassibi, A.; Scherer, A.; Hajimiri, A. A frequency-shift CMOS magnetic biosensor array with single-bead sensitivity and no external magnet. IEEE Int. Solid-State Circuits Conf.-Dig. Techn. Pap. 2009, 9a. [Google Scholar]
- Wang, H.; Shohei, K.; Sideris, C.; Hajimiri, A. An Ultrasensitive CMOS Magnetic Biosensor Array with Correlated Double Counting Noise Suppression. Proceedings of IEEE MTT-S International Microwave Symposium Digest (MTT). IEEE MTT-S International Microwave Symposium, Anaheim, CA, USA, 23–28 May 2010; pp. 616–619.
- Rife, J.C.; Miller, M.M.; Sheehan, P.E.; Tamanaha, C.R.; Tondra, M.; Whitman, L.J. Design and performance of GMR sensors for the detection of magnetic microbeads in biosensors. Sens. Actuators A 2003, 107, 209–218. [Google Scholar]
- Millen, R.L.; Kawaguchi, T.; Granger, M.C.; Porter, M.D.; Tondra, M. Giant magnetoresistive sensors and superparamagnetic nanoparticles: A chip-scale detection strategy for immunosorbent assays. Anal. Chem 2005, 77, 6581–6587. [Google Scholar]
- Shen, W.; Schrag, B.D.; Carter, M.J.; Xie, J.; Xu, C.; Sun, S.; Xiao, G. Quantitative detection of DNA labeled with magnetic nanoparticles using arrays of MgO-based magnetic tunnel junction sensors. J. Appl. Phys. 2008, 103, 07, A306.. [Google Scholar]
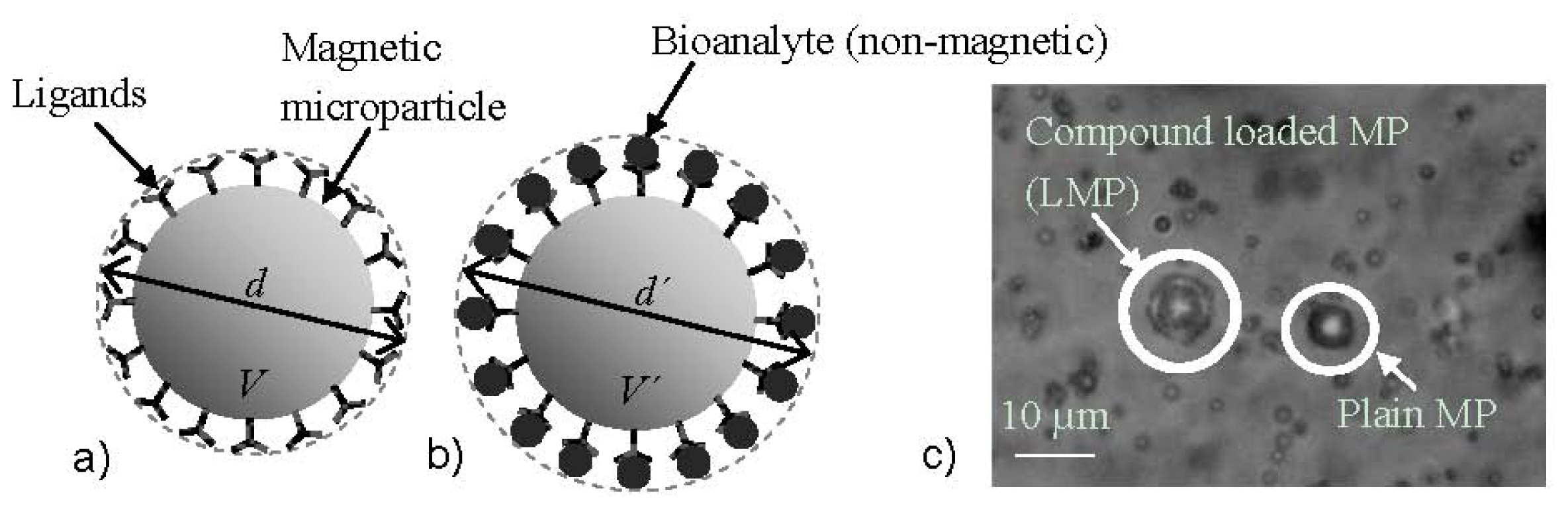
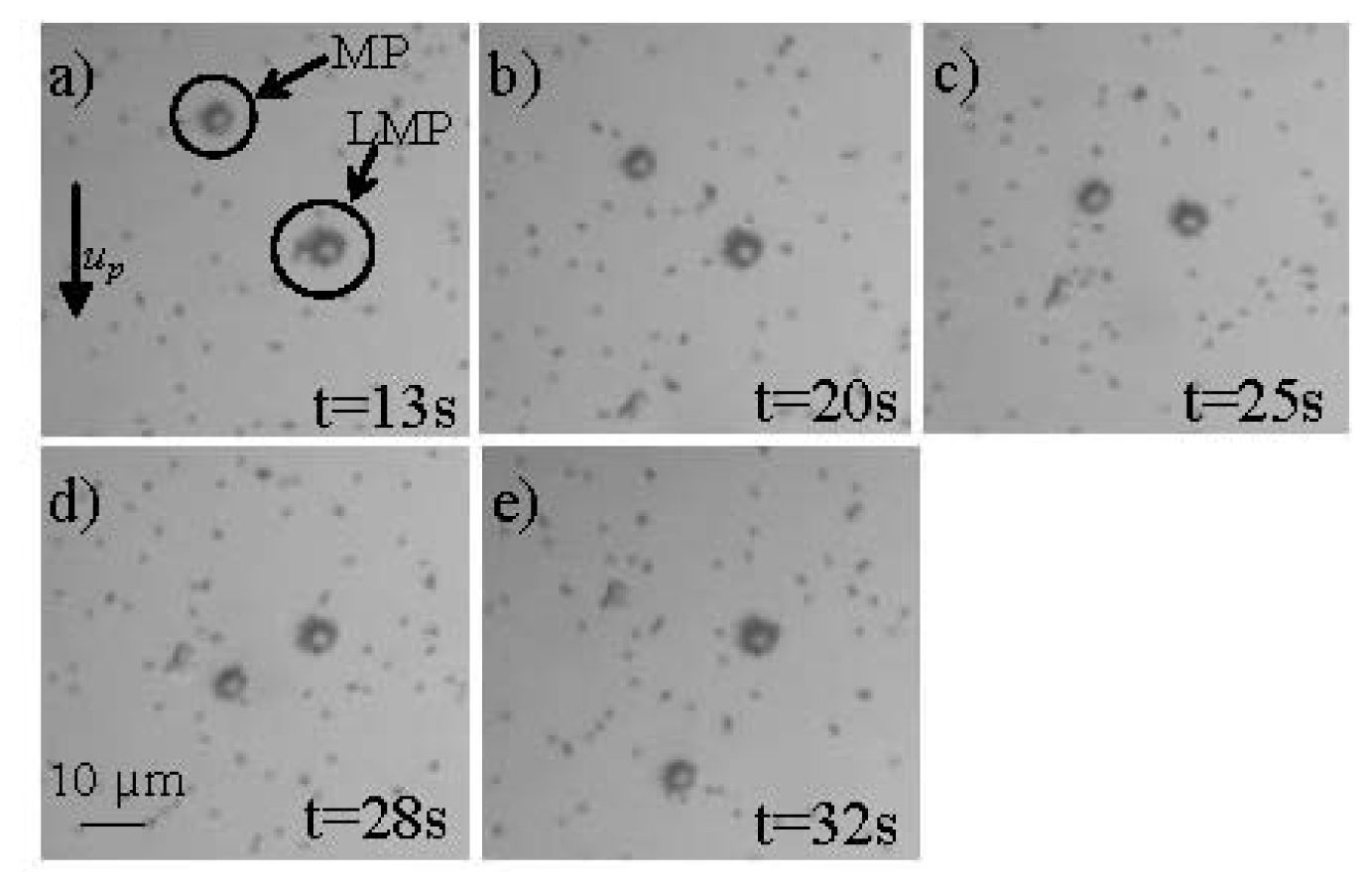
- Giouroudi, I.; van den Driesche, S.; Kosel, J. Biosensing utilizing the motion of magnetic microparticles in a microfluidic system. Procedia Eng. 2010, 5, 824–887. [Google Scholar]
- Gooneratne, C.; Giouroudi, I.; Liang, C.; Kosel, J. A GMR ring-sensor based microsystem for magnetic bead manipulation and detection. J. Appl. Phys. 2011, 109, 07, E517.. [Google Scholar]
- Kokkinis, G.; Keplinger, F.; Kosel, J.; Giouroudi, I. On-Chip Biosensing Utilizing the Motion of Suspended Magnetic Microparticles. Proceedings of the 3rd International Conference of Biosensing Technology, Sitges, Spain, May 2013.
- Dangl, A.; Kokkinis, G.; Keplinger, F.; Giouroudi, I. In vitro Biosensing Based on Magnetically Induced Motion of Magnetic Nanoparticles. Proceedings of the Nanotech Conference & Expo, Washington, WA, USA, 12–16 May 2013; 3.
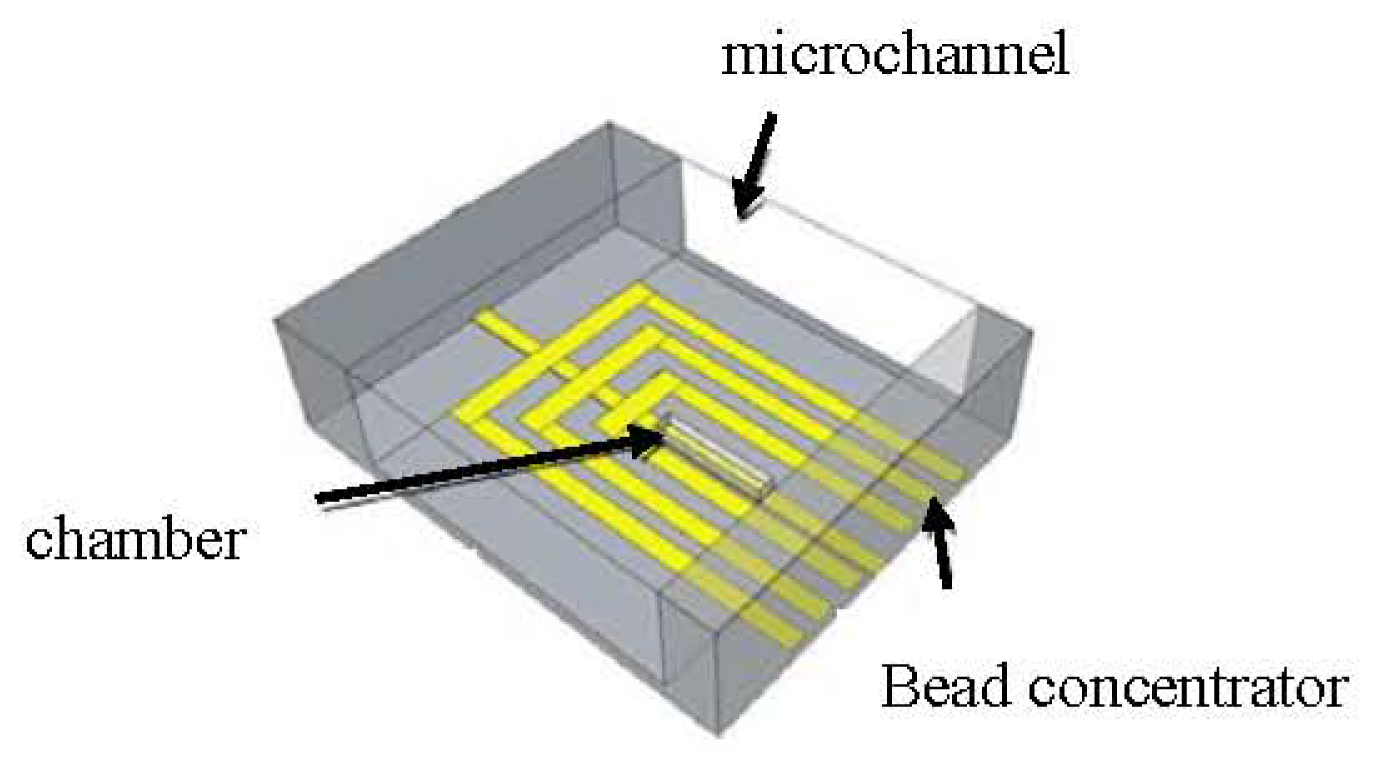
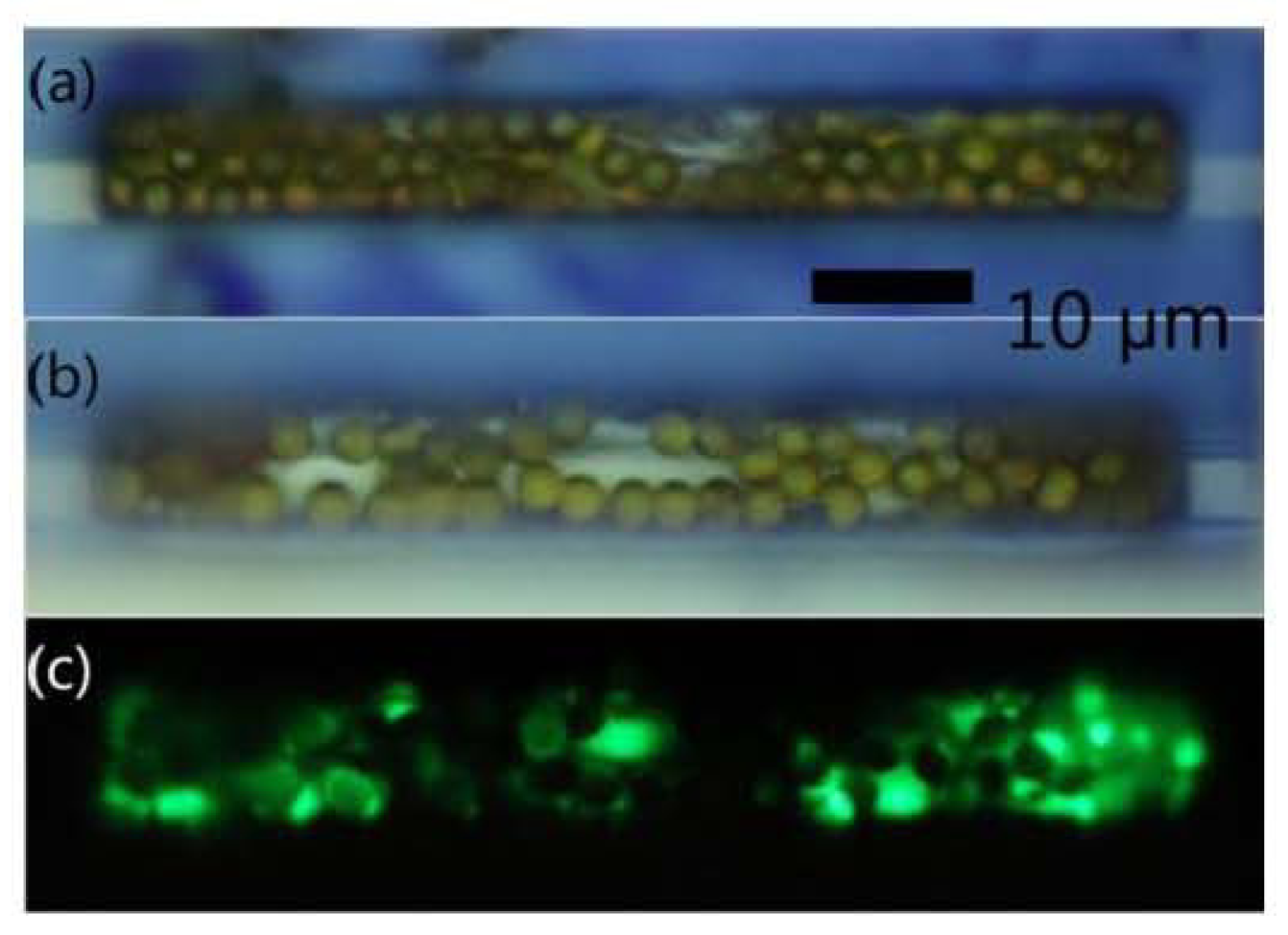
- Giouroudi, I.; Kokkinis, G.; Gooneratne, C.; Kosel, J. Microfluidic Biosensing Device for Controlled Trapping and Detection of Magnetic Microparticles. Proceedings of the 29th Southern Biomedical Engineering Conference Proceedings, Miami, FL, USA, 3–5 May 2013.
- Gooneratne, C.; Yassine, O.; Giouroudi, I.; Kosel, J. Selective manipulation of magnetic beads by a magnetic microchip. IEEE Trans. Magn 2013, 49, 7. [Google Scholar]
- Li, F.; Kosel, J. A magnetic method to concentrate and trap biological targets. IEEE Trans. Magn 2012, 48, 2854–2856. [Google Scholar]
- Loureiro, J.; Ferreira, R.; Cardoso, S.; Germano, J.; Fermon, C.; Arrias, G.; Pannetier-Lecoeur, M.; Rivadulla, F.; Rivas, J. Toward a magnetoresistive chip cytometer: Integrated detection of magnetic beads flowing at cm/s velocities in microfluidic channels. Appl. Phys. Lett 2009, 95, 034104. [Google Scholar]
- Loureiro, J.; Fermon, C.; Pannetier-Lecoeur, M.; Arrias, G.; Ferreira, R.; Cardoso, S.; Freitas, P.P. Magnetoresistive detection of magnetic beads flowing at high speed in microfluidic channels. IEEE Trans. Magn 2009, 45, 4873–4876. [Google Scholar]
- Loureiro, J.; Andrade, P.Z.; Cardoso, S.; da Silva, C.L.; Cabral, J.M.; Freitas, P.P. Magnetoresistive chip cytometer. Lab Chip 2011, 11, 2255–2261. [Google Scholar]
- Melzer, M.; Karnaushenko, D.; Makarov, D.; Baraban, L.; Calvimontes, A.; Mönch, I.; Kaltofen, R.; Mei, Y.F.; Schmidt, O.G. Elastic magnetic sensor with isotropic sensitivity for in-flow detection of magnetic objects. RSC Adv 2012, 2, 2284–2288. [Google Scholar]
- Hall, D.A.; Gaster, R.S.; Osterfeld, S.J.; Murmann, B.; Wang, S.X. GMR biosensor arrays: Correction techniques for reproducibility and enhanced sensitivity. Biosens. Bioelectron 2010, 25, 2177–2181. [Google Scholar]
- Altman, W.R.; Moreland, J.; Russek, S.E.; Han, B.W.; Bright, V.M. Microfluidic Transport and Sensing of Functionalized Superparamagnetic Bead with Integrated Spin Vaves. Proceedings of the 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Seattle, WA, USA, 2–6 October 2011.
- Wu, J.; Gu, M. Microfluidic sensing: State of the art fabrication and detection techniques. J. Biomed. Opt 2011, 16, 080901. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Giouroudi, I.; Keplinger, F. Microfluidic Biosensing Systems Using Magnetic Nanoparticles. Int. J. Mol. Sci. 2013, 14, 18535-18556. https://doi.org/10.3390/ijms140918535
Giouroudi I, Keplinger F. Microfluidic Biosensing Systems Using Magnetic Nanoparticles. International Journal of Molecular Sciences. 2013; 14(9):18535-18556. https://doi.org/10.3390/ijms140918535
Chicago/Turabian StyleGiouroudi, Ioanna, and Franz Keplinger. 2013. "Microfluidic Biosensing Systems Using Magnetic Nanoparticles" International Journal of Molecular Sciences 14, no. 9: 18535-18556. https://doi.org/10.3390/ijms140918535




