The MAPKKK Gene Family in Gossypium raimondii: Genome-Wide Identification, Classification and Expression Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Genome-Wide Identification of the MAPKKK Family in G. raimondii
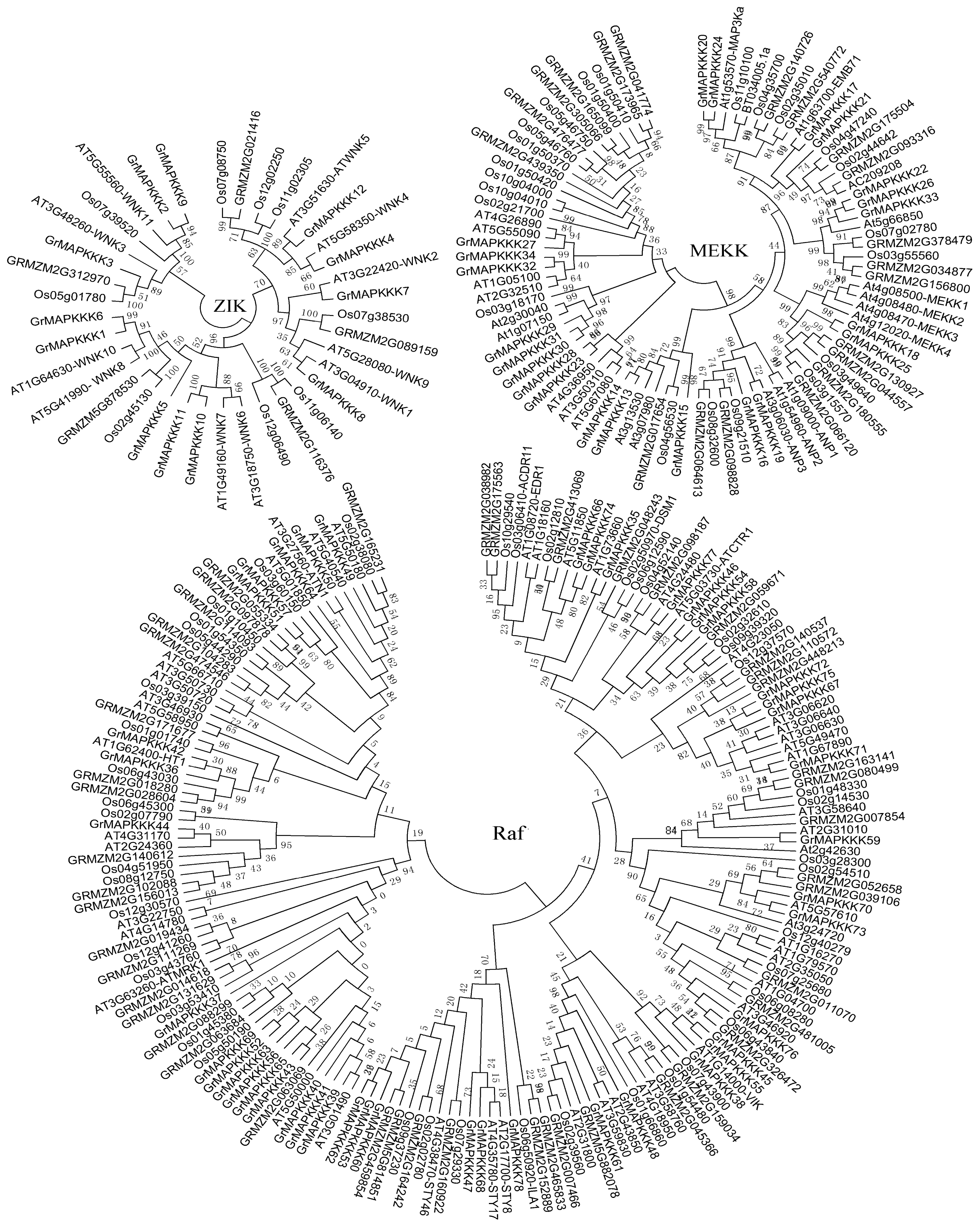
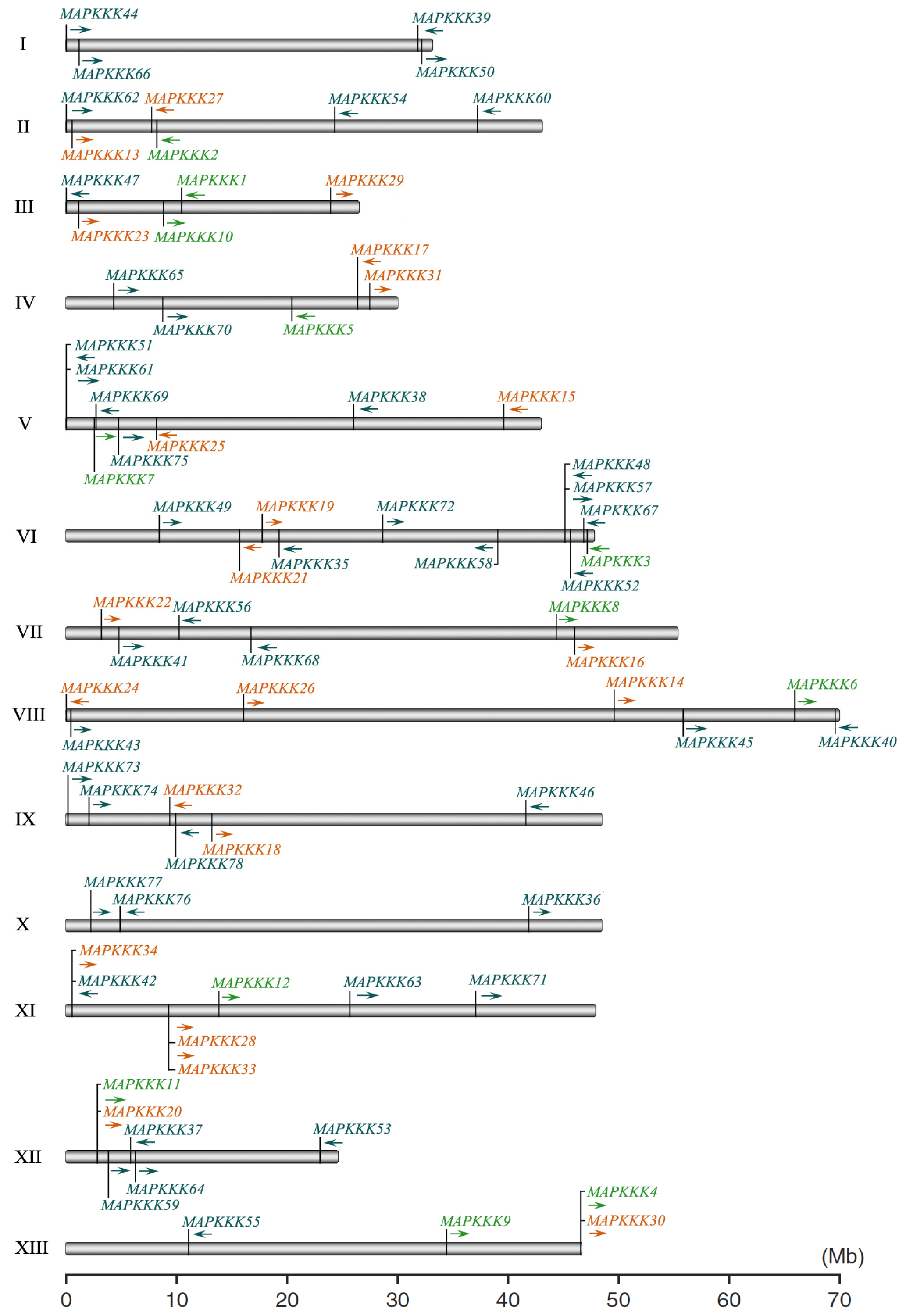
2.2. Phylogenetic Analysis and Genomic Distribution of GrMAPKKKs
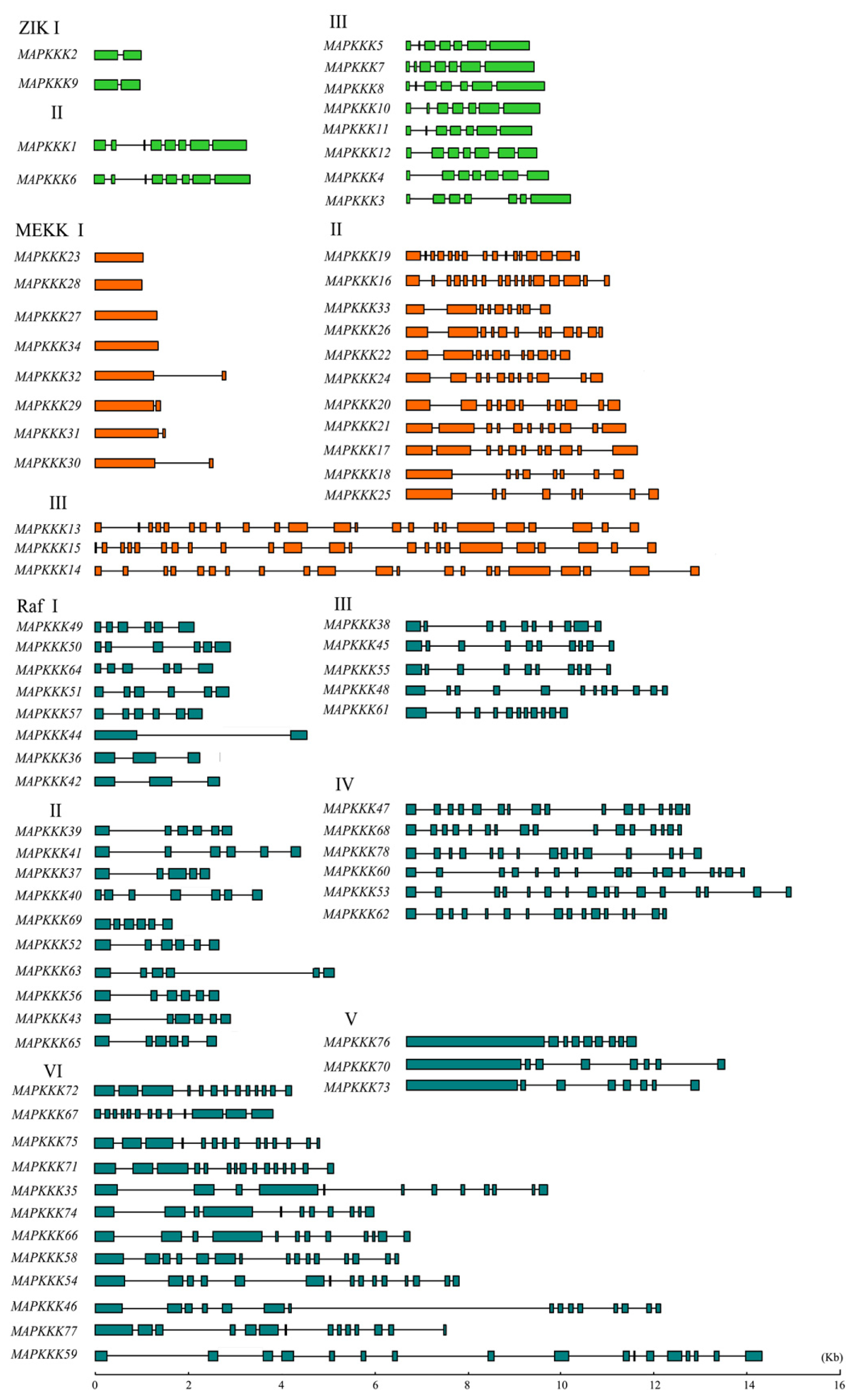
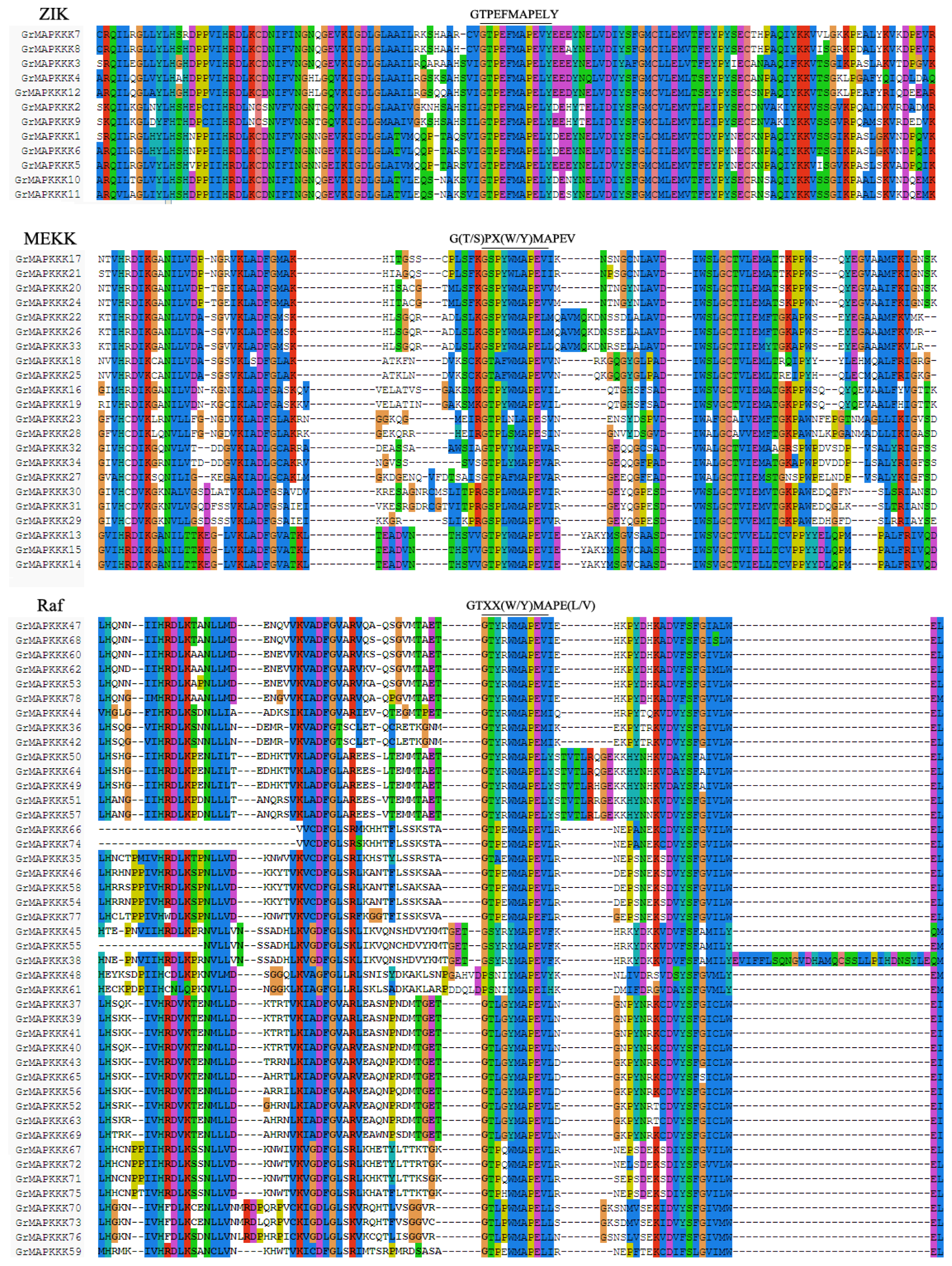
2.3. Gene Structural Organization and Domain Analysis of GrMAPKKKs
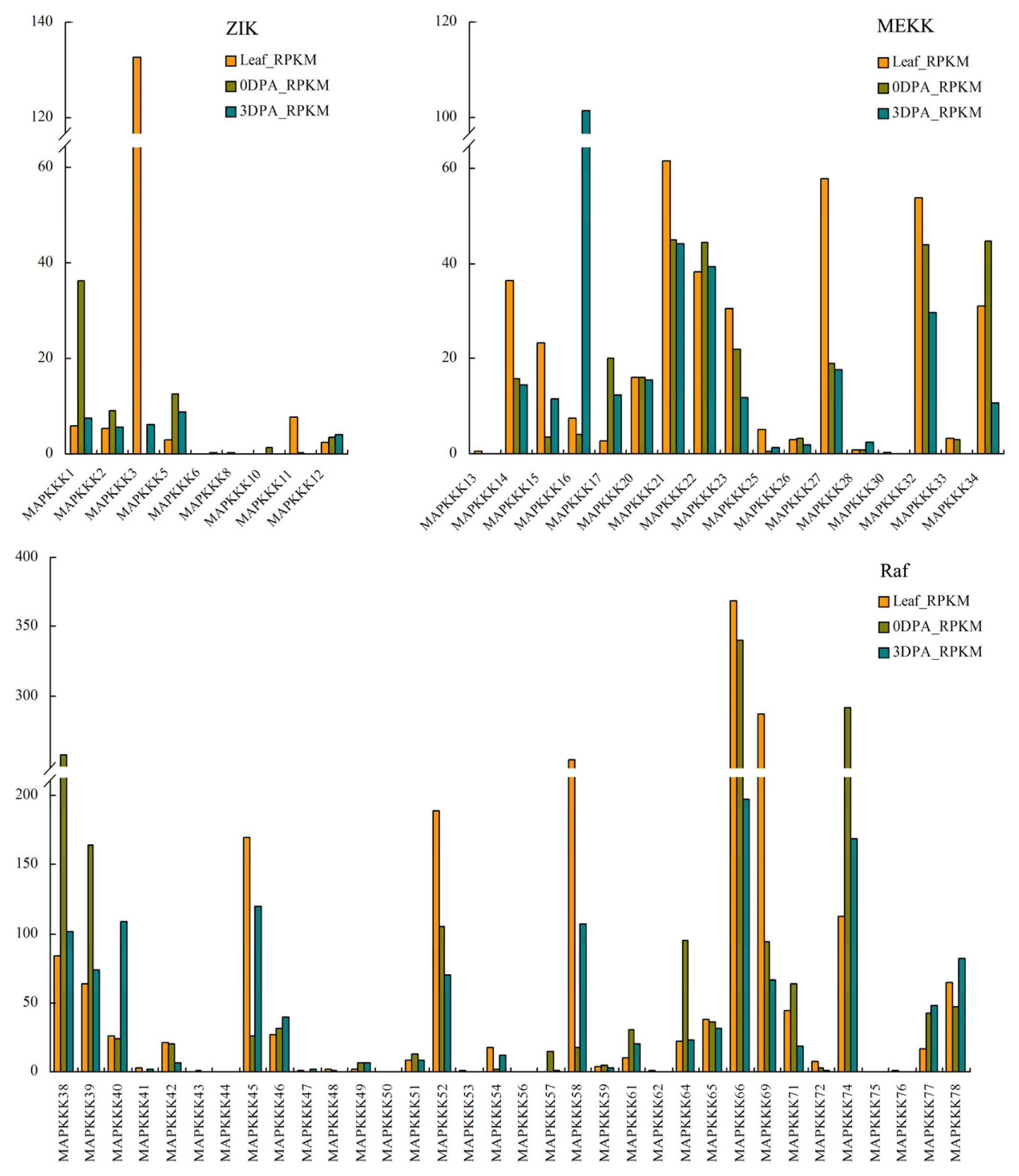
2.4. In Silico Analysis of Expression of MAPKKKs Based on Transcriptome Sequencing Data
3. Experimental Section
3.1. Sequences Data and Database Search
3.2. pI, Mw and Subcellular Localization Predictions for MAPKKKs
3.3. Chromosomal Locations and Gene Structure Analysis
3.4. Phylogenetic Analysis and Gene Duplication of MAPKKK Genes
3.5. Expression Analyses of the MAPKKK Genes
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Nishihama, R.; Banno, H.; Shibata, W.; Hirano, K.; Nakashima, M.; Usami, S.; Machida, Y. Plant homologues of components of MAPK (mitogen-activated protein kinase) signal pathways in yeast and animal cells. Plant Cell Physiol 1995, 36, 749–757. [Google Scholar]
- Mishra, N.S.; Tuteja, R.; Tuteja, N. Signaling through MAP kinase networks in plants. Arch. Biochem. Biophys 2006, 452, 55–68. [Google Scholar]
- Opdenakker, K.; Remans, T.; Vangronsveld, J.; Cuypers, A. Mitogen-activated protein (MAP) kinases in plant metal stress: Regulation and responses in comparison to other biotic and abiotic stresses. Int. J. Mol. Sci 2012, 13, 7828–7853. [Google Scholar]
- Rodriguez, M.C.; Petersen, M.; Mundy, J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol 2010, 61, 621–649. [Google Scholar]
- Yang, S.H.; Sharrocks, A.D.; Whitmarsh, A.J. Transcriptional regulation by the MAP kinase signaling cascades. Gene 2003, 320, 3–21. [Google Scholar]
- Whitmarsh, A.J. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim. Biophys. Acta 2007, 1773, 1285–1298. [Google Scholar]
- Rodriguez, J.; Crespo, P. Working without kinase activity: Phosphotransfer-independent functions of extracellular signal-regulated kinases. Sci. Sign. 2011, 4, re3. [Google Scholar]
- Taj, G.; Agarwal, P.; Grant, M.; Kumar, A. MAPK machinery in plants: Recognition and response to different stresses through multiple signal transduction pathways. Plant Sign. Behave 2010, 5, 1370–1378. [Google Scholar]
- Kieber, J.J.; Rothenberg, M.; Roman, G.; Feldmann, K.A.; Ecker, J.R. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 1993, 72, 427–441. [Google Scholar]
- Frye, C.A.; Tang, D.; Innes, R.W. Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 373–378. [Google Scholar]
- Testerink, C.; Larsen, P.B.; van der Does, D.; van Himbergen, J.A.; Munnik, T. Phosphatidic acid binds to and inhibits the activity of Arabidopsis CTR1. J. Exp. Bot 2007, 58, 3905–3914. [Google Scholar]
- Yoo, S.D.; Sheen, J. MAPK signaling in plant hormone ethylene signal transduction. Plant Sign. Behav 2008, 3, 848–849. [Google Scholar]
- Tang, D.; Christiansen, K.M.; Innes, R.W. Regulation of plant disease resistance, stress responses, cell death, and ethylene signaling in Arabidopsis by the EDR1 protein kinase. Plant Physiol 2005, 138, 1018–1026. [Google Scholar]
- Kim, J.A.; Cho, K.; Singh, R.; Jung, Y.H.; Jeong, S.H.; Kim, S.H.; Lee, J.E.; Cho, Y.S.; Agrawal, G.K.; Rakwal, R.; et al. Rice OsACDR1 (Oryza sativa accelerated cell death and resistance 1) is a potential positive regulator of fungal disease resistance. Mol. Cells 2009, 28, 431–439. [Google Scholar]
- Ning, J.; Li, X.; Hicks, L.M.; Xiong, L. A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol 2010, 152, 876–890. [Google Scholar]
- Ning, J.; Zhang, B.; Wang, N.; Zhou, Y.; Xiong, L. Increased leaf angle1, a Raf-like MAPKKK that interacts with a nuclear protein family, regulates mechanical tissue formation in the Lamina joint of rice. Plant Cell 2011, 23, 4334–4347. [Google Scholar]
- Jin, H.; Axtell, M.J.; Dahlbeck, D.; Ekwenna, O.; Zhang, S.; Staskawicz, B.; Baker, B. NPK1, an MEKK1-like mitogen-activated protein kinase kinase kinase, regulates innate immunity and development in plants. Dev. Cell 2002, 3, 291–297. [Google Scholar]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar]
- Mizoguchi, T.; Irie, K.; Hirayama, T.; Hayashida, N.; Yamaguchi-Shinozaki, K.; Matsumoto, K.; Shinozaki, K. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 765–769. [Google Scholar]
- Pitzschke, A.; Djamei, A.; Bitton, F.; Hirt, H. A major role of the MEKK1-MKK1/2-MPK4 pathway in ROS signalling. Mol. Plant 2009, 2, 120–137. [Google Scholar]
- Melech-Bonfil, S.; Sessa, G. Tomato MAPKKKɛ is a positive regulator of cell-death signaling networks associated with plant immunity. Plant J 2010, 64, 379–391. [Google Scholar]
- Del Pozo, O.; Pedley, K.F.; Martin, G.B. MAPKKKα is a positive regulator of cell death associated with both plant immunity and disease. EMBO J 2004, 23, 3072–3082. [Google Scholar]
- Wrzaczek, M.; Hirt, H. Plant MAP kinase pathways: How many and what for? Biol. Cell 2001, 93, 81–87. [Google Scholar]
- Rao, K.P.; Richa, T.; Kumar, K.; Raghuram, B.; Sinha, A.K. In silico analysis reveals 75 members of mitogen-activated protein kinase kinase kinase gene family in rice. DNA Res 2010, 17, 139–153. [Google Scholar]
- Kong, X.; Lv, W.; Zhang, D.; Jiang, S.; Zhang, S.; Li, D. Genome-wide identification and analysis of expression profiles of maize mitogen-activated protein kinase kinase kinase. PLoS One 2013, 8, e57714. [Google Scholar]
- Paterson, A.H.; Wendel, J.F.; Gundlach, H.; et al. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature 2012, 492, 423–427. [Google Scholar]
- Wang, K.; Wang, Z.; Li, F.; Ye, W.; Wang, J.; Song, G.; Yue, Z.; Cong, L.; Shang, H.; Zhu, S.; et al. The draft genome of a diploid cotton Gossypium raimondii. Nat. Genet 2012, 44, 1098–1103. [Google Scholar]
- Ichimura, K. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002, 7, 301–308. [Google Scholar]
- Chou, K.C.; Shen, H.B. Recent progress in protein subcellular location prediction. Anal. Biochem 2007, 370, 1–16. [Google Scholar]
- Takabatake, R.; Ando, Y.; Seo, S.; Katou, S.; Tsuda, S.; Ohashi, Y.; Mitsuhara, I. MAP kinases function downstream of HSP90 and upstream of mitochondria in TMV resistance gene N-mediated hypersensitive cell death. Plant Cell Physiol 2007, 48, 498–510. [Google Scholar]
- Schauser, L.; Wieloch, W.; Stougaard, J. Evolution of NIN-like proteins in Arabidopsis, rice, and Lotus japonicus. J. Mol. Evolut 2005, 60, 229–237. [Google Scholar]
- Urano, D.; Phan, N.; Jones, J.C.; Yang, J.; Huang, J.; Grigston, J.; Taylor, J.P.; Jones, A.M. Endocytosis of the seven-transmembrane RGS1 protein activates G-protein-coupled signalling in Arabidopsis. Nat. Cell Biol 2012, 14, 1079–1088. [Google Scholar]
- Wang, Y.; Liu, K.; Liao, H.; Zhuang, C.; Ma, H.; Yan, X. The plant WNK gene family and regulation of flowering time in Arabidopsis. Plant Biol 2008, 10, 548–562. [Google Scholar]
- Krysan, P.J.; Jester, P.J.; Gottwald, J.R.; Sussman, M.R. An Arabidopsis mitogen-activated protein kinase kinase kinase gene family encodes essential positive regulators of cytokinesis. Plant Cell 2002, 14, 1109–1120. [Google Scholar]
- Muller, J.; Beck, M.; Mettbach, U.; Komis, G.; Hause, G.; Menzel, D.; Samaj, J. Arabidopsis MPK6 is involved in cell division plane control during early root development, and localizes to the pre-prophase band, phragmoplast, trans-Golgi network and plasma membrane. Plant J 2010, 61, 234–248. [Google Scholar]
- Beck, M.; Komis, G.; Ziemann, A.; Menzel, D.; Samaj, J. Mitogen-activated protein kinase 4 is involved in the regulation of mitotic and cytokinetic microtubule transitions in Arabidopsis thaliana. New Phytol 2011, 189, 1069–1083. [Google Scholar]
- Clark, K.L.; Larsen, P.B.; Wang, X.; Chang, C. Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc. Natl. Acad. Sci. USA 1998, 95, 5401–5406. [Google Scholar]
- Huang, Y.; Li, H.; Hutchison, C.E.; Laskey, J.; Kieber, J.J. Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J 2003, 33, 221–233. [Google Scholar]
- Shi, Y.H.; Zhu, S.W.; Mao, X.Z.; Feng, J.X.; Qin, Y.M.; Zhang, L.; Cheng, J.; Wei, L.P.; Wang, Z.Y.; Zhu, Y.X. Transcriptome profiling, molecular biological, and physiological studies reveal a major role for ethylene in cotton fiber cell elongation. Plant Cell 2006, 18, 651–664. [Google Scholar]
- NCBI. Available online: http://www.ncbi.nlm.nih.gov/ (accessed on 29 December 2012).
- Cotton Genome Project. Available online: http://cgp.genomics.org.cn/page/species/index.jsp/ (accessed on 29 December 2012).
- The Arabidopsis Information Resource. Available online: http://www.arabidopsis.org/ (accessed on 9 January 2013).
- Rice Genome Annotation Project. Available online: ftp://ftp.plantbiology.msu.edu/ (accessed on 10 January 2013).
- PROSITE program. ExPASy server. Available online: http://www.expasy.ch/prosite/ (accessed on 19 June 2013).
- Compute pI/Mw tool. ExPASy server. Available online: http://web.expasy.org/compute_pi/ (accessed on 19 June 2013).
- Bjellqvist, B.; Hughes, G.J.; Pasquali, C.; Paquet, N.; Ravier, F.; Sanchez, J.C.; Frutiger, S.; Hochstrasser, D. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 1993, 14, 1023–1031. [Google Scholar]
- CELLO v2.5. Available online: http://cello.life.nctu.edu.tw/ (accessed on 29 June 2013).
- ClustalW2. Available online: http://www.ebi.ac.uk/Tools/msa/clustalw2/ (accessed on 29 June 2013).
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar]
| Gene name | Accession number | AA | pI | Mw | Predicted subcellular localization |
|---|---|---|---|---|---|
| GrMAPKKK1 | Cotton_D_gene_10030976 | 705 | 5.24 | 79.82 | Nuclear |
| GrMAPKKK2 | Cotton_D_gene_10024942 | 294 | 5.37 | 33.68 | Nuclear |
| GrMAPKKK3 | Cotton_D_gene_10021384 | 613 | 5.09 | 69.23 | Nuclear |
| GrMAPKKK4 | Cotton_D_gene_10025360 | 592 | 6.19 | 67.33 | Nuclear |
| GrMAPKKK5 | Cotton_D_gene_10025689 | 668 | 4.96 | 75.59 | Nuclear |
| GrMAPKKK6 | Cotton_D_gene_10016819 | 686 | 5.17 | 77.71 | Nuclear |
| GrMAPKKK7 | Cotton_D_gene_10011135 | 734 | 4.99 | 83.01 | Nuclear |
| GrMAPKKK8 | Cotton_D_gene_10007538 | 727 | 5.47 | 83.62 | Nuclear |
| GrMAPKKK9 | Cotton_D_gene_10006903 | 299 | 5.21 | 34.12 | Nuclear |
| GrMAPKKK10 | Cotton_D_gene_10028727 | 643 | 5.56 | 72.19 | Nuclear |
| GrMAPKKK11 | Cotton_D_gene_10019741 | 609 | 5.25 | 67.84 | Nuclear |
| GrMAPKKK12 | Cotton_D_gene_10036331 | 593 | 5.36 | 67.67 | Nuclear |
| GrMAPKKK13 | Cotton_D_gene_10018040 | 1370 | 5.96 | 150.94 | Nuclear |
| GrMAPKKK14 | Cotton_D_gene_10034692 | 1399 | 5.86 | 154.46 | Nuclear |
| GrMAPKKK15 | Cotton_D_gene_10029669 | 1397 | 5.94 | 153.50 | Nuclear |
| GrMAPKKK16 | Cotton_D_gene_10017021 | 662 | 5.79 | 72.69 | Nuclear |
| GrMAPKKK17 | Cotton_D_gene_10001555 | 897 | 9.22 | 96.45 | Nuclear |
| GrMAPKKK18 | Cotton_D_gene_10002230 | 577 | 5.41 | 64.07 | Nuclear |
| GrMAPKKK19 | Cotton_D_gene_10003410 | 643 | 6.06 | 70.78 | Nuclear |
| GrMAPKKK20 | Cotton_D_gene_10019751 | 661 | 9.20 | 71.56 | Nuclear |
| GrMAPKKK21 | Cotton_D_gene_10030510 | 896 | 9.29 | 96.69 | Nuclear |
| GrMAPKKK22 | Cotton_D_gene_10032983 | 711 | 8.99 | 78.75 | Nuclear |
| GrMAPKKK23 | Cotton_D_gene_10008602 | 338 | 4.97 | 37.35 | Cytoplasmic |
| GrMAPKKK24 | Cotton_D_gene_10038046 | 660 | 9.05 | 71.32 | Nuclear |
| GrMAPKKK25 | Cotton_D_gene_10039321 | 576 | 5.63 | 63.66 | Nuclear |
| GrMAPKKK26 | Cotton_D_gene_10040437 | 742 | 8.68 | 81.55 | Nuclear |
| GrMAPKKK27 | Cotton_D_gene_10024896 | 437 | 4.65 | 48.84 | Cytoplasmic |
| GrMAPKKK28 | Cotton_D_gene_10030314 | 336 | 5.78 | 36.99 | Cytoplasmic |
| GrMAPKKK29 | Cotton_D_gene_10000305 | 466 | 7.85 | 52.12 | Chloroplast |
| GrMAPKKK30 | Cotton_D_gene_10025330 | 443 | 5.51 | 49.41 | Nuclear |
| GrMAPKKK31 | Cotton_D_gene_10006972 | 495 | 5.63 | 55.42 | Cytoplasmic |
| GrMAPKKK32 | Cotton_D_gene_10033856 | 438 | 5.49 | 48.15 | Nuclear |
| GrMAPKKK33 | Cotton_D_gene_10030328 | 613 | 8.47 | 67.53 | Nuclear |
| GrMAPKKK34 | Cotton_D_gene_10031221 | 446 | 5.58 | 49.38 | Cytoplasmic |
| GrMAPKKK35 | Cotton_D_gene_10040125 | 986 | 5.25 | 108.05 | Nuclear |
| GrMAPKKK36 | Cotton_D_gene_10035121 | 371 | 9.14 | 42.42 | Nuclear |
| GrMAPKKK37 | Cotton_D_gene_10027896 | 374 | 7.92 | 41.92 | Nuclear |
| GrMAPKKK38 | Cotton_D_gene_10037878 | 460 | 8.63 | 51.88 | Mitochondrial |
| GrMAPKKK39 | Cotton_D_gene_10019447 | 381 | 7.92 | 42.44 | Nuclear |
| GrMAPKKK40 | Cotton_D_gene_10030570 | 399 | 5.42 | 44.40 | Nuclear |
| GrMAPKKK41 | Cotton_D_gene_10032760 | 377 | 7.94 | 42.04 | Nuclear |
| GrMAPKKK42 | Cotton_D_gene_10031200 | 374 | 8.92 | 42.57 | Nuclear |
| GrMAPKKK43 | Cotton_D_gene_10038072 | 420 | 7.89 | 46.91 | Cytoplasmic |
| GrMAPKKK44 | Cotton_D_gene_10012774 | 415 | 8.11 | 46.35 | Chloroplast |
| GrMAPKKK45 | Cotton_D_gene_10014886 | 427 | 8.62 | 48.02 | Mitochondrial |
| GrMAPKKK46 | Cotton_D_gene_10028168 | 849 | 6.13 | 94.22 | Nuclear |
| GrMAPKKK47 | Cotton_D_gene_10011865 | 609 | 5.85 | 68.46 | Cytoplasmic |
| GrMAPKKK48 | Cotton_D_gene_10000379 | 540 | 9.03 | 61.20 | Mitochondrial |
| GrMAPKKK49 | Cotton_D_gene_10010262 | 351 | 8.09 | 39.70 | Cytoplasmic |
| GrMAPKKK50 | Cotton_D_gene_10019394 | 354 | 6.57 | 39.98 | Cytoplasmic |
| GrMAPKKK51 | Cotton_D_gene_10002587 | 353 | 8.92 | 39.42 | Cytoplasmic |
| GrMAPKKK52 | Cotton_D_gene_10035493 | 391 | 8.38 | 43.63 | Cytoplasmic |
| GrMAPKKK53 | Cotton_D_gene_10025598 | 563 | 5.91 | 63.78 | Cytoplasmic |
| GrMAPKKK54 | Cotton_D_gene_10013051 | 857 | 6.18 | 94.66 | Nuclear |
| GrMAPKKK55 | Cotton_D_gene_10033568 | 402 | 8.42 | 44.93 | Mitochondrial |
| GrMAPKKK56 | Cotton_D_gene_10035982 | 391 | 8.35 | 43.59 | Nuclear |
| GrMAPKKK57 | Cotton_D_gene_10035556 | 355 | 8.82 | 39.84 | Cytoplasmic |
| GrMAPKKK58 | Cotton_D_gene_10003920 | 860 | 6.49 | 95.59 | Nuclear |
| GrMAPKKK60 | Cotton_D_gene_10005866 | 575 | 5.71 | 65.08 | Cytoplasmic |
| GrMAPKKK61 | Cotton_D_gene_10023083 | 477 | 9.15 | 54.17 | Mitochondrial |
| GrMAPKKK62 | Cotton_D_gene_10018183 | 552 | 6.09 | 62.28 | Cytoplasmic |
| GrMAPKKK63 | Cotton_D_gene_10031629 | 391 | 8.65 | 43.44 | Nuclear |
| GrMAPKKK64 | Cotton_D_gene_10019101 | 352 | 5.98 | 39.79 | Cytoplasmic |
| GrMAPKKK65 | Cotton_D_gene_10003024 | 381 | 7.05 | 42.54 | Cytoplasmic |
| GrMAPKKK66 | Cotton_D_gene_10015094 | 904 | 5.45 | 100.46 | Cytoplasmic |
| GrMAPKKK67 | Cotton_D_gene_10021266 | 782 | 6.87 | 86.35 | Nuclear |
| GrMAPKKK68 | Cotton_D_gene_10034448 | 576 | 6.26 | 65.42 | Nuclear |
| GrMAPKKK69 | Cotton_D_gene_10005280 | 383 | 8.19 | 42.40 | Cytoplasmic |
| GrMAPKKK70 | Cotton_D_gene_10028280 | 1137 | 5.56 | 126.21 | Nuclear |
| GrMAPKKK71 | Cotton_D_gene_10015701 | 848 | 5.88 | 94.81 | Nuclear |
| GrMAPKKK72 | Cotton_D_gene_10012401 | 765 | 6.54 | 84.93 | Nuclear |
| GrMAPKKK73 | Cotton_D_gene_10016031 | 1107 | 5.46 | 122.84 | Cytoplasmic |
| GrMAPKKK74 | Cotton_D_gene_10037238 | 854 | 5.18 | 94.24 | Cytoplasmic |
| GrMAPKKK75 | Cotton_D_gene_10005797 | 743 | 8.17 | 82.52 | Nuclear |
| GrMAPKKK76 | Cotton_D_gene_10004434 | 1360 | 5.18 | 147.72 | Nuclear |
| GrMAPKKK77 | Cotton_D_gene_10011720 | 879 | 6.80 | 97.86 | Nuclear |
| GrMAPKKK78 | Cotton_D_gene_10033954 | 535 | 6.43 | 60.56 | Cytoplasmic |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yin, Z.; Wang, J.; Wang, D.; Fan, W.; Wang, S.; Ye, W. The MAPKKK Gene Family in Gossypium raimondii: Genome-Wide Identification, Classification and Expression Analysis. Int. J. Mol. Sci. 2013, 14, 18740-18757. https://doi.org/10.3390/ijms140918740
Yin Z, Wang J, Wang D, Fan W, Wang S, Ye W. The MAPKKK Gene Family in Gossypium raimondii: Genome-Wide Identification, Classification and Expression Analysis. International Journal of Molecular Sciences. 2013; 14(9):18740-18757. https://doi.org/10.3390/ijms140918740
Chicago/Turabian StyleYin, Zujun, Junjuan Wang, Delong Wang, Weili Fan, Shuai Wang, and Wuwei Ye. 2013. "The MAPKKK Gene Family in Gossypium raimondii: Genome-Wide Identification, Classification and Expression Analysis" International Journal of Molecular Sciences 14, no. 9: 18740-18757. https://doi.org/10.3390/ijms140918740




