Emulsifying Activity and Stability of a Non-Toxic Bioemulsifier Synthesized by Microbacterium sp. MC3B-10
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Microbactan
2.2. Emulsifying Potential
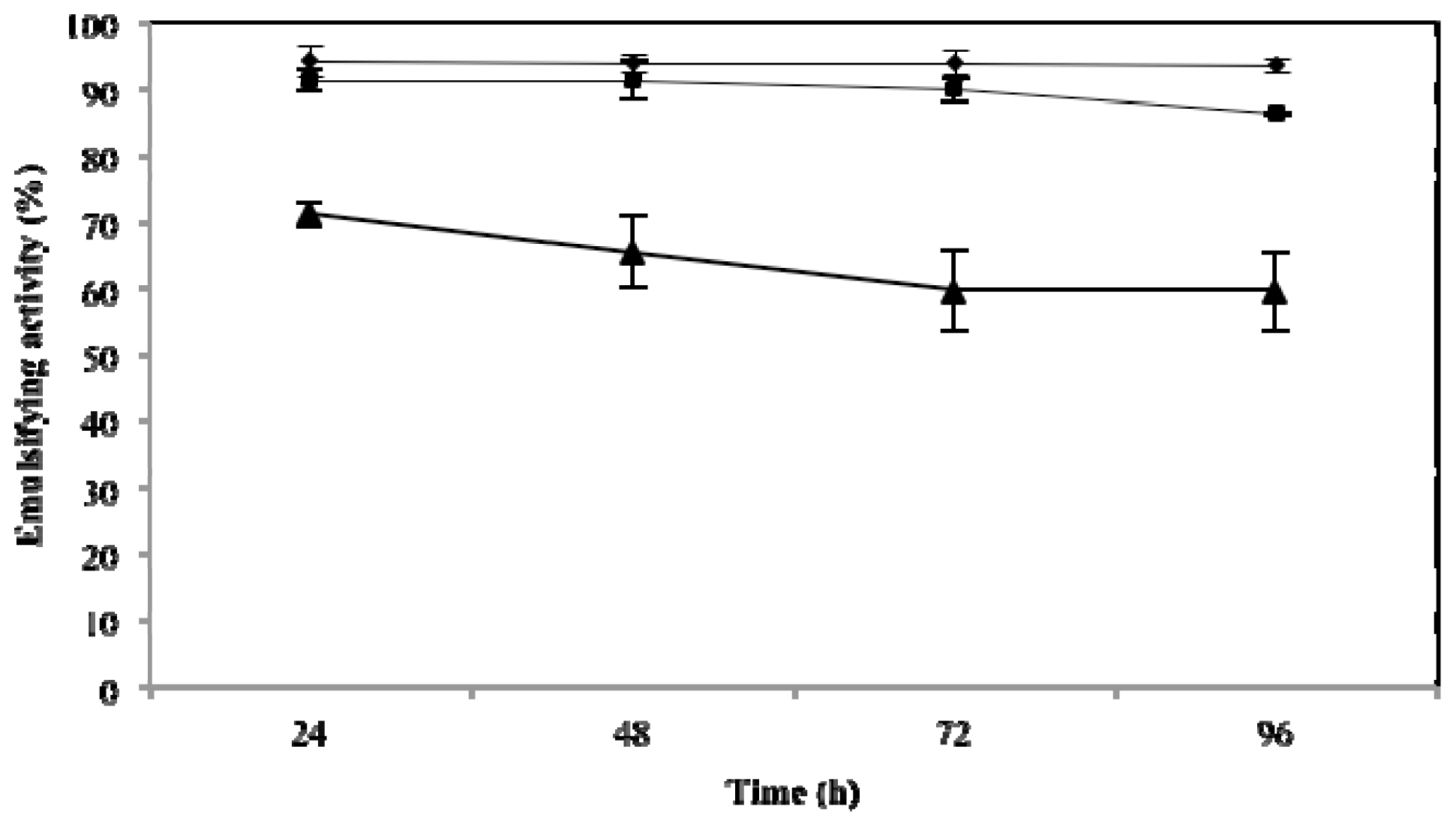
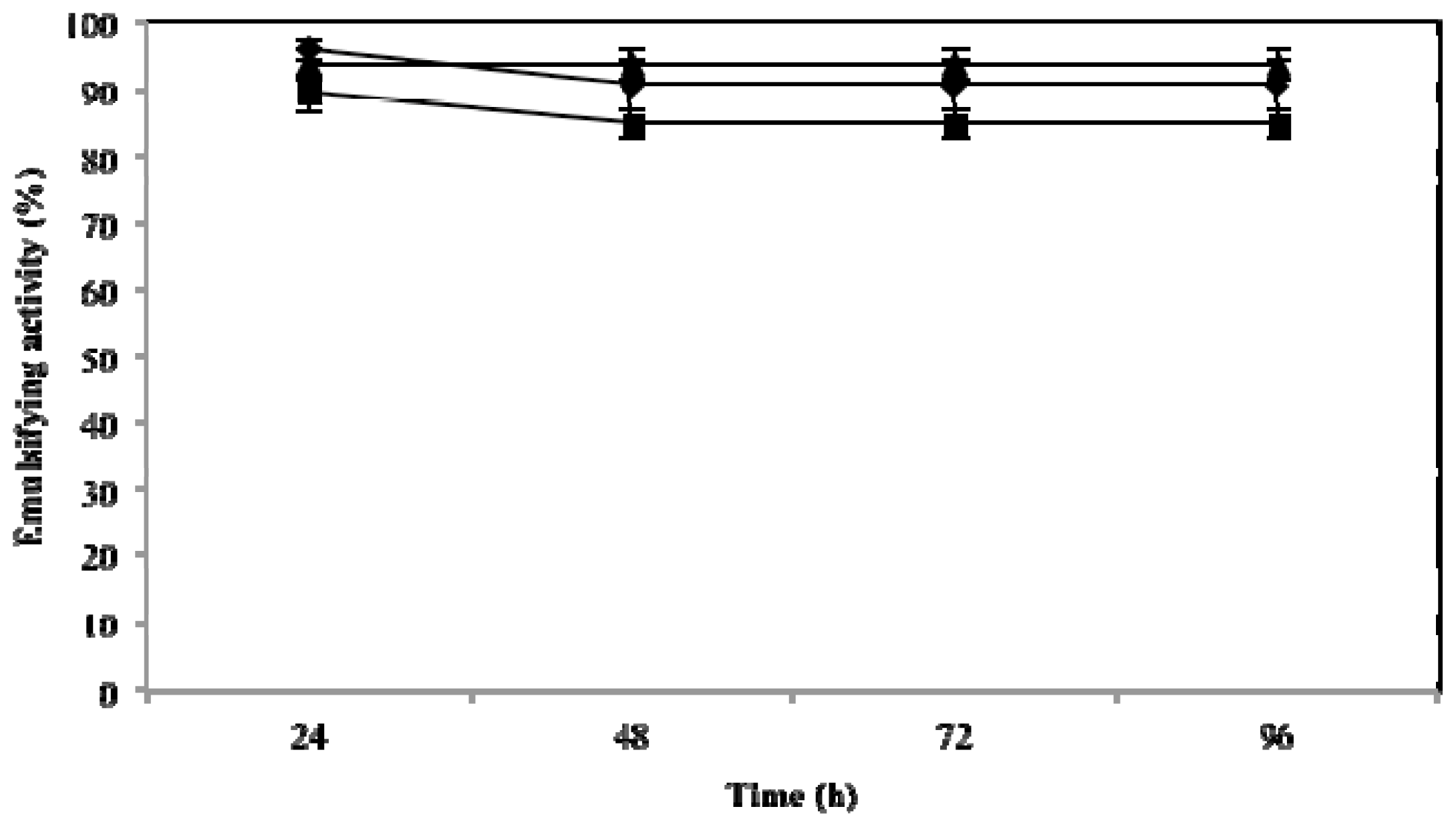
2.3. Effect of Temperature, pH and NaCl on Emulsifying Activity
2.4. Toxicity of Microbactan
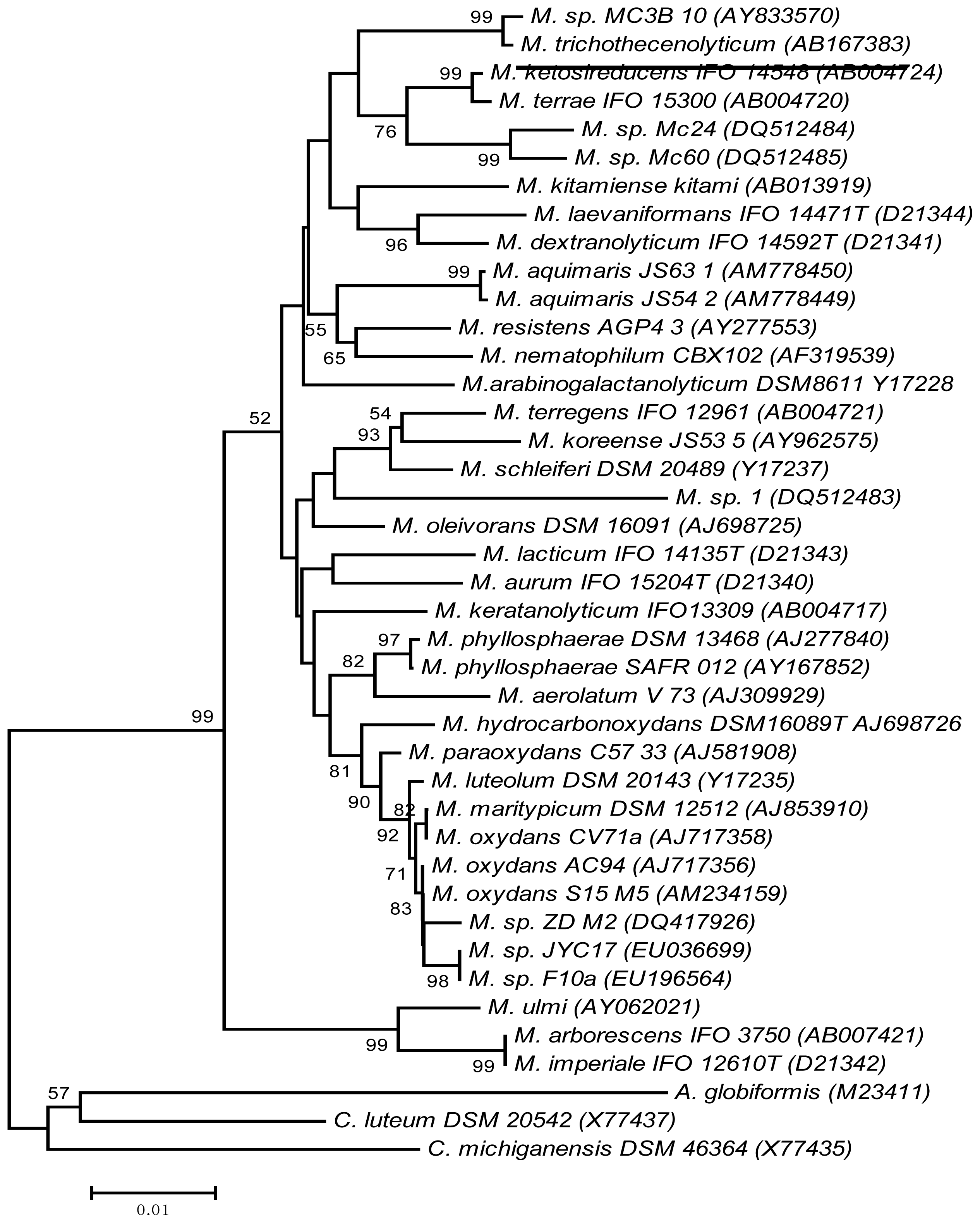
2.5. Phylogenetic Reassessment of Microbacterium sp. MC3B-10
3. Experimental Section
3.1. Production of Microbactan
3.2. Lipid Analysis and Fatty Acid Composition
3.3. High Performance Size Exclusion Chromatography
3.4. Ionic Charge
3.5. Emulsifying Activity
3.6. Effects of Temperature, Salinity and pH on Emulsifying Activity
3.7. Toxicity Test
3.8. Statistical Analysis
3.9. Phylogenetic Reassessment
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ron, E.; Rosenberg, E. Natural roles of biosurfactants. Environ. Microbiol 2001, 3, 229–236. [Google Scholar]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.; Fracchia, L.; Smyth, T.; Marchant, R. Microbial biosurfactants, production, applications and future potential. Appl. Microbiol. Biotechnol 2010, 87, 427–444. [Google Scholar]
- Freitas, F.; Alves, V.; Reis, M. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol 2011, 29, 388–398. [Google Scholar]
- Freitas, F.; Alves, V.; Carvalheira, M.; Costa, N.; Oliveira, R.; Reis, M. Emulsifying behaviour and rheological properties of the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohyd. Polym 2009, 78, 549–556. [Google Scholar]
- Satpute, S.; Banat, I.; Dhakephalkar, P.; Banpurkar, A.; Chopade, B. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv 2010, 28, 436–450. [Google Scholar]
- Gutiérrez, T.; Leo, V.; Walker, G.; Green, D. Emulsifying properties of a glycoprotein extract produced by a marine Flexibacter species strain TG382. Enzyme Microb. Technol 2009, 45, 53–57. [Google Scholar]
- Ortega-Morales, B.O.; Chan-Bacab, M.J.; de la Rosa-García, S.C.; Camacho-Chab, J.C. Valuable processes and products from marine intertidal microbial communities. Curr. Opin. Biotechnol 2010, 21, 346–352. [Google Scholar]
- Ortega-Morales, B.O.; Santiago, J.; Chan-Bacab, M.J.; Miranda, E.; Fardeau, M.; Carrero, J.; Bartolo, P.; Valadéz, A.; Guezennec, J. Characterization of extracellular polymers synthesized by tropical intertidal biofilm bacteria. J. Appl. Microbiol 2007, 102, 254–264. [Google Scholar]
- Beech, I.; Hanjagsit, L.; Kalaji, M.; Neal, A.L.; Zinkevich, V. Chemical and structural characterization of exopolymers produced by Pseudomonas sp. NCIMB 2021 in continuous culture. Microbiology 1999, 145, 1491–1497. [Google Scholar]
- Amaral, P.; Silva, J.; Lehocky, M.; Barros-Timmons, A.; Coelho, M.; Marrucho, I.; Coutinho, J.A.P. Production and characterization of a bioemulsiftier from Yarrowia lipolytica. Process Biochem 2006, 41, 1894–1898. [Google Scholar]
- Luna-Velasco, M.A.; Esparza-García, F.; Cansares-Villanueva, R.O.; Rodríguez-Vázquez, R. Production and properties of a bioemulsifier synthesized by phenanthrene-degrading Penicillum sp. Process Biochem 2007, 42, 310–314. [Google Scholar]
- Maier, R.M. Biosurfactants: Evolution and diversity in bacteria. Adv. Appl. Microbiol 2003, 52, 101–121. [Google Scholar]
- Navon-Venezia, S.; Zosim, Z.; Gottlieb, A.; Legmann, R.; Carmeli, S.; Ron, E.Z.; Rosenberg, E. Alasan, a new bioemulsifier from Acinetobacter radioresistens. Appl. Environ. Microb 1995, 61, 3240–3244. [Google Scholar]
- Thavasi, R.; Jayalakshmi, S.; Balasubramanian, T.; Banat, I. Biosurfactant production by Corynebacterium kutscheri from waste motor lubricant oil and peanut oil cake. Lett. Appl. Microbiol 2007, 45, 686–691. [Google Scholar]
- Thavasi, R.; Jayalakshmi, S.; Balasubramanian, T.; Banat, I. Production and characterization of a glycolipid biosurfactant from Bacillus megaterium using economically cheaper sources. World J. Microbiol. Biotechnol 2007, 24, 917–925. [Google Scholar]
- Whitman, W. Food Emulsifiers: Chemistry, Technology, Functional Properties and Applications; Ten Alps Publishing: London, UK, 1990. [Google Scholar]
- Kim, S.; Ravichandran, D.; Khan, S.; Kim, Y. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioprocess Eng 2008, 13, 511–523. [Google Scholar]
- Kumar, A.; Mody, K.; Jha, B. Evaluation of biosurfactant/bioemulsifier production by a marine bacterium. Bull. Environ. Contam. Toxicol 2007, 79, 617–621. [Google Scholar]
- Willumsen, P.A.; Karlson, U. Screening of bacteria, isolated from PAH-contaminated soils, for production of biosurfactants and bioemulsifiers. Biodegradation 1997, 7, 415–423. [Google Scholar]
- Gutiérrez, T.; Mulloy, B.; Bavington, C.; Black, K.; Green, D. Partial purification and chemical characterization of a glycoprotein (putative hydrocolloid) emulsifier produced by a marine bacterium Antarctobacter. Appl. Microbiol. Biotechnol. 2007, 76, 1017–1026. [Google Scholar]
- Perfumo, A.; Smyth, T.J.; Marchant, R.; Banat, I.M. Production and Roles of Biosurfactants and Bioemulsifiers in Accessing Hydrophobic Substrates. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer-Verlag: Berlin, Germany, 2010; pp. 1502–1510. [Google Scholar]
- Sarubbo, L.; Moura, J.; Campos-Takaki, G. Production and stability studies of the bioemulsifier obtained from a new strain of Candida glabrata UCP 1002. Electron. J. Biotechnol 2006, 9, 400–406. [Google Scholar]
- Rufino, R.; Sarubbo, A.; Campos, G. Enhancement of stability of biosurfactant produced by Candida lipolytica using industrial residue as substrate. World J. Microb. Biotechnol 2007, 23, 729–734. [Google Scholar]
- Ilori, M.O.; Amobi, C.; Odocha, A. Factors affecting biosurfactant production by oil degrading Aeromonas spp. isolated from a tropical environment. Chemosphere 2005, 61, 985–992. [Google Scholar]
- Sarubbo, L.; Farias, C.; Campos-Takaki, G. Co-utilization of canola oil and glucose on the production of a surfactant by Candida lipolytica. Curr. Microbiol 2007, 54, 68–73. [Google Scholar]
- Rosenberg, E.; Rubinovitz, C.; Gottlieb, A.; Rosenhak, S.; Ron, E.Z. Production of biodispersan by Acinetobacter calcoaceticus A2. Appl. Environ. Microb 1988, 54, 317–322. [Google Scholar]
- Pelletier, E.; Delille, D.; Delille, B. Crude oil bioremediation in sub-Antarctic intertidal sediments: Chemistry and toxicity of oiled residues. Mar. Environ. Res 2004, 57, 311–327. [Google Scholar]
- Weyermann, J.; Lochmann, D.; Zimmer, A. A practical note on the use of cytotoxicity assays. Int. J. Pharm 2005, 288, 369–376. [Google Scholar]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLauglin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta. Med 1982, 45, 31–34. [Google Scholar]
- McLaughlin, J.L. Crown-gall Tumors in Potato Discs and Brine Shrimp Lethality: Two Simple Bioassays for Higher Plant Screening and Fractionation. In Methods in Plant Biochemistry; Hostettmann, K., Ed.; Academic Press: London, UK, 1991; Volume 6, pp. 1–32. [Google Scholar]
- Birndorf, H.C.; D’Alossio, J.; Bagshaw, J.C. DNA-dependant RNA-polymerases from Artemia, embryos. Characterization of polymerases I and II from nauplius larvae. Dev. Biol 1975, 45, 34–43. [Google Scholar]
- Perfeito, J.P.; Santos, M.L.; López, K.S.; Paula, J.E.; Silveira, D. Characterization and biological properties of Pouteria torta extracts. Rev. Bras. Farmacogn 2005, 15, 183–186. [Google Scholar]
- Stackebrandt, E.; Ebers, J. Taxonomic parameters revisited: Tarnished gold standards. Microbiol. Today 2006, 33, 152–155. [Google Scholar]
- Gneiding, K.; Frodl, R.; Funke, G. Identities of Microbacterium spp. encountered in human clinical specimens. J. Clin. Microbiol 2008, 46, 3646–3652. [Google Scholar]
- Takeuchi, M.; Hatano, K. Proposal of six new species in the genus Microbacterium and transfer of Flavobacterium marinotypicum ZoBell and Upham to the genus Microbacterium as Microbacterium maritypicum comb. nov. Int. J. Syst. Bacteriol 1998, 48, 973–982. [Google Scholar]
- Aniszewski, E.; Silva, R.; Faria, F.; Gomes, S.; Soares, A. Bioemulsifier production by Microbacterium sp. strains isolated from mangrove and their application to remove cadmiun and zinc from hazardous industrial residue. Braz. J. Microbiol 2010, 41, 235–245. [Google Scholar]
- Burmølle, M.; Webb, J.; Rao, D.; Hansen, L.; Sørensen, S.; Kjelleberg, S. Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl. Environ. Microb 2006, 72, 3916–3925. [Google Scholar]
- Schippers, A.; Bosecker, K.; Sproer, C.; Schumann, P. Microbacterium oleivorans sp. nov. and Microbacterium hydrocarbonoxydans sp. nov., novel crude-oil-degrading Gram-positive bacteria. Int. J. Syst. Evol. Microbiol 2005, 55, 655–660. [Google Scholar]
- Franzetti, A.; Gandolfi, I.; Bertolini, V.; Raimondi, C.; Piscitello, M.; Papacchini, M.; Bestetti, G. Phylogenetic characterization of bioemulsifier-producing bacteria. Int. Biodeterior. Biodegrad 2011, 65, 1095–1099. [Google Scholar]
- Izard, J.; Limberger, R. Rapid screening method for quantification of bacterial cell lipids from whole cells. J. Microbiol. Meth 2003, 55, 411–418. [Google Scholar]
- Ganfield, M.C.; Pieringer, R.A. Phosphatidylkojibiosyl diglyceride. The covalently linked lipid constituent of the membrane lipoteichoic acid from Streptococcus faecalis (faecium) ATCC 9790. J. Biol. Chem 1975, 250, 702–709. [Google Scholar]
- Rezanka, T.; Vokoun, J.; Slavicek, J.; Podojil, M. Determination of fatty acids in algae by capillary gas chromatography-mass spectrometry. J. Chromatogr. 1983, 268, 71–78. [Google Scholar]
- Van Oss, C.J. Specifically impermeable precipitate membranes formed through double diffusion in gels: Behavior with complex forming and with simple systems. J. Colloid Interf. Sci 1968, 27, 684–690. [Google Scholar]
- Ortega-Morales, B.O.; Chan-Bacab, M.J.; Miranda-Tello, E.; Fardeau, M.L.; Carrero, J.C.; Stein, T. Antifouling activity of sessile bacilli derived from marine surface. J. Ind. Microbiol. Biotechnol. 2008, 35, 9–15. [Google Scholar]
- Solís, P.; Wright, C.; Anderson, M.; Gupta, M.; Phillipson, D.A. A Microwell cytotoxic using Artemia salina (brine shrimp). Planta Med 1993, 59, 250–252. [Google Scholar]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol 2007, 24, 1596–1599. [Google Scholar]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar]

| Hydrophobic substrate | Microbactan a | Synthetic surfactants a | Biopolymers a | ||
|---|---|---|---|---|---|
| Tween 80 | Triton-X-100 | Gum arabic | Xanthan gum | ||
| Benzene | 76.9 ± 2.4 | 98.7 ± 0.6 | 54.3 ± 2.0 | 80.7 ± 3.0 | 64.7 ± 1.3 |
| Xylene | 81.5 ± 3.4 | 94.7 ± 6.1 | 98.2 ± 0.4 | 72.6 ± 3.4 | 76.8 ± 1.7 |
| Crude oil | 76.5 ± 2.1 | 82.9 ± 0.6 | 100 | 54.7 ± 3.7 | 95.0 ± 0.9 |
| Motor oil | 96.3 ± 0.1 | 54.9 ± 1.4 | 68.5 ± 6 | 95.2 ± 1.1 | 89.5 ± 3.3 |
| Sunflower oil | 84.4 ± 5.4 | 100 | 100 | 77.8 ± 1.5 | 62.4 ± 2.4 |
| Corn oil | 81.1 ± 1.6 | 100 | 100 | 0 | 90.6 ± 1.9 |
| Olive oil | 76.1 ± 0.3 | 100 | 100 | 96.6 ± 0.1 | 76.6 ± 5.4 |
| Mineral oil | 0 | 95.9 ± 5.6 | 100 | 60.3 ± 2.8 | 86.5 ± 3.8 |
| Hydrophobic substrate | Microbactan a | Synthetic surfactants a | Biopolymers a | ||
|---|---|---|---|---|---|
| Tween 80 | Triton-X-100 | Gum arabic | Xanthan gum | ||
| Benzene | 75.3 ± 3.8 | 93.2 ± 5 | 54.3 ± 2.1 | 78.9 ± 0 | 61.7 ± 1.4 |
| Xylene | 75.4 ± 0.5 | 94.1 ± 5 | 97.9 ± 0.5 | 63.7 ± 3 | 75 ± 1.6 |
| Crude oil | 72 ± 4.8 | 85.5 ± 5 | 100 ± 0 | 53.2 ± 2.4 | 93.8 ± 2.5 |
| Motor oil | 96.3 ± 0.1 | 53.5 ± 2.3 | 59 ± 4.2 | 92.8 ± 1.8 | 83.7 ± 3.2 |
| Sunflower oil | 83.8 ± 3.4 | 100 | 100 | 77.8 ± 2.1 | 62.4 ± 2.4 |
| Corn oil | 81.1 ± 1.6 | 100 | 100 | 0 | 91.5 ± 0.8 |
| Olive oil | 76.1 ± 0.5 | 100 | 100 | 96.6 ± 0.6 | 78.2 ± 2.6 |
| Mineral oil | 0 | 91.2 ± 2.3 | 100 ± 0 | 56.8 ± 2.3 | 82.9 ± 2 |
| Surfactant | LC50 (μg/mL) |
|---|---|
| Microbactan | >1000 |
| Triton X-100 | 100.3 ± 3.8 |
| Tween 80 | >1000 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Camacho-Chab, J.C.; Guézennec, J.; Chan-Bacab, M.J.; Ríos-Leal, E.; Sinquin, C.; Muñiz-Salazar, R.; De la Rosa-García, S.D.C.; Reyes-Estebanez, M.; Ortega-Morales, B.O. Emulsifying Activity and Stability of a Non-Toxic Bioemulsifier Synthesized by Microbacterium sp. MC3B-10. Int. J. Mol. Sci. 2013, 14, 18959-18972. https://doi.org/10.3390/ijms140918959
Camacho-Chab JC, Guézennec J, Chan-Bacab MJ, Ríos-Leal E, Sinquin C, Muñiz-Salazar R, De la Rosa-García SDC, Reyes-Estebanez M, Ortega-Morales BO. Emulsifying Activity and Stability of a Non-Toxic Bioemulsifier Synthesized by Microbacterium sp. MC3B-10. International Journal of Molecular Sciences. 2013; 14(9):18959-18972. https://doi.org/10.3390/ijms140918959
Chicago/Turabian StyleCamacho-Chab, Juan Carlos, Jean Guézennec, Manuel Jesús Chan-Bacab, Elvira Ríos-Leal, Corinne Sinquin, Raquel Muñiz-Salazar, Susana Del C. De la Rosa-García, Manuela Reyes-Estebanez, and Benjamín Otto Ortega-Morales. 2013. "Emulsifying Activity and Stability of a Non-Toxic Bioemulsifier Synthesized by Microbacterium sp. MC3B-10" International Journal of Molecular Sciences 14, no. 9: 18959-18972. https://doi.org/10.3390/ijms140918959





