In Vitro Phosphorylation Does not Influence the Aggregation Kinetics of WT α-Synuclein in Contrast to Its Phosphorylation Mutants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Construction and Production of the α-SYN Phosphorylation Mutants S129A/D and Y125F/E
2.2. In Vitro Aggregation Properties of the Phosphorylation Mutants
2.3. In Vitro Phosphorylation of α-SYN on Different Residues
2.4. Structural Properties of in Vitro Phosphorylated α-SYN
2.5. The Influence of pS129- and pY125-α-SYN on Its in Vitro Fibril Formation
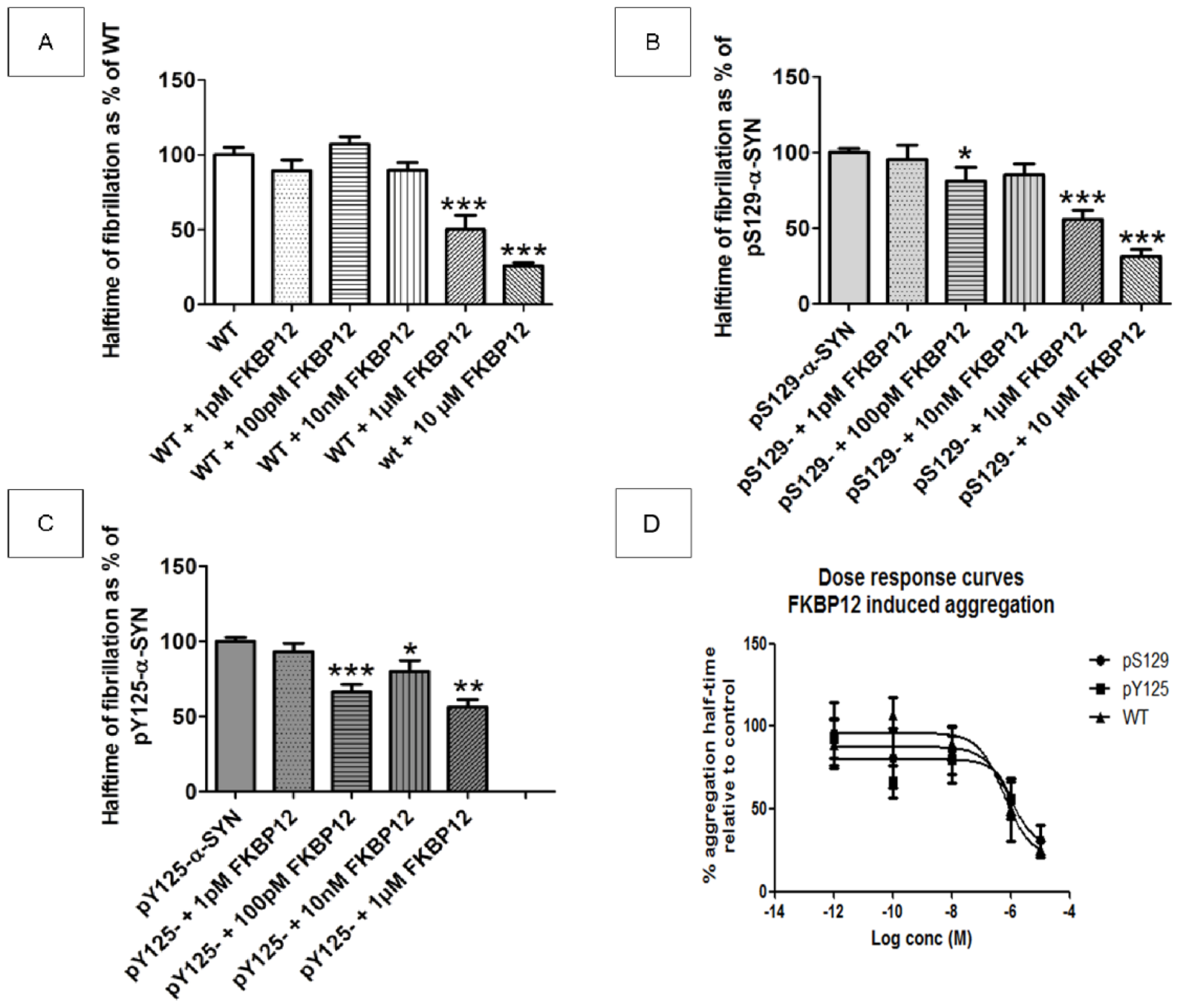
2.6. Does FKBP12 Still Accelerate the Fibrillization of Phosphorylated α-SYN?
2.7. Discussion
2.7.1. The Y125 Phosphorylation Mutants Display Different Aggregation Properties Compared to in Vitro Phosphorylated α-SYN
2.7.2 . In Vitro Phosphorylation of α-SYN on S129 or Y125 Does not Affect the Fibrillization Process
2.7.3. FKBP12 Mediated Acceleration of Fibrillization Is Unaltered for Phosphorylated α-SYN
3. Experimental Section
3.1. Purification of α-Synuclein (α-SYN)
3.2. Construction of Phosphorylation Mutants of α-Synuclein (α-SYN)
3.3. SDS PAGE
3.4. Fluorescence Correlation Spectroscopy (FCS)
3.5. Circular Dichroism
3.6. Turbidity and Thioflavin T Measurements
3.7. Data Fitting and Statistical Analysis of ThioT and Turbidity Data
3.8. Phosphorylation Assay
3.9. Mass Spectrometry
3.10. Western Blot Analysis
3.11. Transmission Electron Microscopy (TEM)
3.12. Peptidyl Prolyl Cis-Trans Isomerase Activity of FKBP12
4. Conclusions
Supporting Information
Fluorescent Labeling
Fluorescence Correlation Spectroscopy (FCS)
Mass Spectrometry
Peptidyl prolyl Cis-Trans Isomerase Activity of FKBP12
Acknowledgments
Conflicts of Interest
References
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar]
- Baba, M.; Nakajo, S.; Tu, P.H.; Tomita, T.; Nakaya, K.; Lee, V.M.; Trojanowski, J.Q.; Iwatsubo, T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am. J. Pathol 1998, 152, 879–884. [Google Scholar]
- Bertoncini, C.W.; Jung, Y.S.; Fernandez, C.O.; Hoyer, W.; Griesinger, C.; Jovin, T.M.; Zweckstetter, M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proc. Natl. Acad. Sci. USA 2005, 102, 1430–1435. [Google Scholar]
- Dedmon, M.M.; Lindorff-Larsen, K.; Christodoulou, J.; Vendruscolo, M.; Dobson, C.M. Mapping long-range interactions in alpha-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J. Am. Chem. Soc 2005, 127, 476–477. [Google Scholar]
- Abeliovich, A.; Schmitz, Y.; Farinas, I.; Choi-Lundberg, D.; Ho, W.H.; Castillo, P.E.; Shinsky, N.; Verdugo, J.M.; Armanini, M.; Ryan, A.; et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 2000, 25, 239–252. [Google Scholar]
- Lundblad, M.; Decressac, M.; Mattsson, B.; Bjorklund, A. Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proc. Natl. Acad. Sci. USA 2012, 109, 3213–3219. [Google Scholar]
- Cooper, A.A.; Gitler, A.D.; Cashikar, A.; Haynes, C.M.; Hill, K.J.; Bhullar, B.; Liu, K.; Xu, K.; Strathearn, K.E.; Liu, F.; et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 2006, 313, 324–328. [Google Scholar]
- Nemani, V.M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M.K.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 2010, 65, 66–79. [Google Scholar]
- Kahle, P.J.; Neumann, M.; Ozmen, L.; Haass, C. Physiology and pathophysiology of alpha-synuclein: Cell culture and transgenic animal models based on a Parkinson’s disease-associated protein. Ann. N. Y. Acad. Sci 2000, 920, 33–41. [Google Scholar]
- Scott, D.A.; Tabarean, I.; Tang, Y.; Cartier, A.; Masliah, E.; Roy, S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J. Neurosci 2010, 30, 8083–8095. [Google Scholar]
- Scott, D.; Roy, S. Alpha-synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J. Neurosci 2012, 32, 10129–10135. [Google Scholar]
- Anderson, J.P.; Walker, D.E.; Goldstein, J.M.; de Laat, R.; Banducci, K.; Caccavello, R.J.; Barbour, R.; Huang, J.; Kling, K.; Lee, M.; et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem 2006, 281, 29739–29752. [Google Scholar]
- Fujiwara, H.; Hasegawa, M.; Dohmae, N.; Kawashima, A.; Masliah, E.; Goldberg, M.S.; Shen, J.; Takio, K.; Iwatsubo, T. Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol 2002, 4, 160–164. [Google Scholar]
- Hirai, Y.; Fujita, S.C.; Iwatsubo, T.; Hasegawa, M. Phosphorylated alpha-synuclein in normal mouse brain. FEBS Lett 2004, 572, 227–232. [Google Scholar]
- Paleologou, K.E.; Schmid, A.W.; Rospigliosi, C.C.; Kim, H.-Y.; Lamberto, G.R.; Fredenburg, R.A.; Lansbury, P.T.; Fernandez, C.O.; Eliezer, D.; Zweckstetter, M.; et al. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J. Biol. Chem 2008, 283, 16895–16905. [Google Scholar]
- Chen, L.; Feany, M.B. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat. Neurosci 2005, 8, 657–663. [Google Scholar]
- Chau, K.Y.; Ching, H.L.; Schapira, A.H.; Cooper, J.M. Relationship between alpha synuclein phosphorylation, proteasomal inhibition and cell death: Relevance to Parkinson’s disease pathogenesis. J. Neurochem 2009, 110, 1005–1013. [Google Scholar]
- Azeredo da Silveira, S.; Schneider, B.L.; Cifuentes-Diaz, C.; Sage, D.; Abbas-Terki, T.; Iwatsubo, T.; Unser, M.L.; Aebischer, P. Phosphorylation does not prompt, nor prevent, the formation of alpha-synuclein toxic species in a rat model of Parkinson’s disease. Hum. Mol. Genet 2009, 18, 872–887. [Google Scholar]
- Gorbatyuk, O.S.; Li, S.; Sullivan, L.F.; Chen, W.; Kondrikova, G.; Manfredsson, F.P.; Mandel, R.J.; Muzyczka, N. The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc. Natl. Acad. Sci. USA 2008, 105, 763–768. [Google Scholar]
- McFarland, N.R.; Fan, Z.; Xu, K.; Schwarzschild, M.A.; Feany, M.B.; Hyman, B.T.; McLean, P.J. Alpha-synuclein S129 phosphorylation mutants do not alter nigrostriatal toxicity in a rat model of Parkinson disease. J. Neuropathol. Exp. Neurol 2009, 68, 515–524. [Google Scholar]
- Okochi, M.; Walter, J.; Koyama, A.; Nakajo, S.; Baba, M.; Iwatsubo, T.; Meijer, L.; Kahle, P.J.; Haass, C. Constitutive phosphorylation of the Parkinson’s disease associated alpha-synuclein. J. Biol. Chem 2000, 275, 390–397. [Google Scholar]
- Waxman, E.A.; Giasson, B.I. Specificity and regulation of casein kinase-mediated phosphorylation of alpha-synuclein. J. Neuropathol. Exp. Neurol 2008, 67, 402–416. [Google Scholar]
- Arawaka, S.; Wada, M.; Goto, S.; Karube, H.; Sakamoto, M.; Ren, C.-H.; Koyama, S.; Nagasawa, H.; Kimura, H.; Kawanami, T.; et al. The role of G-protein-coupled receptor kinase 5 in pathogenesis of sporadic Parkinson’s disease. J. Neurosci. Off. J. Soc. Neurosci 2006, 26, 9227–9238. [Google Scholar]
- Pronin, A.N.; Morris, A.J.; Surguchov, A.; Benovic, J.L. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J. Biol. Chem 2000, 275, 26515–26522. [Google Scholar]
- Inglis, K.J.; Chereau, D.; Brigham, E.F.; Chiou, S.S.; Schobel, S.; Frigon, N.L.; Yu, M.; Caccavello, R.J.; Nelson, S.; Motter, R.; et al. Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J. Biol. Chem 2009, 284, 2598–2602. [Google Scholar]
- Mbefo, M.K.; Paleologou, K.E.; Boucharaba, A.; Oueslati, A.; Schell, H.; Fournier, M.; Olschewski, D.; Yin, G.; Zweckstetter, M.; Masliah, E.; et al. Phosphorylation of synucleins by members of the Polo-like kinase family. J. Biol. Chem 2010, 285, 2807–2822. [Google Scholar]
- Negro, A.; Brunati, A.M.; Donella-Deana, A.; Massimino, M.L.; Pinna, L.A. Multiple phosphorylation of alpha-synuclein by protein tyrosine kinase Syk prevents eosin-induced aggregation. J. Off. Publ. Feder. Am. Soc. Exp. Biol 2002, 16, 210–212. [Google Scholar]
- Ellis, C.E.; Schwartzberg, P.L.; Grider, T.L.; Fink, D.W.; Nussbaum, R.L. Alpha-synuclein is phosphorylated by members of the Src family of protein-tyrosine kinases. J. Biol. Chem 2001, 276, 3879–3884. [Google Scholar]
- Nakamura, T.; Yamashita, H.; Takahashi, T.; Nakamura, S. Activated Fyn phosphorylates alpha-synuclein at tyrosine residue 125. Biochem. Biophys. Res. Commun 2001, 280, 1085–1092. [Google Scholar]
- Ahn, B.H.; Rhim, H.; Kim, S.Y.; Sung, Y.M.; Lee, M.Y.; Choi, J.Y.; Wolozin, B.; Chang, J.S.; Lee, Y.H.; Kwon, T.K.; et al. Alpha-Synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells. J. Biol. Chem 2002, 277, 12334–12342. [Google Scholar]
- Chen, L.; Periquet, M.; Wang, X.; Negro, A.; McLean, P.J.; Hyman, B.T.; Feany, M.B. Tyrosine and serine phosphorylation of alpha-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J. Clin. Investig 2009, 119, 3257–3265. [Google Scholar]
- Deleersnijder, A.; van Rompuy, A.S.; Desender, L.; Pottel, H.; Buee, L.; Debyser, Z.; Baekelandt, V.; Gerard, M. Comparative analysis of different peptidyl-prolyl isomerases reveals FK506-binding protein 12 as the most potent enhancer of alpha-synuclein aggregation. J. Biol. Chem 2011, 286, 26687–26701. [Google Scholar]
- Gerard, M.; Debyser, Z.; Desender, L.; Baert, J.; Brandt, I.; Baekelandt, V.; Engelborghs, Y. FK506 binding protein 12 differentially accelerates fibril formation of wild type alpha-synuclein and its clinical mutants A30P or A53T. J. Neurochem 2008, 106, 121–133. [Google Scholar]
- Gerard, M.; Deleersnijder, A.; Daniels, V.; Schreurs, S.; Munck, S.; Reumers, V.; Pottel, H.; Engelborghs, Y.; van den Haute, C.; Taymans, J.M.; et al. Inhibition of FK506 binding proteins reduces alpha-synuclein aggregation and Parkinson’s disease-like pathology. J. Neurosci 2010, 30, 2454–2463. [Google Scholar]
- Hong, D.P.; Fink, A.L.; Uversky, V.N. Structural characteristics of alpha-synuclein oligomers stabilized by the flavonoid baicalein. J. Mol. Biol 2008, 383, 214–223. [Google Scholar]
- Nath, S.; Meuvis, J.; Hendrix, J.; Carl, S.A.; Engelborghs, Y. Early aggregation steps in alpha-synuclein as measured by FCS and FRET: Evidence for a contagious conformational change. Biophys. J 2010, 98, 1302–1311. [Google Scholar]
- Morar, A.S.; Olteanu, A.; Young, G.B.; Pielak, G.J. Solvent-induced collapse of alpha-synuclein and acid-denatured cytochrome c. Protein Sci 2001, 10, 2195–2199. [Google Scholar]
- Paleologou, K.E.; Oueslati, A.; Shakked, G.; Rospigliosi, C.C.; Kim, H.Y.; Lamberto, G.R.; Fernandez, C.O.; Schmid, A.; Chegini, F.; Gai, W.P.; et al. Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J. Neurosci 2010, 30, 3184–3198. [Google Scholar]
- Gerard, M.; Debyser, Z.; Desender, L.; Kahle, P.J.; Baert, J.; Baekelandt, V.; Engelborghs, Y. The aggregation of alpha-synuclein is stimulated by FK506 binding proteins as shown by fluorescence correlation spectroscopy. FASEB J 2006, 20, 524–526. [Google Scholar]
- Meuvis, J.; Gerard, M.; Desender, L.; Baekelandt, V.; Engelborghs, Y. The conformation and the aggregation kinetics of alpha-synuclein depend on the proline residues in its C-terminal region. Biochemistry 2010, 49, 9345–9352. [Google Scholar]
- Kuwahara, T.; Tonegawa, R.; Ito, G.; Mitani, S.; Iwatsubo, T. Phosphorylation of alpha-synuclein protein at Ser-129 reduces neuronal dysfunction by lowering its membrane binding property in Caenorhabditis elegans. J. Biol. Chem 2012, 287, 7098–7109. [Google Scholar]
- Visanji, N.P.; Wislet-Gendebien, S.; Oschipok, L.W.; Zhang, G.; Aubert, I.; Fraser, P.E.; Tandon, A. Effect of Ser-129 phosphorylation on interaction of alpha-synuclein with synaptic and cellular membranes. J. Biol. Chem 2011, 286, 35863–35873. [Google Scholar]
- Hashimoto, M.; Hsu, L.J.; Sisk, A.; Xia, Y.; Takeda, A.; Sundsmo, M.; Masliah, E. Human recombinant NACP/alpha-synuclein is aggregated and fibrillated in vitro: Relevance for Lewy body disease. Brain Res 1998, 799, 301–306. [Google Scholar]
- Uversky, V.N.; Oldfield, C.J.; Dunker, A.K. Showing your ID: Intrinsic disorder as an ID for recognition, regulation and cell signaling. J. Mol. Recognit 2005, 18, 343–384. [Google Scholar]
- Yamin, G.; Uversky, V.N.; Fink, A.L. Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS Lett 2003, 542, 147–152. [Google Scholar]
- Lu, Y.; Prudent, M.; Fauvet, B.; Lashuel, H.A.; Girault, H.H. Phosphorylation of alpha-synuclein at Y125 and S129 alters its metal binding properties: Implications for understanding the role of alpha-synuclein in the pathogenesis of Parkinson’s disease and related disorders. ACS Chem. Neurosci 2011, 2, 667–675. [Google Scholar]
- Hejjaoui, M.; Butterfield, S.; Fauvet, B.; Vercruysse, F.; Cui, J.; Dikiy, I.; Prudent, M.; Olschewski, D.; Zhang, Y.; Eliezer, D.; et al. Elucidating the role of C-terminal post-translational modifications using protein semisynthesis strategies: Alpha-synuclein phosphorylation at tyrosine 125. J. Am. Chem. Soc 2012, 134, 5196–5210. [Google Scholar]
- Ulrih, N.P.; Barry, C.H.; Fink, A.L. Impact of Tyr to Ala mutations on alpha-synuclein fibrillation and structural properties. Biochim. Biophys. Acta 2008, 1782, 581–585. [Google Scholar]
- Gothel, S.F.; Marahiel, M.A. Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell. Mol. Life Sci 1999, 55, 423–436. [Google Scholar]
- Edlich, F.; Weiwad, M.; Wildemann, D.; Jarczowski, F.; Kilka, S.; Moutty, M.C.; Jahreis, G.; Lucke, C.; Schmidt, W.; Striggow, F.; et al. The specific FKBP38 inhibitor N-(N′,N′-dimethylcarboxamidomethyl)cycloheximide has potent neuroprotective and neurotrophic properties in brain ischemia. J. Biol. Chem 2006, 281, 14961–14970. [Google Scholar]
- Gold, B.G.; Udina, E.; Bourdette, D.; Navarro, X. Neuroregenerative and neuroprotective actions of neuroimmunophilin compounds in traumatic and inflammatory neuropathies. Neurol. Res 2004, 26, 371–380. [Google Scholar]
- Gold, B.G.; Zeleny-Pooley, M.; Wang, M.S.; Chaturvedi, P.; Armistead, D.M. A nonimmunosuppressant FKBP-12 ligand increases nerve regeneration. Exp. Neurol 1997, 147, 269–278. [Google Scholar]
- Van Raaij, M.E.; Segers-Nolten, I.M.; Subramaniam, V. Quantitative morphological analysis reveals ultrastructural diversity of amyloid fibrils from alpha-synuclein mutants. Biophys. J 2006, 91, L96–L98. [Google Scholar]
- Kumar, A.; Greggio, E.; Beilina, A.; Kaganovich, A.; Chan, D.; Taymans, J.M.; Wolozin, B.; Cookson, M.R. The Parkinson’s disease associated LRRK2 exhibits weaker in vitro phosphorylation of 4E-BP compared to autophosphorylation. PLoS One 2010, 5, e8730. [Google Scholar]
- Schapira, A.H. Etiology of Parkinson’s disease. Neurology 2006, 66, S10–S23. [Google Scholar]
- Takahashi, M.; Ko, L.W.; Kulathingal, J.; Jiang, P.; Sevlever, D.; Yen, S.H. Oxidative stress-induced phosphorylation, degradation and aggregation of alpha-synuclein are linked to upregulated CK2 and cathepsin D. Eur. J. Neurosci 2007, 26, 863–874. [Google Scholar]
- Vancraenenbroeck, R.; Lobbestael, E.; de Maeyer, M.; Baekelandt, V.; Taymans, J.M. Kinases as targets for Parkinson’s disease: from genetics to therapy. CNS Neurol. Disord. Drug Targets 2011, 10, 724–740. [Google Scholar]
- Kostka, M.T.; Hogen, K.M.; Danzer, J.; Levin, M.; Habeck, A.; Wirth, R.; Wagner, C.G.; Glabe, S.; Finger, U.; Heinzelmann, P.; et al. Single particle characterization of iron-induced pore-forming alpha-synuclein oligomers. J. Biol. Chem 2008, 283, 10992–11003. [Google Scholar]
- Petrásek, Z.; Schwille, P. Precise measurement of diffusion coefficients using scanning fluorescence correlation spectroscopy. Biophys. J 2008, 94, 1437–1448. [Google Scholar]
- Kullertz, G.; Luthe, S.; Fischer, G. Semiautomated microtiter plate assay for monitoring peptidylprolyl cis/trans isomerase activity in normal and pathological human sera. Clin. Chem 1998, 44, 502–508. [Google Scholar]
- Olsen, J.V.; Vermeulen, M.; Santamaria, A.; Kumar, C.; Miller, M.L.; Jensen, L.J.; Gnad, F.; Cox, J.; Jensen, T.S.; Nigg, E.A.; et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci. Signal 2010. [Google Scholar] [CrossRef]





© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schreurs, S.; Gerard, M.; Derua, R.; Waelkens, E.; Taymans, J.-M.; Baekelandt, V.; Engelborghs, Y. In Vitro Phosphorylation Does not Influence the Aggregation Kinetics of WT α-Synuclein in Contrast to Its Phosphorylation Mutants. Int. J. Mol. Sci. 2014, 15, 1040-1067. https://doi.org/10.3390/ijms15011040
Schreurs S, Gerard M, Derua R, Waelkens E, Taymans J-M, Baekelandt V, Engelborghs Y. In Vitro Phosphorylation Does not Influence the Aggregation Kinetics of WT α-Synuclein in Contrast to Its Phosphorylation Mutants. International Journal of Molecular Sciences. 2014; 15(1):1040-1067. https://doi.org/10.3390/ijms15011040
Chicago/Turabian StyleSchreurs, Sarah, Melanie Gerard, Rita Derua, Etienne Waelkens, Jean-Marc Taymans, Veerle Baekelandt, and Yves Engelborghs. 2014. "In Vitro Phosphorylation Does not Influence the Aggregation Kinetics of WT α-Synuclein in Contrast to Its Phosphorylation Mutants" International Journal of Molecular Sciences 15, no. 1: 1040-1067. https://doi.org/10.3390/ijms15011040



