Molecular Cloning, Bioinformatics Analysis and Expression of Insulin-Like Growth Factor 2 from Tianzhu White Yak, Bos grunniens
Abstract
:1. Introduction
2. Results and Discussion
2.1. Result
2.1.1. Cloning and Characterization of the Full Coding Region of the Yak IGF2 Gene
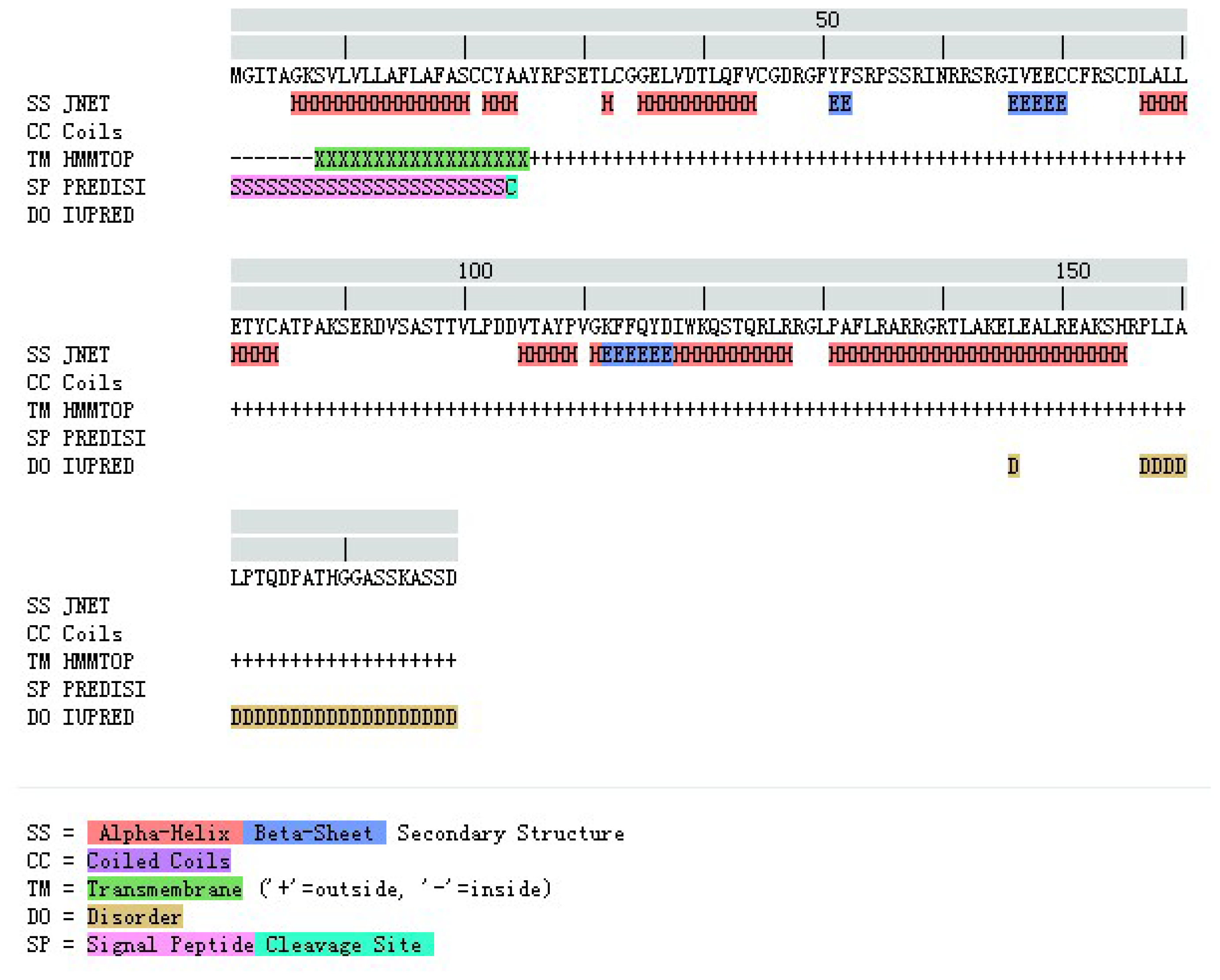
2.1.2. Amino Acid Composition and Protein Secondary Structure
2.1.3. Multiple Sequence Alignment
2.1.4. 3D Structure Prediction
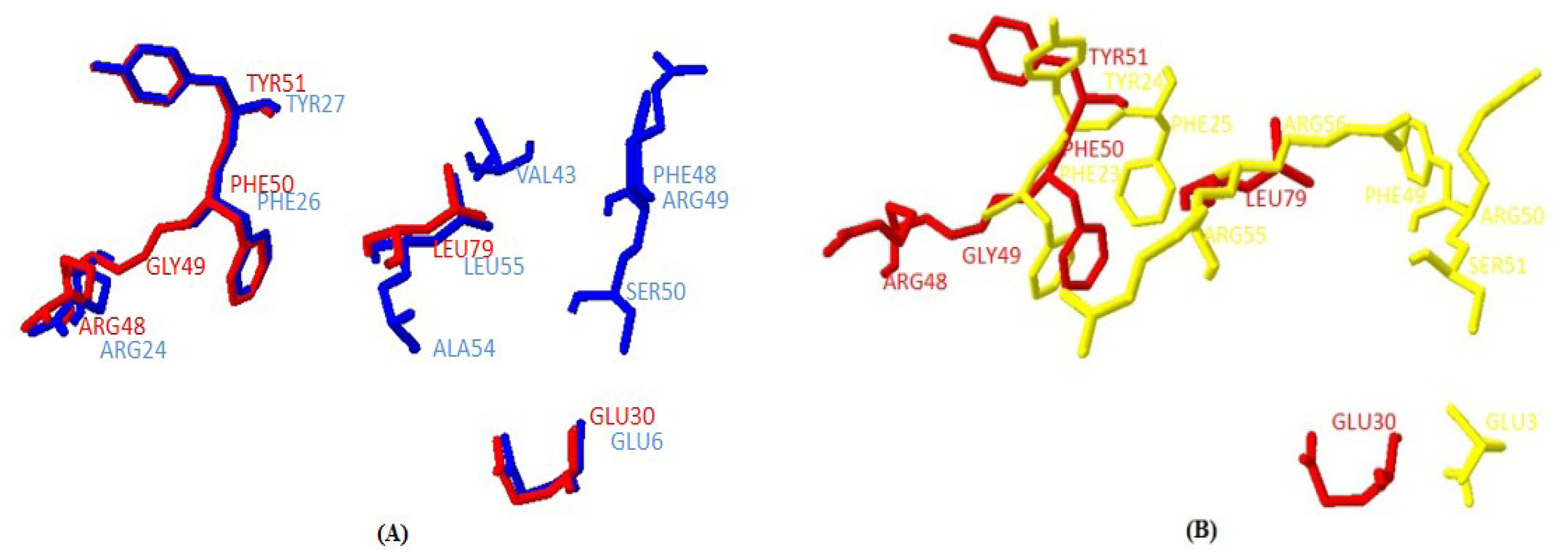
2.1.5. Similarities between Structure of Yak IGF2 and Other Mammalian IGF2
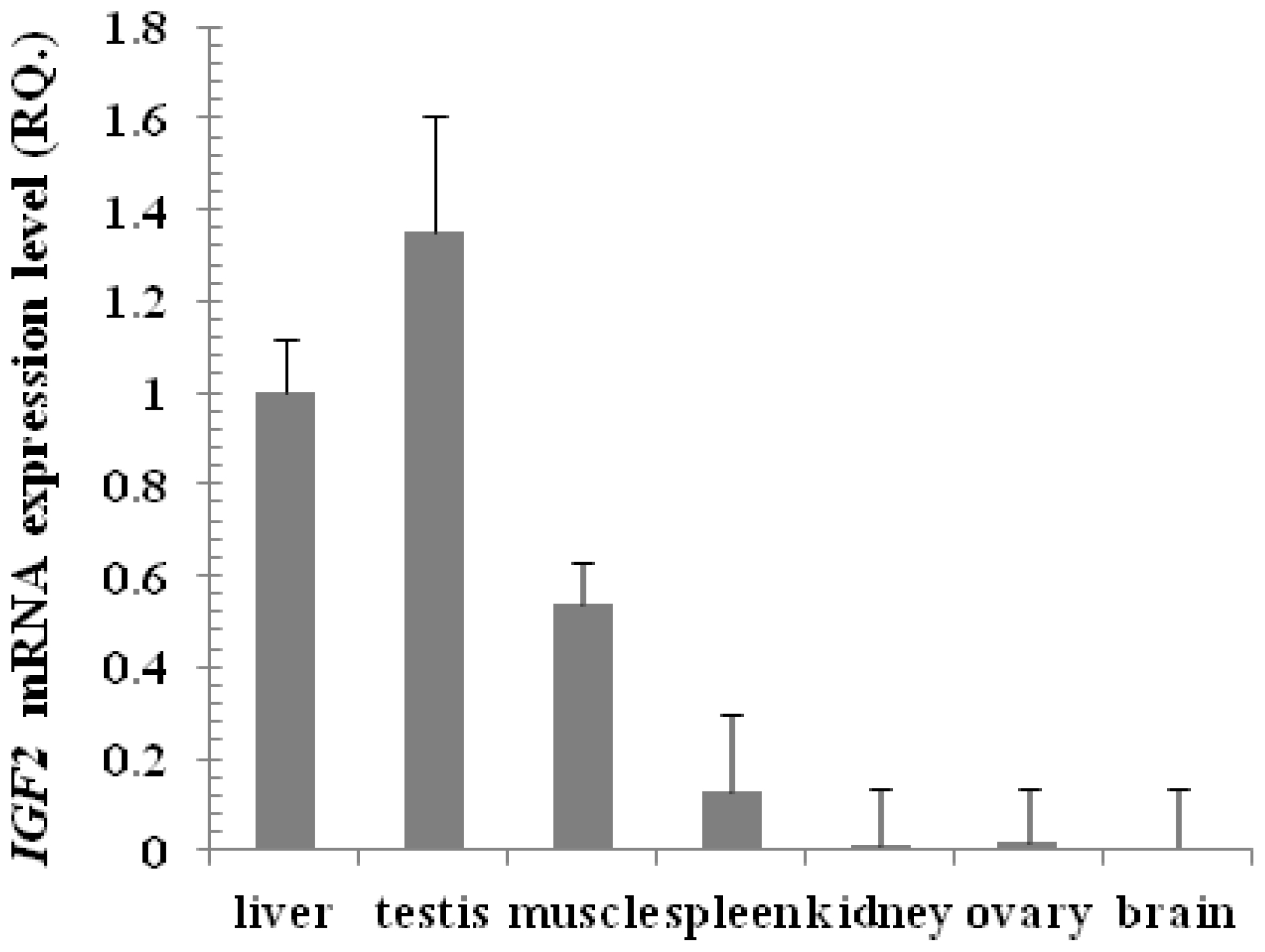
2.1.6. Expression of IGF2 Gene by Real Time PCR in Tianzhu White Yak
2.2. Discussion
3. Experimental Section
3.1. Brief Introduction of Methods
3.2. Samples and Materials
3.3. RNA Isolation and cDNA Synthesis
3.4. Synthesis and Confirmation of Partial cDNA of IGF2 Gene
3.5. Rapid Amplification of cDNA Ends (RACE), Cloning and Sequencing
3.6. Analysis and Alignment of cDNA Sequence
3.7. Protein and mRNA Secondary Structure Prediction
3.8. Multiple Sequence Alignment and Phylogenetic Analysis
3.9. Yak IGF2 Protein 3D Structure Prediction
3.10. Real Time PCR Assays for IGF2 Gene Expression in Yak
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hu, Q.; Ma, T.; Wang, K.; Xu, T.; Liu, J.; Qiu, Q. The Yak genome database: An integrative database for studying yak biology and high-altitude adaption. BMC Genomics 2012, 13, 600. [Google Scholar]
- Lu, C.; Lam, H.N.; Menon, R.K. New members of the insulin family: Regulators of metabolism, growth and now reproduction. Pediatr. Res 2005, 57, 70–73. [Google Scholar]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev 1995, 16, 3–34. [Google Scholar]
- O’Dell, S.D.; Day, I.N. Molecules in focus Insulin-like growth factor II (IGF-II). Int. J. Biochem. Cell Biol 1998, 30, 767–771. [Google Scholar]
- Salmon, W., Jr.; Daughaday, W. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J. Lab. Clin. Med. 1957, 49, 825–836. [Google Scholar]
- Sussenbach, J.S. The gene structure of the insulin-like growth factor family. Prog. Growth Factor Res 1989, 1, 33–48. [Google Scholar]
- Blundell, T.L.; Bedarkar, S.; Humbel, R.E. Tertiary structures, receptor binding, and antigenicity of insulinlike growth factors. Fed. Proc 1983, 42, 2592–2597. [Google Scholar]
- Martinez, J.; Potier, P. Peptide hormones as prohormones. Trends Pharmacol. Sci 1986, 7, 139–147. [Google Scholar]
- Ferry, R.J., Jr.; Cerri, R.W.; Cohen, P. Insulin-like growth factor binding proteins: New proteins, new functions. Horm. Res 1999, 51, 53–67. [Google Scholar]
- Grizard, J.; Dardevet, D.; Papet, I.; Mosoni, L.; Mirand, P.P.; Attaix, D.; Tauveron, I.; Bonin, D.; Arnal, M. Nutrient regulation of skeletal muscle protein metabolism in animals: The involvement of hormones and substrates. Nutr. Res. Rev 1995, 8, 67–91. [Google Scholar]
- Fazio, S.; Palmieri, E.A.; Biondi, B.; Cittadini, A.; Sacca, L. The role of the GH-IGF-I axis in the regulation of myocardial growth: From experimental models to human evidence. Eur. J. Endocrinol 2000, 142, 211–216. [Google Scholar]
- Olney, R.C.; Mougey, E.B. Expression of the components of the insulin-like growth factor axis across the growth-plate. Mol. Cell. Endocrinol 1999, 156, 63–71. [Google Scholar]
- Baker, J.; Liu, J.-P.; Robertson, E.J.; Efstratiadis, A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993, 75, 73–82. [Google Scholar]
- Wang, Y.; Nishida, S.; Sakata, T.; Elalieh, H.Z.; Chang, W.; Halloran, B.P.; Doty, S.B.; Bikle, D.D. Insulin-like growth factor-I is essential for embryonic bone development. Endocrinology 2006, 147, 4753–4761. [Google Scholar]
- De Los Rios, P.; Hill, D. Cellular localization and expression of insulin-like growth factors (IGFs) and IGF binding proteins within the epiphyseal growth plate of the ovine fetus: Possible functional implications. Can. J. Physiol. Pharmacol 1999, 77, 235–249. [Google Scholar]
- Reinecke, M.; Schmid, A.C.; Heyberger-Meyer, B.; Hunziker, E.B.; Zapf, J. Effect of growth hormone and insulin-like growth factor I (IGF-I) on the expression of IGF-I messenger ribonucleic acid and peptide in rat tibial growth plate and articular chondrocytes in vivo. Endocrinology 2000, 141, 2847–2853. [Google Scholar]
- PROTEAN, version 5.07; DNASTAR Inc.: Madison, WI, USA, 2003.
- MAFFT, version 7.113; Computational Biology Research Center (CBRC): Tokyo, Japan, 2013.
- Jalview, version 2.8; University of Dundee: Scotland, UK, 2012.
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics. 2006, 22, pp. 195–201. Available online: http://beta.swissmodel.expasy.org/workspace (accessed on 29 September 2013).
- Denley, A.; Cosgrove, L.J.; Booker, G.W.; Wallace, J.C.; Forbes, B.E. Molecular interactions of the IGF system. Cytokine Growth Factor Rev 2005, 16, 421–439. [Google Scholar]
- PyMOL, version 0.99; Schrödinger: New York, NY, USA, 2006.
- EMBL-EBI. Available online: http://www.ebi.ac.uk/msd-srv/ssm/cgi-bin/ssmserver (accessed on 29 September 2013).
- Rosenzweig, S.A. What’s new in the IGF-binding proteins? Growth Horm. IGF Res 2004, 14, 329–336. [Google Scholar]
- Torres, A.M.; Forbes, B.E.; Aplin, S.E.; Wallace, J.C.; Francise, G.L.; Norton, R.S. Solution structure of human insulin-like growthfactor II. Relationship to receptor and binding protein interactions. J. Mol. Biol 1995, 248, 385–401. [Google Scholar]
- Pavelic, J.; Matijevic, T.; Knezevic, J. Biological & physiological aspects of action of insulin-like growth factor peptide family. Indian J. Med. Res 2007, 125, 511–522. [Google Scholar]
- Pace, C.N.; Grimsley, G.; Thomson, J.; Barnett, B. Conformational stability and activity of ribonuclease T1 with zero, one, and two intact disulfide bonds. J. Biol. Chem 1988, 263, 11820–11825. [Google Scholar]
- Krissinel, E.; Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Cryst 2004, D60, 2256–2268. [Google Scholar]
- NCBI Nucleotide Program. Available online: http://www.ncbi.nlm.nih.gov/nuccore/?term=IGF2 (accessed on 28 September 2013).
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA 5.1, version 5.1; Tokyo, Japan, 2013. [Google Scholar]
- Oligo 7.0, version 7.0; Molecular Biology Insights, Inc.: Cascade, CO, USA, 2013.
- Primer 5, version 5.0; PREMIER Biosoft: Palo Alto, CA, USA, 1994.
- NCBI Open Reading Frame Finder Available Program. Available online: http://www.ncbi.nlm.nih.gov/gorf/gorf.html (accessed on 28 September 2013).
- NCBI BLAST Program. Available online: http://www.ncbi.nlm.nih.gov/blast (accessed on 28 September 2013).
- Quick2D, Bioinformatics Toolkit, version n.a. Available online: http://toolkit.tuebingen.mpg.de/quick2_d (accecssed on 28 September 2013).
- Antigenic Properties Predict Program. Available online: http://imed.med.ucm.es/Tools/antigenic.pl (accessed on 28 September 2013).
- Elrobh, M.S.; Alanazi, M.S.; Khan, W.; Abduljaleel, Z.; Al-Amri, A.; Bazzi, M.D. Molecular cloning and characterization of cDNA encoding a putative stress-induced heat-shock protein from camelus dromedarius. Int. J. Mol. Sci 2011, 12, 4214–4236. [Google Scholar]
- George, S.A.; Khan, S.; Briggs, H.; Abelson, J.L. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology 2010, 35, 607–612. [Google Scholar]
- Mitani, T.; Akane, A.; Tokiyasu, T.; Yoshimura, S.; Okii, Y.; Yoshida, M. Identification of animal species using the partial sequences in the mitochondrial 16S rRNA gene. Leg. Med 2009, 11, 449–450. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res 1997, 25, 3389–3402. [Google Scholar]
- Yang, B.; Xue, T.; Zhao, J.; Kommidi, C.; Soneja, J.; Li, J.; Will, R.; Sharp, B.; Kenyon, R.; Crasta, O. Sobral BWS: Bioinformatics Web Services; The 2006 International Conference on Bioinformatics & Computational Biology (BIOCOMP): Las Vegas, NV, USA, 2006; pp. 258–264. [Google Scholar]
- Emanuelsson, O.; Brunak, S.; von Heijne, G.; Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc 2007, 2, 953–971. [Google Scholar]
- Parker, J.; Guo, D.; Hodges, R. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: Correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry 1986, 25, 5425–5432. [Google Scholar]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy—The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 2003, 31, 3784–3788. [Google Scholar]
- Blow, N. Transcriptomics: The digital generation. Nature 2009, 458, 239–242. [Google Scholar]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Johansson, M.U.; Zoete, V.; Michielin, O.; Guex, N. Defining and searching for structural motifs using DeepView/Swiss-PdbViewer. BMC Bioinforma 2012, 13, 173. [Google Scholar]
- Ataya, F.S. Cloning, phylogenetic analysis and 3D modeling of a putative lysosomal acid lipase from the camel Camelus dromedarius. Molecules 2012, 17, 10399–10413. [Google Scholar]
- Kolaskar, A.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic on protein antigens. FEBS Lett 1990, 276, 172–174. [Google Scholar]
- Karplus, P.; Schulz, G. Prediction of chain flexibility in proteins. Naturwissenschaften 1985, 72, 212–213. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar]










| Amimo Acid | Number Count | % By Weight | % By Frequency |
|---|---|---|---|
| Ala (A) | 20 | 7.22 | 11.17 |
| Arg (R) | 18 | 14.28 | 10.06 |
| Asn (N) | 1 | 0.58 | 0.56 |
| Asp (D) | 9 | 5.26 | 5.03 |
| Cys (C) | 8 | 4.19 | 4.47 |
| Gln (Q) | 5 | 3.26 | 2.79 |
| Glu (E) | 9 | 5.90 | 5.03 |
| Gly (G) | 12 | 3.48 | 6.70 |
| His (H) | 2 | 1.39 | 1.12 |
| Ile (I) | 5 | 2.87 | 2.79 |
| Pyl (O) | 0 | 0 | 0 |
| Leu (L) | 19 | 10.92 | 10.61 |
| Lys (K) | 7 | 4.56 | 3.91 |
| Met(M) | 1 | 0.67 | 0.56 |
| Phe (F) | 9 | 6.73 | 5.03 |
| Pro (P) | 9 | 4.44 | 5.03 |
| Ser (S) | 17 | 7.52 | 9.50 |
| Thr (T) | 12 | 6.16 | 6.70 |
| Trp (W) | 1 | 0.95 | 0.56 |
| Tyr (Y) | 6 | 4.97 | 3.35 |
| Val (V) | 9 | 4.53 | 5.03 |
| Sec (U) | 0 | 0 | 0 |
| IGF-2 | (NCBI Reference Sequence) | No. of Residues | Total Score | Coverage (%) | Identity (%) | Positive (%) | E-Value | pI | Mean Value | Gaps (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bos grunniens | KF682139 | 179 | 364 | 100 | 100 | 100 | 2e- 134 | 8.82 | 19.68 | 0 |
| Bos taurus | AY957981.1 | 179 | 362 | 100 | 99 | 99 | 4e- 133 | 8.65 | 19.64 | 0 |
| Bubalus bubalis | KC107769.1 | 135 | 278 | 75 | 100 | 100 | 3e- 101 | 9.23 | 13.77 | 0 |
| Bos primigenius | AF283002.1 | 149 | 305 | 83 | 100 | 100 | 2e- 111 | 10.50 | 15.11 | 0 |
| Ovis aries | M89788.1 | 179 | 331 | 100 | 96 | 97 | 5e -121 | 8.49 | 19.62 | 0 |
| Capra hircus | DQ645739.1 | 179 | 350 | 100 | 96 | 97 | 9e -129 | 8.49 | 19.65 | 0 |
| Cervus elaphus | EF177491.1 | 179 | 335 | 100 | 92 | 94 | 1e -122 | 8.67 | 19.72 | 0 |
| Sus scrofa | HQ450757.1 | 181 | 289 | 98 | 84 | 88 | 2e -104 | 9.99 | 20.31 | 0 |
| Homo sapiens | XM005252900.1 | 180 | 309 | 100 | 84 | 88 | 1e -112 | 9.34 | 20.14 | 0 |
| Structure | (PDB) | No. of Residues | Aligned Residues | RMSD | Q-Score | p-Score | Z-Score |
|---|---|---|---|---|---|---|---|
| Predicted yak IGF2 | human IGF1 (1H02) | 64 | 49 | 1.61 | 0.45 | 1.8 | 3.8 |
| Predicted yak IGF2 | human IGF2 (1IGL) | 67 | 65 | 0.09 | 0.97 | 10.8 | 9.7 |
| NO. | Start Position | End Position | Peptide | Peptide Length |
|---|---|---|---|---|
| 1 | 6 | 27 | GKSVLVLLAFLAFSCCYAAYR | 22 |
| 2 | 29 | 48 | SETLCGGELVDTLQFVCGDR | 20 |
| 3 | 67 | 87 | VEECCFRSCDLALLETYCATP | 21 |
| 4 | 93 | 119 | DVSASTTVLPDDVTAYPVGKFFQYDIW | 27 |
| 5 | 127 | 135 | RRGLPAFLR | 9 |
| 6 | 155 | 164 | HRPLIALPTQ | 10 |
| Function | Software and website |
|---|---|
| To search nucleotide sequence | http://www.ncbi.nlm.nih.gov/nuccore/ [29] |
| To find conserved sequence | MEGA 5.1/DNAstar/DNAMAN [30] |
| To design primers in the conserved sequence | Oligo 7.0/Primer Premier 5 [31,32] |
| To find open reading frame | http://www.ncbi.nlm.nih.gov/gorf/gorf.html [33] |
| To analysis amino sequence | PROTEAN program/DNAMAN [17,30] |
| To analysis identity of amino acid | http://blast.ncbi.nlm.nih.gov/Blast.cgi [34] |
| To alignmultiple sequence | MAFFT Multiple program/Sequence Alignment [18] |
| To analysis phylogenetic | MAFFT Multiple program/Sequence Alignment/MEGA 5.1 [18] |
| To predict secondary structure | Bioinformatics Toolkit Quick2D program [35] |
| To predict 3D structure | http://swissmodel.expasy.org/ [20] |
| To analysis 3D structure | Swiss-PDB Viewer program [20] |
| To predict antigenic properties | http://imed.med.ucm.es/Tools/antigenic.pl [36] |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Q.; Gong, J.; Wang, X.; Wu, X.; Li, Y.; Ma, Y.; Zhang, Y.; Zhao, X. Molecular Cloning, Bioinformatics Analysis and Expression of Insulin-Like Growth Factor 2 from Tianzhu White Yak, Bos grunniens. Int. J. Mol. Sci. 2014, 15, 504-524. https://doi.org/10.3390/ijms15010504
Zhang Q, Gong J, Wang X, Wu X, Li Y, Ma Y, Zhang Y, Zhao X. Molecular Cloning, Bioinformatics Analysis and Expression of Insulin-Like Growth Factor 2 from Tianzhu White Yak, Bos grunniens. International Journal of Molecular Sciences. 2014; 15(1):504-524. https://doi.org/10.3390/ijms15010504
Chicago/Turabian StyleZhang, Quanwei, Jishang Gong, Xueying Wang, Xiaohu Wu, Yalan Li, Youji Ma, Yong Zhang, and Xingxu Zhao. 2014. "Molecular Cloning, Bioinformatics Analysis and Expression of Insulin-Like Growth Factor 2 from Tianzhu White Yak, Bos grunniens" International Journal of Molecular Sciences 15, no. 1: 504-524. https://doi.org/10.3390/ijms15010504




