Atrazine Molecular Imprinted Polymers: Comparative Analysis by Far-Infrared and Ultraviolet Induced Polymerization
Abstract
:1. Introduction
2. Results and Discussion
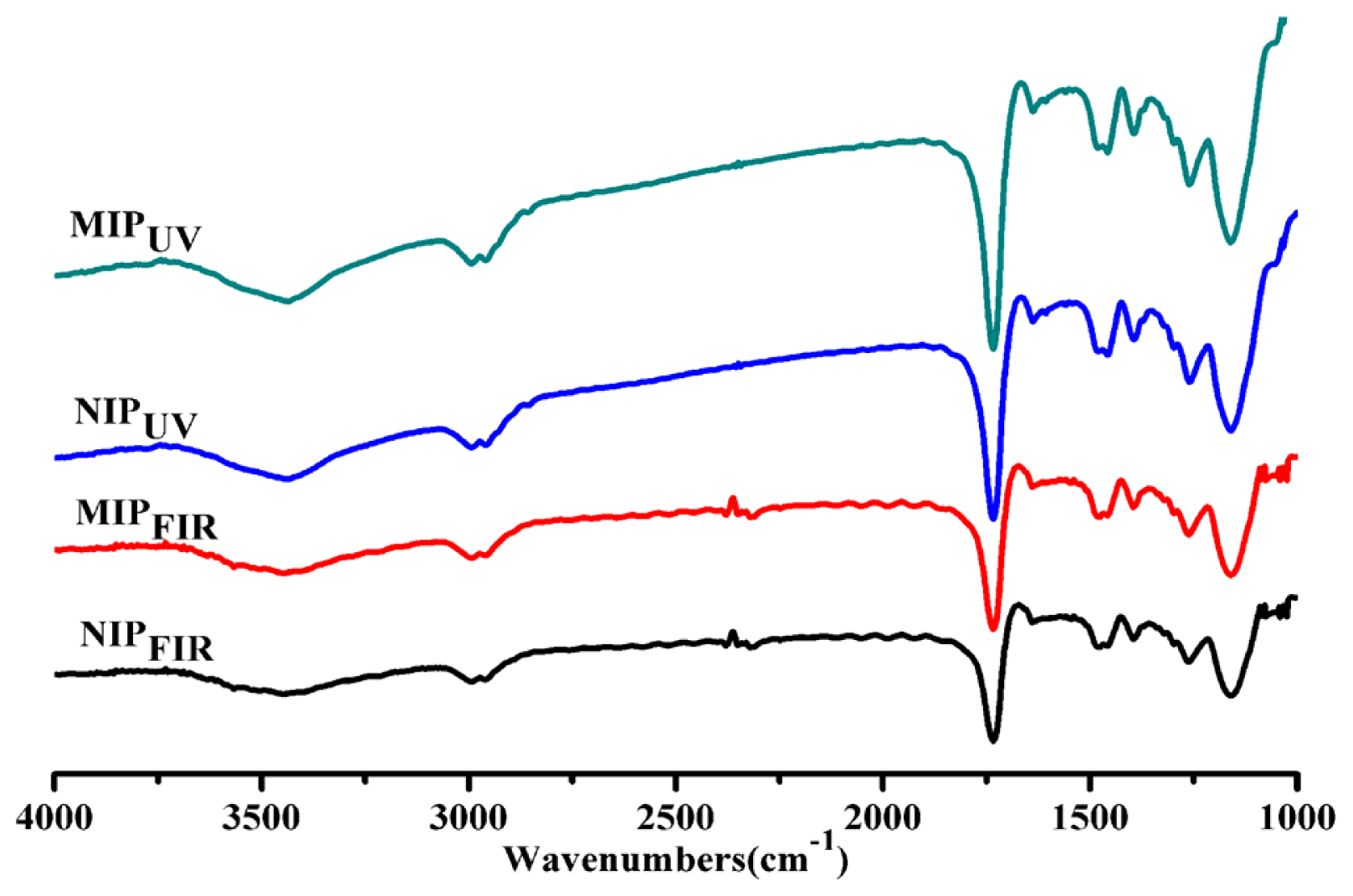
2.1. Physical Characterization of the Synthesized Polymers
2.2. Adsorption Capacity of MIPs and NIPs
2.3. Scatchard Analysis
2.4. Selectivity Experiments for the Prepared Polymers
2.5. Enrichment of Atrazine Using both MIPs, NIPs and C18 as SPE Cartridges
3. Experimental Section
3.1. Reagents and Materials
3.2. Preparation of Atrazine MIPs by FIR Radiation and UV-Induced Polymerization
3.3. Preparation of SPE Cartridges Using the MIPs and NIPs as the Sorbents
3.4. Affinity and Specificity Study of Both MIPs
3.5. MIP-SPE for Atrazine Standard Solutions and Real Samples
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wulff, G.; Sarhan, A. Use of polymers with enzyme-analogous structures for the resolution of racemates. Angew. Chem. Int. Ed. Engl 1972, 11, 341–342. [Google Scholar]
- Vlatakis, G.; Andersson, L.I.; Müller, R.; Mosbach, K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361, 645–647. [Google Scholar]
- Claude, B.; Nehmé, R.; Morin, P. Analysis of urinary neurotransmitters by capillary electrophoresis: Sensitivity enhancement using field-amplified sample injection and molecular imprinted polymer solid phase extraction. Anal. Chim. Acta 2011, 699, 242–248. [Google Scholar]
- Zheng, Y.Q.; Liu, Y.H.; Guo, H.B.; He, L.M.; Fang, B.H.; Zeng, Z.L. Molecularly imprinted solid-phase extraction for determination of tilmicosin in feed using high performance liquid chromatography. Anal. Chim. Acta 2011, 690, 269–274. [Google Scholar]
- Lin, Z.; Yang, F.; He, X.; Zhao, X.; Zhang, Y. Preparation and evaluation of a macroporous molecularly imprinted hybrid silica monolithic column for recognition of proteins by high performance liquid chromatography. J. Chromatogr. A 2009, 1216, 8612–8622. [Google Scholar]
- Sambe, H.; Hoshina, K.; Haginaka, J. Molecularly imprinted polymers for triazine herbicides prepared by multi-step swelling and polymerization method: Their application to the determination of methylthiotriazine herbicides in river water. J. Chromatogr. A 2007, 1152, 130–137. [Google Scholar]
- Yin, J.F.; Yang, G.L.; Chen, Y. Rapid and efficient chiral separation of nateglinide and its l-enantiomer on monolithic molecularly imprinted polymers. J. Chromatogr. A 2005, 1090, 68–75. [Google Scholar]
- Chen, Z.Y.; Xu, L.; Liang, Y.; Zhao, M.P. pH sensitive water soluble nanospheric imprinted hydrogels prepared as horseradish peroxidase mimetic enzymes. Adv. Mater 2010, 22, 1488–1492. [Google Scholar]
- Visnjevski, A.; Schomacker, R.; Yilmaz, E.; Bruggemann, O. Synthesis and application of a carbamazepine-imprinted polymer for solid-phase extraction from urine and wastewater. Catal. Commun 2005, 6, 601–606. [Google Scholar]
- Shen, X.T.; Zhu, L.H.; Li, J.; Tang, H.Q. Synthesis of molecular imprinted polymer coated photocatalysts with high selectivity. Chem. Commun 2007, 11, 1163–1165. [Google Scholar]
- Matsui, J.; Sodeyama, T.; Saiki, Y.; Miyazawa, T.; Yamada, T.; Tamaki, K.; Murashima, T. Face-to-face porphyrin moieties assembled with spacing for pyrazine recognition in molecularly imprinted polymers. Biosens. Bioelectron 2009, 25, 635–639. [Google Scholar]
- Basabe-Desmonts, L.; Reinhoudt, D.N.; Crego-Calama, M. Design of fluorescent materials for chemical sensing. Chem. Soc. Rev 2007, 36, 993–1017. [Google Scholar]
- Sellergren, B. Direct drug determination by selective sample enrichment on an imprinted polymer. Anal. Chem 1994, 66, 1578–1582. [Google Scholar]
- Javanbakht, M.; Namjumanesh, M.H.; Akbari-Adergani, B. Molecularly imprinted solid-phase extraction for the selective determination of bromhexine in human serum and urine with high performance liquid chromatography. Talanta 2009, 80, 133–138. [Google Scholar]
- Sérgio, F.; Rodrigues, R.T. Biosensors and rapid diagnostic tests on the frontier between analytical and clinical chemistry for biomolecular diagnosis of dengue disease: A review. Anal. Chem. Acta 2011, 687, 28–42. [Google Scholar]
- Caldorera-Moore, M.; Peppas, N.A. Micro-and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Adv. Drug Delivery Rev 2009, 61, 1391–1401. [Google Scholar]
- Matsui, J.; Okada, M.; Tsuruoka, M.; Takeuchi, T. Solid-phase extraction of a triazine herbicide using a molecularly imprinted synthetic receptor. Anal. Commun 1997, 34, 85–87. [Google Scholar]
- Prasad, K.; Prathish, K.P.; Gladis, J.M.; Naidu, G.R.K.; Prasada, R.T. Molecularly imprinted polymer (biomimetic) based potentiometric sensor for atrazine. Sensors Actuators B 2007, 123, 65–70. [Google Scholar]
- Guzzella, L.; Pozzoni, F.; Baggiani, C. Synthesis and characterization of a propazine imprinted polymer for the extraction of triazines herbicides. Water Sci. Technol 2008, 57, 139–144. [Google Scholar]
- Matsui, J.; Miyoshi, Y.; Doblhoff-Dier, O.; Takeuchi, T. A molecularly imprinted synthetic polymer receptor selective for atrazine. Anal. Chem 1995, 67, 4404–4408. [Google Scholar]
- Lanza, F.; Sellergren, B. Molecularly imprinted polymers via high-throughput and combinatorial techniques. Macromol. Rapid Commun 2004, 25, 59–68. [Google Scholar]
- Imma, F.; Lanza, F.; Tolokan, A.; Viola, H.; Sellergren, B.; Horvai, G.; Barceló, D. Selective trace enrichment of chlorotriazine pesticides from natural waters and sediment samples using terbuthylazine molecularly imprinted polymers. Anal. Chem 2000, 72, 3934–3941. [Google Scholar]
- Wiesbrock, F.; Hoogenboom, R.; Abeln, C.H.; Schubert, U.S. Single-Mode microwave ovens as new reaction devices: Accelerating the living polymerization of 2-ethyl-2-oxazoline. Macromol. Rapid Commun 2004, 25, 1739–1764. [Google Scholar]
- Bogdal, D.; Penczek, P.; Pielichowski, J.; Prociak, A. Microwave assisted synthesis, crosslinking, and processing of polymeric materials. Adv. Polym. Sci 2003, 163, 193–263. [Google Scholar]
- Bjarnason, B.; Chimuka, L.; Ramstrom, O. On-line solid-phase extraction of triazine herbicides using a molecularly imprinted polymer for selective sample enrichment. Anal. Chem 1999, 71, 2152–2156. [Google Scholar]
- Koeber, R.; Fleischer, C.; Lanza, F.; Boos, K.S.; Sellergren, B.; Barcelo, D. Evaluation of a multidimensional solid-phase extraction platform for highly selective on-line cleanup and high-throughput LC-MS analysis of triazines in river water samples using molecularly imprinted polymers. Anal. Chem 2001, 73, 2437–2444. [Google Scholar]
- Zhang, Y.P.; Qi, N.; Chen, N.; Chen, J.; Zhou, X.M.; Bai, L.Y. Preparation and evaluation of melamine molecularly imprinted polymers by thermal- and photo-initiation methods. J. Chin. Chem. Soc 2013, 60, 204–211. [Google Scholar]
- Sierra, J.; Palacios, J.; Vivaldo-Lima, E. Effect of microwave activation on polymerization rate and molecular weight development in emulsion polymerization of methyl methacrylate. J. Macromol. Sci. A 2006, 43, 589–600. [Google Scholar]
- Syu, M.J.; Deng, J.H.; Nian, Y.M. Towards bilirubin imprinted poly(methacrylic acid-co-ethylene glycol dimethylacrylate) for the specific binding of á-bilirubin. Anal. Chim. Acta 2004, 504, 167–177. [Google Scholar]
- Kobayashi, T.; Wang, H.Y.; Fujii, N. Molecular imprint membranes of polyacrylonitrile copolymers with different acrylic acid segments. Anal. Chim. Acta 1998, 365, 81–88. [Google Scholar]
- Sreenivasulu Reddy, P.; Kobayashi, T.; Fujii, N. Recognition characteristics of dibenzofuran by molecularly imprinted polymers made of common polymers. J. Eur. Polymer 2002, 38, 779–785. [Google Scholar]
- Fan, J.; Wei, Y.; Wang, J.; Wu, C.; Shi, H. Study of molecularly imprinted solid-phase extraction of diphenylguanidine and its structural analogs. Anal. Chim. Acta 2009, 639, 42–50. [Google Scholar]
- Yoshiyuki, W.; Hosoya, K.; Tanaka, N.; Takuya, K.; Takuya, K.; Masatoshi, M. Novel surface modified molecularly imprinted polymer focused on the removal of interference in environmental water samples for chromatographic determination. J. Chromatogr. A 2005, 1073, 363–370. [Google Scholar]
- Lopez, C.; Claude, B.; Morin, P.; Max, J.P.; Pena, R.; Ribet, J.P. Synthesis and study of a molecularly imprinted polymer for the specific extraction of indole alkaloids from Catharanthus roseus extracts. Anal. Chim. Acta 2011, 683, 198–205. [Google Scholar]
- Turner, N.W.; Holdsworth, C.I.; Donne, S.W.; Adam, M.; Bowyer, M.C. Microwave induced MIP synthesis: comparative analysis of thermal and microwave induced polymerisation of caffeine imprinted polymers. New J. Chem 2010, 34, 686–692. [Google Scholar]







| Polymer | Total pore volume (cm3/g) | Surface area (m2/g) | Total porosity (%) |
|---|---|---|---|
| MIPFIR | 0.44 | 198.92 | 64.58 |
| NIPFIR | 0.39 | 145.74 | 52.93 |
| MIPUV | 0.52 | 213.92 | 83.64 |
| NIPUV | 0.48 | 179.25 | 77.13 |
| Polymer | Linear regression equation | Ka1 × 10−4 (mol/L) | Qmax1 (μmol/g) | Ka2 × 10−4 (mol/L) | Qmax2 (μmol/g) | |
|---|---|---|---|---|---|---|
| High-affinity | Low-affinity | |||||
| MIPFIR | Q/C = 66.39 − 1.86Q | Q/C = 35.23 − 0.31Q | 5.38 | 35.70 | 32.30 | 113.93 |
| NIPFIR | Q/C = 117.13 − 10.89Q | Q/C = 34.95 − 1.69Q | 0.92 | 10.76 | 5.93 | 20.70 |
| MIPUV | Q/C = 67.23 − 1.37Q | Q/C = 40.26 − 0.13Q | 7.33 | 49.25 | 76.50 | 307.78 |
| NIPUV | Q/C = 83.02 − 4.41Q | Q/C = 54.04 − 2.04Q | 2.27 | 18.96 | 4.90 | 26.49 |
| Substrate | QMIP (μmol/g) | QNIP (μmol/g) | IF | SI | ||||
|---|---|---|---|---|---|---|---|---|
| FIR | UV | FIR | UV | FIR | UV | FIR | UV | |
| atrazine | 27.12 | 36.12 | 12.96 | 17.47 | 2.09 | 2.07 | 1.00 | 1.00 |
| cyromazine | 20.71 | 28.57 | 12.43 | 17.64 | 1.67 | 1.62 | 0.74 | 0.79 |
| metamitron | 12.02 | 14.29 | 10.85 | 11.25 | 1.11 | 1.27 | 0.49 | 0.62 |
| simazine | 18.55 | 25.52 | 11.59 | 15.66 | 1.60 | 1.63 | 0.71 | 0.79 |
| ametryn | 15.13 | 23.87 | 11.07 | 16.13 | 1.37 | 1.48 | 0.61 | 0.72 |
| terbutryn | 14.01 | 22.61 | 11.31 | 16.87 | 1.24 | 1.34 | 0.55 | 0.65 |
| Polymer | Standard addition amount (mg/L) | Determined (mg/L) | Recovery rate (%) | RSD (n = 3) (%) |
|---|---|---|---|---|
| MIPFIR | 0.5 | 0.47 | 94.7 | 5.28 |
| 1 | 0.97 | 97.1 | 5.12 | |
| 5 | 4.89 | 97.8 | 4.85 | |
| 10 | 9.08 | 90.8 | 3.27 | |
| 20 | 19.21 | 90.1 | 2.76 | |
| MIPUV | 0.5 | 0.47 | 94.6 | 7.13 |
| 1 | 0.95 | 95.3 | 4.52 | |
| 5 | 4.92 | 98.4 | 4.13 | |
| 10 | 9.44 | 94.4 | 3.47 | |
| 20 | 20.37 | 101.9 | 5.92 | |
| C18 | 0.5 | 0.45 | 90.3 | 7.49 |
| 1 | 0.86 | 86.4 | 3.78 | |
| 5 | 4.56 | 91.2 | 4.26 | |
| 10 | 8.95 | 89.5 | 2.51 | |
| 20 | 18.96 | 94.8 | 2.17 | |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, J.; Bai, L.-Y.; Liu, K.-F.; Liu, R.-Q.; Zhang, Y.-P. Atrazine Molecular Imprinted Polymers: Comparative Analysis by Far-Infrared and Ultraviolet Induced Polymerization. Int. J. Mol. Sci. 2014, 15, 574-587. https://doi.org/10.3390/ijms15010574
Chen J, Bai L-Y, Liu K-F, Liu R-Q, Zhang Y-P. Atrazine Molecular Imprinted Polymers: Comparative Analysis by Far-Infrared and Ultraviolet Induced Polymerization. International Journal of Molecular Sciences. 2014; 15(1):574-587. https://doi.org/10.3390/ijms15010574
Chicago/Turabian StyleChen, Jun, Lian-Yang Bai, Kun-Feng Liu, Run-Qiang Liu, and Yu-Ping Zhang. 2014. "Atrazine Molecular Imprinted Polymers: Comparative Analysis by Far-Infrared and Ultraviolet Induced Polymerization" International Journal of Molecular Sciences 15, no. 1: 574-587. https://doi.org/10.3390/ijms15010574




