Synthetic Chalcones with Potent Antioxidant Ability on H2O2-Induced Apoptosis in PC12 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry

2.2. Effects of Chalcones on the Viability of PC12 Cells Exposed to H2O2
| Compound | R2 | Compound | R2 |
|---|---|---|---|
| 1a | CH3– | 2b | CH3CH2– |
| 1b | CH3CH2– | 2c |  |
| 1c |  | 2d | 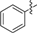 |
| 1d | CH2=CH– | 2e |  |
| 2a | CH3– | 2f |  |

2.3. Active Compounds Dose-Dependently Increase the Viability of PC12 Cells Exposed to H2O2

2.4. 1 and 1d Effectively Attenuate H2O2-Induced Cell Apoptosis

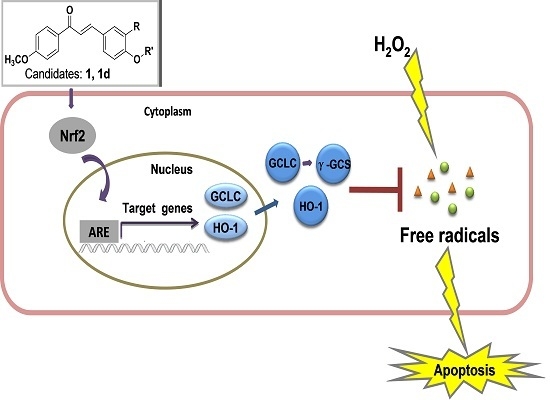
2.5. Active Compounds Significantly Elevate the Expression of Antioxidant Genes GCLC and HO-1
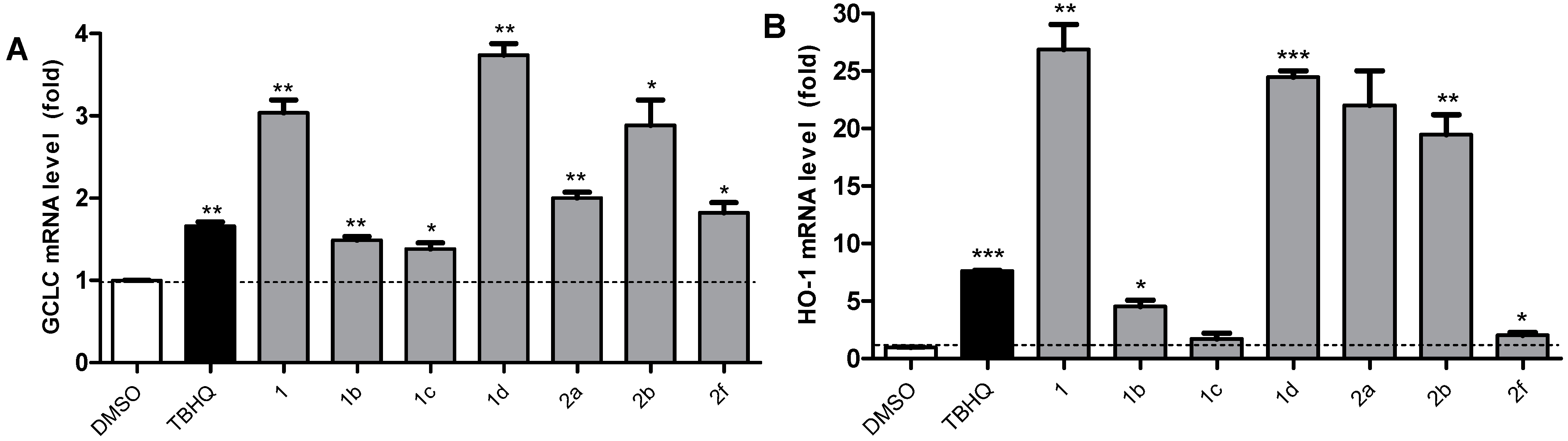
2.6. 1 and 1d Up-Regulate γ-GCS and HO-1 Protein Levels in PC12 Cells

2.7. Induction of HO-1 and GCLC Expression Is Responsible for the Antioxidant Activities of 1 and 1d

3. Experimental Section
3.1. Chemical Synthesis
3.2. Biology
3.2.1. Reagents and Cell Culture
3.2.2. Cell Viability Assay
3.2.3. Hoechst Staining
3.2.4. RNA Extraction and qRT-PCR
3.2.5. Western Blot Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, M.A.; Perry, G.; Richey, P.L.; Sayre, L.M.; Anderson, V.E.; Beal, M.F; Kowall, N. Oxidative damage in Alzheimer’s. Nature 1996, 382, 120–121. [Google Scholar] [CrossRef]
- Baird, L; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- Sashidhara, K.V; Kumar, A; Kumar, M; Sarkar, J; Sinha, S. Synthesis and in vitro evaluation of novel coumarin-chalcone hybrids as potential anticancer agents. Bioorg. Med. Chem. Lett. 2010, 20, 7205–7211. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, V.; Kumar, P. Heterocyclic chalcone analogues as potential anticancer agents. Anticancer Agents Med. Chem. 2013, 13, 422–432. [Google Scholar]
- Singh, P.; Raj, R.; Kumar, V.; Mahajan, M.P.; Bedi, P.M.; Kaur, T.; Saxena, A.K. 1,2,3-Triazole tethered β-lactam-chalcone bifunctional hybrids: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2012, 47, 594–600. [Google Scholar] [CrossRef]
- Jin, F.; Jin, X.Y.; Jin, Y.L.; Sohn, D.W.; Kim, S.A.; Sohn, D.H.; Kim, Y.C.; Kim, H.S. Structural requirements of 2',4',6'-tris(methoxymethoxy) chalcone derivatives for anti-inflammatory activity: The importance of a 2'-hydroxy moiety. Arch. Pharm. Res. 2007, 30, 1359–1367. [Google Scholar] [CrossRef]
- Okunrobo, L.O.; Usifoh, C.O.; Uwaya, J.O. Anti-inflammatory and gastroprotective properties of some chalcones. Acta Pol. Pharm. 2006, 63, 195–199. [Google Scholar]
- Lacka, I.; Konieczny, M.T.; Bulakowska, A.; Rzymowski, T.; Milewski, S. Antifungal action of the oxathiolone-fused chalcone derivative. Mycoses 2011, 54, e407–e414. [Google Scholar] [CrossRef]
- Tran, T.D.; Nguyen, T.T.; Do, T.H.; Huynh, T.N.; Tran, C.D.; Thai, K.M. Synthesis and anti-bacterial activity of some heterocyclic chalcone analogues alone and in combination with anti-biotics. Molecules 2012, 17, 6684–6696. [Google Scholar] [CrossRef]
- Detsi, A.; Majdalani, M.; Ontogiorgis, K.C.A.; Hadjipavlou-Litina, D.; Kefalas, P. Natural and synthetic 2'-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg. Med. Chem. 2009, 17, 8073–8085. [Google Scholar] [CrossRef]
- Shenvi, S.; Kumar, K.; Hatti, K.S.; Rijesh, K.; Diwakar, L.; Reddy, G.C. Synthesis, anticancer and antioxidant activities of 2,4,5-trimethoxy chalcones and analogues from asaronaldehyde: Structure-activity relationship. Eur. J. Med. Chem. 2013, 62, 435–442. [Google Scholar] [CrossRef]
- Srivastava, S.; Sonkar, R.; Mishra, S.K.; Tiwari, A.; Balaramnavar, V.M.; Mir, S.; Bhatia, G.; Saxena, A.K.; Lakshmi, V. Antidyslipidemic and antioxidant effects of novel Lupeol-derived chalcones. Lipids 2013, 48, 1017–1027. [Google Scholar] [CrossRef]
- Jin, C.; Liang, Y.J.; He, H.; Fu, L. Synthesis and antitumor activity of novel chalcone derivatives. Biomed. Pharmacother. 2013, 67, 215–217. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Cai, Y.; Pan, Y.; Ye, F.; Zhang, Y.; Zhao, Y.; Yang, S.; Li, X.; Liang, G. Evaluation and discovery of novel synthetic chalcone derivatives as anti-inflammatory agents. J. Med. Chem. 2011, 54, 4147–4159. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Gawande, N.M.; Khobragade, C.N. Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, anti-oxidant and antimicrobial agents. Bioorg. Med. Chem. 2009, 17, 8168–8173. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Totre, J.V.; Khobragade, C.N. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2010, 18, 1364–1370. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Patil, S.A.; Gacche, R.N.; Korbad, B.L.; Hote, B.S.; Kinkar, S.N.; Jalde, S.S. Synthesis and biological evaluation of nitrogen-containing chalcones as possible anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. Lett. 2010, 20, 730–733. [Google Scholar] [CrossRef]
- Gacche, R.; Khsirsagar, M.; Kamble, S.; Bandgar, B.; Dhole, N.; Shisode, K.; Chaudhari, A. Antioxidant and anti-inflammatory related activities of selected synthetic chalcones: Structure-activity relationship studies using computational tools. Chem. Pharm. Bull. 2008, 56, 897–901. [Google Scholar] [CrossRef]
- Isa, N.M.; Abdelwahab, S.I.; Mohan, S.; Abdul, A.B.; Sukari, M.A.; Taha, M.M.; Syam, S.; Narrima, P.; Cheah, S.; Ahmad, S.; et al. In vitro anti-inflammatory, cytotoxic and antioxidant activities of boesenbergin A, a chalcone isolated from Boesenbergia rotunda (L.) (fingerroot). Braz. J. Med. Biol. Res. 2012, 45, 524–530. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.; Ham, H.; Jeong, H.S.; Lee, J. Antioxidant and cytoprotective effects of oligomeric and polymeric procyanidin fractions from defatted grape seed in PC12 cells. J. Med. Food 2012, 15, 490–494. [Google Scholar] [CrossRef]
- Si, C.L.; Shen, T.; Jiang, Y.Y.; Wu, L.; Yu, G.J.; Ren, X.D.; Xu, G.H.; Hu, W.C. Anti-oxidant properties and neuroprotective effects of isocampneoside II on hydrogen peroxide-induced oxidative injury in PC12 cells. Food. Chem. Toxicol. 2013, 59, 145–152. [Google Scholar] [CrossRef]
- Tanaka, A.; Hamada, N.; Fujita, Y.; Itoh, T.; Nozawa, Y.; Iinuma, M.; Ito, M. A novel kavalactone derivative protects against H2O2-induced PC12 cell death via Nrf2/ARE activation. Bioorg. Med. Chem. 2010, 18, 3133–3139. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Yuan, J.; Lei, A. Copper-catalyzed oxidative coupling of alkenes with aldehydes: Direct access to α, β-unsaturated ketones. Angew. Chem. Int. Ed. 2013, 52, 2256–2259. [Google Scholar] [CrossRef]
- Jian, Z.; Li, K.; Liu, L.; Zhang, Y.; Zhou, Z.; Li, C.; Gao, T. Heme oxygenase-1 protects human melanocytes from H2O2-induced oxidative stress via the Nrf2-ARE pathway. J. Investig. Dermatol. 2011, 131, 1420–1427. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M. Heme oxygenase-1: Molecular mechanisms of gene expression in oxygen-related stress. Antioxid. Redox Signal. 2002, 4, 625–632. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-Z.; Cheng, C.-C.; Shen, L.-L.; Wang, Z.-K.; Wu, S.-B.; Li, W.-L.; Chen, S.-H.; Zhou, R.-P.; Qiu, P.-H. Synthetic Chalcones with Potent Antioxidant Ability on H2O2-Induced Apoptosis in PC12 Cells. Int. J. Mol. Sci. 2014, 15, 18525-18539. https://doi.org/10.3390/ijms151018525
Wu J-Z, Cheng C-C, Shen L-L, Wang Z-K, Wu S-B, Li W-L, Chen S-H, Zhou R-P, Qiu P-H. Synthetic Chalcones with Potent Antioxidant Ability on H2O2-Induced Apoptosis in PC12 Cells. International Journal of Molecular Sciences. 2014; 15(10):18525-18539. https://doi.org/10.3390/ijms151018525
Chicago/Turabian StyleWu, Jian-Zhang, Chan-Chan Cheng, Lai-Lai Shen, Zhan-Kun Wang, Shou-Biao Wu, Wu-Lan Li, Su-Hua Chen, Rong-Ping Zhou, and Pei-Hong Qiu. 2014. "Synthetic Chalcones with Potent Antioxidant Ability on H2O2-Induced Apoptosis in PC12 Cells" International Journal of Molecular Sciences 15, no. 10: 18525-18539. https://doi.org/10.3390/ijms151018525