Long-Term Spinal Ventral Root Reimplantation, but not Bone Marrow Mononuclear Cell Treatment, Positively Influences Ultrastructural Synapse Recovery and Motor Axonal Regrowth
Abstract
:1. Introduction
2. Results
2.1. Ventral Root Avulsion (VRA) Implantation Preserved Synaptic Inputs by Ultrastructural Analysis of Spinal Cord


2.1.1. Preferential Loss of Excitatory Terminals after Avulsion

2.1.2. Pattern of the Terminal Distribution after Avulsion


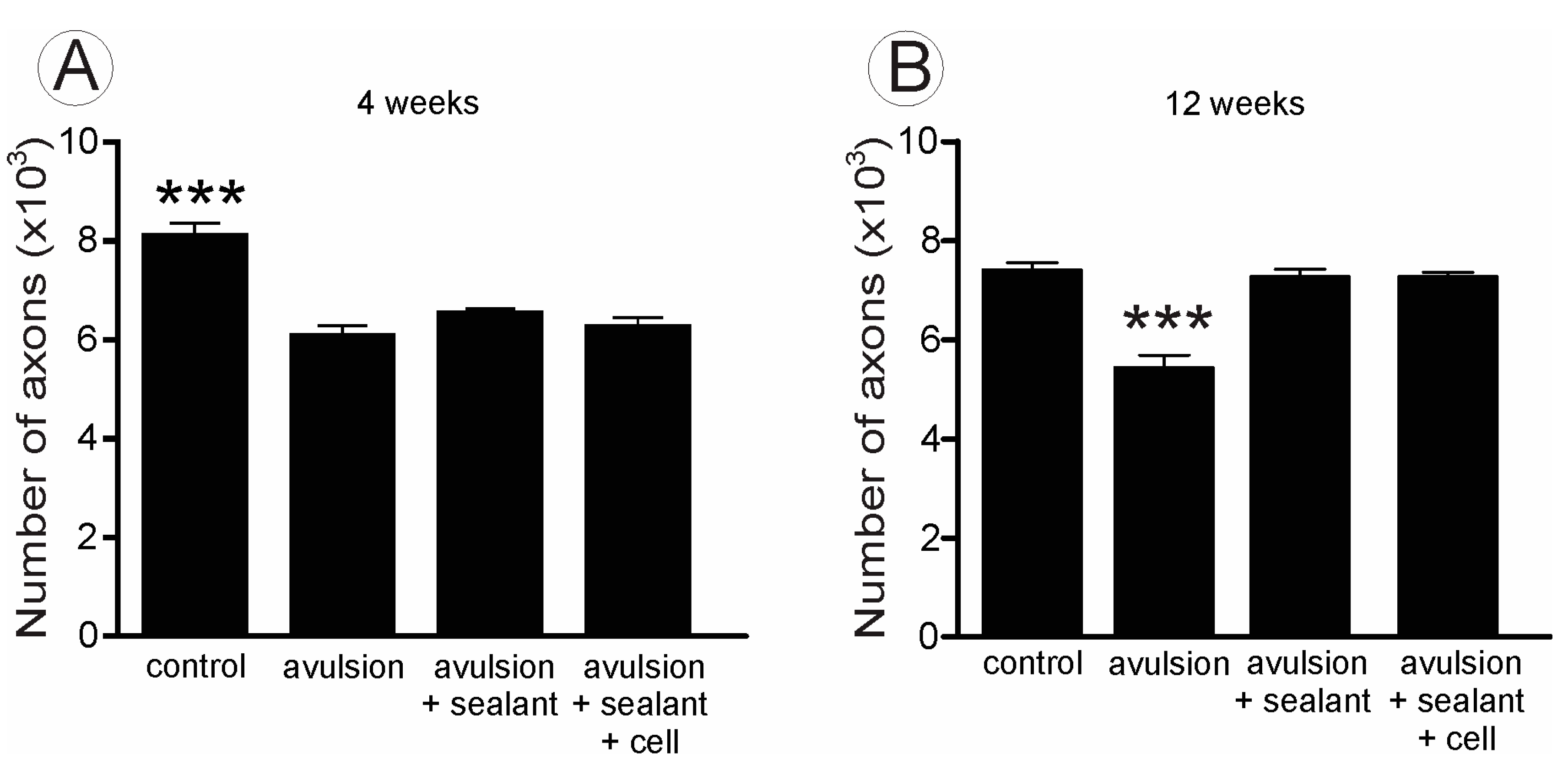
2.2. VRA Implantation Promotes Axonal Regeneration Observed by Histological Analysis of the Sciatic Nerve


| Groups | Fiber Diameter (µm) | “g” Ratio |
|---|---|---|
| Control | 7.59 ± 0.40 | 0.66 ± 0.03 |
| Avulsion | 7.32 ± 0.23 | 0.69 ± 0.02 |
| Avulsion + Implant | 7.25 ± 0.14 | 0.65 ± 0.02 |
| Avulsion + Implant + MC | 7.73 ± 0.28 | 0.62 ± 0.02 |
3. Discussion
4. Experimental Section
4.1. Experimental Animals
4.2. VRA
4.3. Reimplantation of the Motor Roots
4.4. Mononuclear Cells (MC) Transplantation
4.5. Specimen Preparation
4.6. Synaptic Analysis of the Ultrathin Sections
4.7. Sciatic Nerve Regeneration
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thatte, M.R.; Babhulkar, S.; Hiremath, A. Brachial plexus injury in adults: Diagnosis and surgical treatment strategies. Ann. Indian Acad. Neurol. 2013, 16, 26–33. [Google Scholar]
- Carlstedt, T. Nerve root replantation. Neurosurg. Clin. N. Am. 2009, 20, 39–50. [Google Scholar]
- Koliatsos, V.E.; Price, W.L.; Pardo, C.A.; Price, D.L. Ventral root avulsion: An experimental model of death of adult motor neurons. J. Comp. Neurol. 1994, 342, 35–44. [Google Scholar]
- Chu, T.H.; Wu, W. Neurotrophic factor treatment after spinal root avulsion injury. Cent. Nerv. Syst. Agents Med. Chem. 2009, 9, 40–55. [Google Scholar]
- Brannstrom, T.; Kellerth, J.O. Changes in synaptology of adult cat spinal α-motoneurons after axotomy. Exp. Brain Res. 1998, 118, 1–13. [Google Scholar]
- Cullheim, S.; Carlstedt, T.; Risling, M. Axon regeneration of spinal motoneurons following a lesion at the cord-ventral root interface. Spinal Cord 1999, 37, 811–819. [Google Scholar]
- Hoang, T.X.; Nieto, J.H.; Dobkin, B.H.; Tillakaratne, N.J.; Havton, L.A. Acute implantation of an avulsed lumbosacral ventral root into the rat conus medullaris promotes neuroprotection and graft reinnervation by autonomic and motor neurons. Neuroscience 2006, 138, 1149–1160. [Google Scholar]
- Cullheim, S.; Carlstedt, T.; Linda, H.; Risling, M.; Ulfhake, B. Motoneurons reinnervate skeletal muscle after ventral root implantation into the spinal cord of the cat. Neuroscience 1989, 29, 725–733. [Google Scholar]
- Chang, H.Y.; Havton, L.A. Surgical implantation of avulsed lumbosacral ventral roots promotes restoration of bladder morphology in rats. Exp. Neurol. 2008, 214, 117–124. [Google Scholar]
- Hallin, R.G.; Carlstedt, T.; Nilsson-Remahl, I.; Risling, M. Spinal cord implantation of avulsed ventral roots in primates: Correlation between restored motor function and morphology. Exp. Brain Res. 1999, 124, 304–310. [Google Scholar]
- Risling, M.; Linda, H.; Cullheim, S.; Franson, P. A persistent defect in the blood-brain barrier after ventral funiculus lesion in adult cats: Implications for CNS regeneration? Brain Res. 1989, 494, 13–21. [Google Scholar]
- Sjogren, A.M.; Thelestam, M.; Blomqvist, L.; Linda, H.; Remahl, S.; Risling, M. Extravasation of staphylococcal α-toxin in normal and injured CNS regions lacking blood-brain barrier function: Observations after ventral root replantation. Brain Res. 1991, 559, 276–282. [Google Scholar]
- Frisen, J.; Fried, K.; Sjogren, A.M.; Risling, M. Growth of ascending spinal axons in CNS scar tissue. Int. J. Dev. Neurosci. 1993, 11, 461–475. [Google Scholar]
- Lopes-Filho, J.D.; Caldas, H.C.; Santos, F.C.; Mazzer, N.; Simoes, G.F.; Kawasaki-Oyama, R.S.; Abbud-Filho, M.; Oliveira, A.R.; Toboga, S.R.; Chueire, A.G. Microscopic evidences that bone marrow mononuclear cell treatment improves sciatic nerve regeneration after neurorrhaphy. Microsc. Res. Tech. 2011, 74, 355–363. [Google Scholar]
- Kachramanoglou, C.; Li, D.; Andrews, P.; East, C.; Carlstedt, T.; Raisman, G.; Choi, D. Novel strategies in brachial plexus repair after traumatic avulsion. Br. J. Neurosurg. 2011, 25, 16–27. [Google Scholar]
- Spejo, A.B.; Carvalho, J.L.; Goes, A.M.; Oliveira, A.L. Neuroprotective effects of mesenchymal stem cells on spinal motoneurons following ventral root axotomy: Synapse stability and axonal regeneration. Neuroscience 2013, 250, 715–732. [Google Scholar]
- Li, Y.; Yamamoto, M.; Raisman, G.; Choi, D.; Carlstedt, T. An experimental model of ventral root repair showing the beneficial effect of transplanting olfactory ensheathing cells. Neurosurgery 2007, 60, 734–740. [Google Scholar]
- Barbizan, R.; Castro, M.V.; Barraviera, B.; Ferreira, R.S., Jr.; Oliveira, A.L. Influence of delivery method on neuroprotection by bone marrow mononuclear cell therapy following ventral root reimplantation with fibrin sealant. PLoS One 2014, 9, e105712. [Google Scholar]
- Cullheim, S.; Wallquist, W.; Hammarberg, H.; Linda, H.; Piehl, F.; Carlstedt, T.; Risling, M. Properties of motoneurons underlying their regenerative capacity after axon lesions in the ventral funiculus or at the surface of the spinal cord. Brain Res. Rev. 2002, 40, 309–316. [Google Scholar]
- Hammarberg, H.; Piehl, F.; Risling, M.; Cullheim, S. Differential regulation of trophic factor receptor mRNAs in spinal motoneurons after sciatic nerve transection and ventral root avulsion in the rat. J. Comp. Neurol. 2000, 426, 587–601. [Google Scholar]
- Carlstedt, T.; Risling, M.; Linda, H.; Cullheim, S.; Ulfhake, B.; Sjogren, A.M. Regeneration after spinal nerve root injury. Restor. Neurol. Neurosci. 1990, 1, 289–295. [Google Scholar]
- Carlstedt, T.; Linda, H.; Cullheim, S.; Risling, M. Reinnervation of hind limb muscles after ventral root avulsion and implantation in the lumbar spinal cord of the adult rat. Acta Physiol. Scand. 1986, 128, 645–646. [Google Scholar]
- Purves, D.; Lichtman, J.W. Formation and maintenance of synaptic connections in autonomic ganglia. Physiol. Rev. 1978, 58, 821–862. [Google Scholar]
- Purves, D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J. Physiol. 1975, 252, 429–463. [Google Scholar]
- Takata, M.; Nagahama, T. Synaptic efficacy of inhibitory synapses in hypoglossal motoneurons after transection of the hypoglossal nerves. Neuroscience 1983, 10, 23–29. [Google Scholar]
- Delgadogarcia, J.M.; Delpozo, F.; Spencer, R.F.; Baker, R. Behavior of neurons in the abducens nucleus of the alert cat III. Axotomized motoneurons. Neuroscience 1988, 24, 143–160. [Google Scholar]
- Barbizan, R.; Castro, M.V.; Rodrigues, A.C.; Barraviera, B.; Ferreira, R.S.; Oliveira, A.L. Motor recovery and synaptic preservation after ventral root avulsion and repair with a fibrin sealant derived from snake venom. PLoS One 2013, 8, e63260. [Google Scholar]
- Linda, H.; Shupliakov, O.; Ornung, G.; Ottersen, O.P.; Storm-Mathisen, J.; Risling, M.; Cullheim, S. Ultrastructural evidence for a preferential elimination of glutamate-immunoreactive synaptic terminals from spinal motoneurons after intramedullary axotomy. J. Comp. Neurol. 2000, 425, 10–23. [Google Scholar]
- Linda, H.; Risling, M.; Cullheim, S. “Dendraxons” in regenerating motoneurons in the cat: Do dendrites generate new axons after central axotomy? Brain Res. 1985, 358, 329–333. [Google Scholar]
- Lang, E.M.; Asan, E.; Plesnila, N.; Hofmann, G.O.; Sendtner, M. Motoneuron survival after C7 nerve root avulsion and replantation in the adult rabbit: Effects of local ciliary neurotrophic factor and brain-derived neurotrophic factor application. Plastic Reconstr. Surg. 2005, 115, 2042–2050. [Google Scholar]
- Ikeda, M.; Oka, Y. The relationship between nerve conduction velocity and fiber morphology during peripheral nerve regeneration. Brain Behav. 2012, 2, 382–390. [Google Scholar]
- Waxman, S.G. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve 1980, 3, 141–150. [Google Scholar]
- Waxman, S.G. Pathophysiology of nerve conduction: Relation to diabetic neuropathy. Ann. Intern. Med. 1980, 92, 297–301. [Google Scholar]
- De Freria, C.M.; Barbizan, R.; de Oliveira, A.L. Granulocyte colony stimulating factor neuroprotective effects on spinal motoneurons after ventral root avulsion. Synapse 2012, 66, 128–141. [Google Scholar]
- Barbizan, R.; Oliveira, A.L. Impact of acute inflammation on spinal motoneuron synaptic plasticity following ventral root avulsion. J. Neuroinflam. 2010, 7, 29. [Google Scholar]
- Scorisa, J.M.; Zanon, R.G.; Freria, C.M.; de Oliveira, A.L. Glatiramer acetate positively influences spinal motoneuron survival and synaptic plasticity after ventral root avulsion. Neurosci. Lett. 2009, 451, 34–39. [Google Scholar]
- Rodrigues Hell, R.C.; Silva Costa, M.M.; Goes, A.M.; Oliveira, A.L. Local injection of BDNF producing mesenchymal stem cells increases neuronal survival and synaptic stability following ventral root avulsion. Neurobiol. Dis. 2009, 33, 290–300. [Google Scholar]
- Oliveira, A.L.; Langone, F. GM-1 ganglioside treatment reduces motoneuron death after ventral root avulsion in adult rats. Neurosci. Lett. 2000, 293, 131–134. [Google Scholar]
- Barros, L.C.; Soares, A.M.; Costa, F.L.; Rodrigues, V.M.; Fuly, A.L.; Giglio, J.R.; Gallacci, M.; Thomazini-Santos, I.A.; Barraviera, S.R.C.S.; Barraviera, B.; et al. Biochemical and biological evaluation of gyroxin isolated from Crotalus durissus terrificus venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 23–33. [Google Scholar]
- Barros, L.C.; Ferreira, R.S., Jr.; Barraviera, S.R.; Stolf, H.O.; Thomazini-Santos, I.A.; Mendes-Giannini, M.J.; Toscano, E.; Barraviera, B. A new fibrin sealant from Crotalus durissus terrificus venom: Applications in medicine. J. Toxicol. Environ. Health Part B Crit. Rev. 2009, 12, 553–571. [Google Scholar]
- Gasparotto, V.P.; Landim-Alvarenga, F.C.; Oliveira, A.L.; Simoes, G.F.; Lima-Neto, J.F.; Barraviera, B.; Ferreira, R.S., Jr. A new fibrin sealant as a three-dimensional scaffold candidate for mesenchymal stem cells. Stem Cell Res. Ther. 2014, 5, 78. [Google Scholar]
- Conradi, S. Observations on the ultrastructure of the axon hillock and initial axon segment of lumbosacral motoneurons in the cat. Acta Physiol. Scand. Suppl. 1969, 332, 65–84. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbizan, R.; Castro, M.V.; Ferreira, R.S., Jr.; Barraviera, B.; Oliveira, A.L.R. Long-Term Spinal Ventral Root Reimplantation, but not Bone Marrow Mononuclear Cell Treatment, Positively Influences Ultrastructural Synapse Recovery and Motor Axonal Regrowth. Int. J. Mol. Sci. 2014, 15, 19535-19551. https://doi.org/10.3390/ijms151119535
Barbizan R, Castro MV, Ferreira RS Jr., Barraviera B, Oliveira ALR. Long-Term Spinal Ventral Root Reimplantation, but not Bone Marrow Mononuclear Cell Treatment, Positively Influences Ultrastructural Synapse Recovery and Motor Axonal Regrowth. International Journal of Molecular Sciences. 2014; 15(11):19535-19551. https://doi.org/10.3390/ijms151119535
Chicago/Turabian StyleBarbizan, Roberta, Mateus V. Castro, Rui Seabra Ferreira, Jr., Benedito Barraviera, and Alexandre L. R. Oliveira. 2014. "Long-Term Spinal Ventral Root Reimplantation, but not Bone Marrow Mononuclear Cell Treatment, Positively Influences Ultrastructural Synapse Recovery and Motor Axonal Regrowth" International Journal of Molecular Sciences 15, no. 11: 19535-19551. https://doi.org/10.3390/ijms151119535





