Characterization of Flavonoid Glycosides from Fenugreek (Trigonella foenum-graecum) Crude Seeds by HPLC–DAD–ESI/MS Analysis
Abstract
:1. Introduction
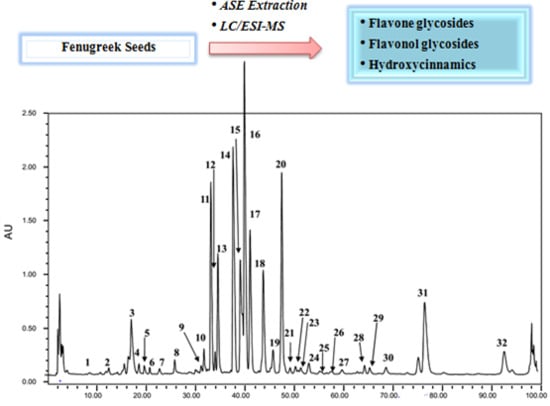
2. Results and Discussion

| Peak | Rt (min) | λmax (nm) | [M − H]− | Fragment Signals (m/z) | Compound Identification |
|---|---|---|---|---|---|
| 9 | 31.74 | 234, 272, 334 | 593 | 473, 383, 353 | apigenin 6,8-di C-hexoside (vicenin 2 isomer) |
| 11 | 33.65 | 232, 272, 334 | 593 | 473, 383, 353 | apigenin 6,8-di C-glucoside (vicenin 2) |
| 13 | 35.06 | 232, 270, 336 | 593 | 473, 383, 353 | apigenin 6,8-di C-hexoside (vicenin 2 isomer) |
| 14 | 38.12 | 232, 270, 336 | 563 | 443, 383, 353 | apigenin 8- C-xyloside-6-C-glucoside (vicenin 3) |
| 17 | 41.53 | 230, 270, 336 | 563 | 443, 383, 353 | apigenin 6- C-xyloside-8-C-glucoside (vicenin 1) |
| 19 | 46.17 | 232, 272, 338 | 577 | 503, 473, 383, 353 | apigenin 8- C-rhamnoside-6-C-glucoside |
| 31 | 77.08 | 232, 270, 316 | 593 | 447, 429, 309, 285 | kaempferol 7- O-rhamnosyl-(1→2)-glucoside |

2.1. Non-Acylated Flavone and Flavonol Glycosides
2.2. Acylated Flavone and Flavonol Glycosides
| Peak | Rt (min) | λmax (nm) | [M − H]− | Fragment Signals (m/z) | Compound Identification |
|---|---|---|---|---|---|
| 10 | 32.28 | 234, 334 | 771 | 593, 503, 473, 383, 353 | apigenin 6- C-glucosyl 8-C-(2''-O-dihydroferuloyl)-glucoside |
| 12 | 34.51 | 232, 270, 336 | 749 | 593, 503, 473, 383, 353 | vicenin derivative |
| 15 | 39.58 | 234, 270, 348 | 895 | 563, 447, 357, 327, 284 | luteolin 7- O-[6''-dihydrogalloyl]-glucosyl-8-C-pentosyl-(1→6)-glucoside |
| 16 | 40.40 | 270, 346 | 895 | 563, 447, 357, 339, 327, 285 | luteolin 7-O-[6''-dihydrogalloyl]-glucosyl-8- C-pentosyl-(1→2)-glucoside |
| 18 | 44.22 | 232, 268, 336 | 863 | 563, 443, 323, 311 | apigenin 7- O-(2''-dihydrogalloyl)-rhamonsyl-6-C-(2'''-pentosy)-glucoside |
| 20 | 47.94 | 234, 270, 336 | 863 | 563, 443, 323, 311, 283 | apigenin 7- O-(2''-dihydrogalloyl)-rhamonsyl-6-C-(2'''-pentosy)-glucoside |
| 21 | 49.62 | 232, 270, 336 | 725 | 533, 443, 413, 383, 353 | apigenin 6- C-pentosyl 8-C-(2''-O-hydroxyferuloyl)-pentoside |
| 22 | 50.69 | 232, 272, 340 | 759 | 593, 473, 383, 353 | apigenin 6- C-glucosyl 8-C-(6''-O-methoxygalloyl)-glucoside |
| 23 | 51.74 | 234, 270, 336 | 863 | 563, 431, 323, 283 | apigenin and 7- O-(6''-dihydrogalloyl)-rhamonsyl-6-C-(2'''-pentosy)-glucoside |
| 24 | 53.33 | 232, 316 | 877 | 563, 473, 447, 327, 285 | kaempferol 7- O-(6''-galloyl)-glucosyl 6-C-(2'''pentosyl)-rhamnoside |
| 25 | 55.50 | 232, 270, 346 | 877 | 533, 447, 357, 339, 305, 285 | luteolin 7- O-(2''-galloyl)-glucosyl 6-C-(2'''pentosyl)-rhamnoside |
| 26 | 58.02 | 232, 270, 338 | 893 | 577, 473, 383, 353 | apigenin 7- O-(6''-dihydrogalloyl)-glucosyl-8-C-rhamnosyl-6-C-glucoside |
| 27 | 60.02 | 230, 270, 338 | 893 | 577, 473, 383, 353 | apigenin 7- O-(2''-dihydrogalloyl)-glucosyl-8-C-rhamnosyl-6-C-glucoside |
| 28 | 64.59 | 232, 270, 344 | 925 | 605, 563, 443, 383, 353 | luteolin 7- O-(6''-quinoyl)-rhamnosyl-6-C-pentosyl-8-C,O-(6'''acetyl)-glucoside |
| 29 | 65.60 | 232, 270, 344 | 547 | 487, 457, 383, 353, 283 | luteolin 8- C-(2''-malonyl)-glucoside |
| 30 | 68.87 | 270, 344 | 935 | 651, 547, 461, 327, 285 | luteolin 7- O-(2''dihydrogalloyl)-pentosyl-4'-O-(2''',6'''-malonyl-pentosyl)-rhamnoside |
| 32 | 93.20 | 232, 270, 318 | 1133 | 1063, 917, 577, 164, 293 | kaempferol 7- O-(2''',6''',2''-malonyl)-rhamonsyl-diglucosyl-3-O-(6'''''rhamnosyl)-rhamnoside |
2.3. Hydroxycinnamic Acids
2.4. Quantitation of Flavonoids Glycoside in Crude Fenugreek Seeds
| Peak | Rt (min) | λmax (nm) | [M − H]− | Fragment Signals (m/z) | Compound Identification |
|---|---|---|---|---|---|
| 1 | 9.23 | 228, 264 | 827 | 665, 545, 383, 341, 281, 221, 179, 146, 129, 110 | tricaffeoyl-glucosyl-glucoside |
| 2 | 13.09 | 232, 276 | 695 | 619, 407, 363, 309,180, 167,128 | tricaffeoyl-hydroxyferulic acid |
| 3 | 17.65 | 248 | 888 | 863, 452, 431, 171, 137 | dihydrogallic acid derivative |
| 4 | 19.22 | 228, 260 | 947 | 765, 483, 382, 266, 205, 167, 115 | disynapoyl-hydro feruloyl-feruloyl-hydrocaffeic acid |
| 5 | 20.28 | 236, 278, 328 | 447 | 224, 152, 136, 108 | galloyl-coumaric acid pentoside |
| 6 | 21.40 | 230, 296 | 499 | 377, 273, 163, 119 | caffeoyl-coumaroyl-quinic acid |
| 7 | 23.38 | 240, 334, 346 | 801 | 671, 477, 399, 323, 261, 144, 119 | dicaffeoyl-protocatechuic acid diglucoside |
| 8 | 26.46 | 220, 234, 316 | 837 | 647, 625, 587, 452, 395, 347, 317, 293, 165, 132, 128, 115 | unidentified |
| Peak Number | Quantitative Chemical Composition (%) | Peak Number | Quantitative Chemical Composition (%) | Peak Number | Quantitative Chemical Composition (%) |
|---|---|---|---|---|---|
| 1 | 0.11 | 12 | 0.66 | 23 | 0.26 |
| 2 | 0.15 | 13 | 5.60 | 24 | 0.80 |
| 3 | 3.10 | 14 | 14.41 | 25 | 0.08 |
| 4 | 0.45 | 15 | 3.76 | 26 | 0.05 |
| 5 | 0.43 | 16 | 15.80 | 27 | 0.38 |
| 6 | 0.27 | 17 | 8.82 | 28 | 0.45 |
| 7 | 0.34 | 18 | 6.59 | 29 | 0.44 |
| 8 | 0.70 | 19 | 1.18 | 30 | 0.62 |
| 9 | 0.27 | 20 | 12.02 | 31 | 8.71 |
| 10 | 0.82 | 21 | 0.31 | 32 | 2.51 |
| 11 | 9.61 | 22 | 0.31 |
3. Materials and Methods
3.1. General
3.2. Plant Materials
3.3. Accelerated Solvent Extractor (ASE)
3.4. HPLC–DAD–ESI/MS Analyses
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Basch, E.; Ulbricht, C.; Kuo, G.; Szapary, P.; Smith, M. Therapeutic applications of fenugreek. Altern. Med. Rev. 2003, 8, 20–27. [Google Scholar] [PubMed]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Sharma, R.D.; Raghuram, T.C.; Rao, N.S. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur. J. Clin. Nutr. 1990, 44, 301–306. [Google Scholar] [PubMed]
- Zia, T.; Nazrul Hasnain, S.; Hasan, S.K. Evaluation of the oral hypoglycaemic effect of Trigonella foenum-graecum L in normal mice. J. Ethnopharmacol. 2001, 75, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.; Madar, Z. The effect of an ethanol extract derived from fenugreek (Trigonella foenum-graecum) on bile acid absorption and cholecterol levels in rats. Br. J. Nutr. 1993, 69, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Fenugreek (Trigonella foenum-graecum): A review of health beneficial physiological effects. Food Rev. Int. 2006, 22, 203–224. [Google Scholar] [CrossRef]
- Sujapandian, R.; Anuradha, V.V.; Viswanathan, P. Gastroprotective effect of fenugreek seeds (Trigonella foenum-graecum) on experimental gastric ulcer in rats. J. Ethnopharmacl. 2002, 81, 393–397. [Google Scholar] [CrossRef]
- Amin, A.; Alkaabi, A.; Al-Falasi, S.; Daoud, S.A. Chemopreventive activities of Trigonella foenum-graecum (fenugreek) against breast cancer. Cell Biol. Int. 2005, 29, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchy, N.S.; Glenn, K.C.; Gnanasambandam, R.; Johnson, M.G. Natural antioxidant extract from fenugreek (Trigonella foenum graecum) for ground beef patties. J. Food Sci. 1996, 61, 516–519. [Google Scholar] [CrossRef]
- Kavirasan, S.; Naik, G.H.; Gangabhagirathi, R.; Anuradha, C.V.; Priyadarsini, K.I. In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum-graecum) seeds. Food Chem. 2007, 103, 31–37. [Google Scholar] [CrossRef]
- Ahmadiani, A.; Javan, M.; Semnanian, S.; Bharat, E.; Kamalinejad, M. Anti-inflammatory and antipyretic effects of Trigonella foenum-graecum leaves extract in the rat. J. Ethnopharmacol. 2001, 75, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Petit, P.; Sauvaire, Y.; Ponsin, G.; Manteghetti, M.; Fave, A.; Ribes, G. Effect of a fenugreek seed extraction on feeding behaviour in the rat: Metabolic-endocrine correlates. Pharmacol. Biochem. Behav. 1993, 45, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, G.A. Fenugreek—The Genus Trigonella; Taylor and Francis: London, UK; New York, NY, USA, 2002; pp. 1–255. [Google Scholar]
- Wagner, H.; Iyengar, M.A.; Horhammer, L. Vicenin-1 and-2 in the seeds of Trigonella foenum graecum. Phytochemistry 1973, 12, 2548. [Google Scholar] [CrossRef]
- Huang, W.Z.; Liang, X. Determination of two flavone glycosides in the seeds of Trigonella foenum-graecum L. from various production localities. J. Plant Res. Environ. 2000, 9, 53–54. [Google Scholar]
- Rayyan, S.; Fossen, T.; Andersen, U.M. Flavone C-glycosides from seeds of fenugreek, Trigonella foenum-graecum L. J. Agric. Food Chem. 2010, 58, 7211–7217. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kakani, R.; Nair, M.G. Compounds in functional food fenugreek spice exhibit anti-inflammatory and antioxidant activities. Food Chem. 2012, 131, 1187–1192. [Google Scholar] [CrossRef]
- Kenny, O.; Smyth, T.J.; Hewage, C.M.; Brunton, N.P. Antioxidant properties and quantitative UPLC–MS analysis of phenolic compounds from extracts of fenugreek (Trigonella foenum-graecum) seeds and bitter melon (Momordica charantia) fruit. Food Chem. 2013, 141, 4295–4302. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Cais Han, J.; Li, J.; Zhao, Y.; Zheng, J.; Namba, T.; Kadota, S.; Tezuka, Y.; Fan, W. Studies on flavonoids from fenugreek (Trigonella foenum graecum L). Zhongguo Zhong Yao Za Zhi 1998, 23, 614–639. (In Chinese) [Google Scholar] [PubMed]
- Fazli, F.R.Y.; Hardman, R. The spice fenugreek (Trigonella foenum-graecum L.): Its commercial varieties of seed as a source of diosgenin. Trop. Sci. 1968, 10, 66–78. [Google Scholar]
- Sauvaire, Y.; Ribes, G.; Baccou, J.C.; Loubatieerres-Mariani, M.M. Implications of steroidal sapogenins in the hypocholesterolemic effect of fenugreek. Lipids 1991, 26, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.G.; Zaman, M.S.; Mir, Z.; Mir, P.S.; Achary, S.N.; Mears, G.J.; Elder, J.L. Analysis of steroidal sapogenins from amber fenugreek (Trigonella foenum-graecum) by capillary gas chromatography and combined gas chromatography/mass spectrometry. J. Agric. Food Chem. 1997, 45, 753–759. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Nanavaty, K.; Gumashta, A. Effect of cooking procedures on the free and total niacin content of certain food. J. Nutr. Diet. 1964, 1, 276–280. [Google Scholar]
- Bellakhdar, J. La Pharmacopée Marocaine Traditionnelle, Médecine Arabe Ancienne et Savoirs Populaires; Ibis Press: Paris, France, 1997; p. 764. (In French) [Google Scholar]
- Truchado, P.; Vit, P.; Ferreres, F.; Tomas-Barberan, F. Liquid chromatography–tandem mass spectrometry analysis allows the simultaneous characterization of C-glycosyl and O-glycosyl flavonoids in stingless bee honeys. J. Chromatogr. A 2011, 1218, 7601–7607. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yue, Y.; Tang, F.; Wang, J.; Yao, X.; Sun, J. A comparison of C-glycosidic flavonoid isomers by electrospray ionization quadrupole time-of-flight tandem mass spectrometry in negative and positive ion mode. Int. J. Mass Spectrom. 2013, 333, 59–66. [Google Scholar] [CrossRef]
- Talhi, O.; Silva, A.M.S. Advances in C-glycosylflavonoid research. Curr. Org. Chem. 2012, 16, 859–896. [Google Scholar] [CrossRef]
- Colombo, R.; Yariwake, J.H.; McCullagh, M. Study of C- and O-glycosylflavones in sugarcane extracts using liquid chromatography—Exact mass measurement mass spectrometry. J. Br. Chem. Soc. 2008, 19, 483–490. [Google Scholar] [CrossRef]
- Bakhtiar, A.; Gleye, J.; Moulis, C.; Fourasté, I. Desorption chemical ionisation mass spectrometry of C-glycosylflavones. Phytochem. Anal. 1994, 5, 86–89. [Google Scholar] [CrossRef]
- Grayer, R.J.; Kite, G.C.; Abou-Zaid, M.; Archer, L.J. The application of atmospheric pressure chemical ionisation liquid chromatography-mass spectrometry in the chemotaxonomic study of flavonoids: Characterisation of flavonoids from Ocimum gratissimum var. Gratissimum. Phytochem. Anal. 2000, 11, 257–267. [Google Scholar] [CrossRef]
- March, R.E.; Lewars, E.G.; Stadey, C.G.; Miao, X.S.; Zhao, X.; Metcalfe, C.D. A comparison of flavonoid glycosides by electrospray tandem mass spectrometry. Mass Spectrom. 2006, 248, 61–85. [Google Scholar]
- Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N. Identification and quantification of flavonoid glycosides from fenugreek (Trigonella foenum-graecum) germinated seeds by LC–DAD–ESI/MS analysis. J. Food Comp. Anal. 2014, 35, 21–29. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Ferreres, F.; Llorach, R.; Gil-Izquierdo, A. Characterization of the interglycosidic linkage in di-, tri-, tetra- and penta-glycosylated flavonoids and differentiation of positional isomers by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.M.; Claeys, M. Characterization and differentiation of diglycosyl flavonoids by positive ion fast atom bombardment and tandem mass spectrometry. Biol. Mass Spectrom. 1994, 23, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Waridel, P.; Wolfender, J.L.; Ndjoko, K.; Hobby, K.R.; Major, H.J.; Hostettmann, K. Evaluation of quadrupole time-of-flight tandem mass spectrometry and ion-trap multiple-stage mass spectrometry for the differentiation of C-glycosidic flavonoid isomers. J. Chromatogr. A 2001, 926, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Vukics, V.; Kery, A.; Bonn, G.K.; Guttman, A. Major flavonoid components of heartsease (Viola tricolor L.) and their antioxidant activities. Anal. Bioanal. Chem. 2008, 390, 1917–1925. [Google Scholar] [CrossRef]
- Es-Safi, N.; Kerhoas, L.; Einhorn, J.; Ducrot, P.H. Application of ESI/MS, CID/MS and tandem MS/MS to the fragmentation study of eriodictyol 7-O-glucosyl-(1→2)-glucoside and luteolin 7-O-glucosyl-(1→2)-glucoside. Int. J. Mass Spectrom. 2005, 247, 93–100. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The ultraviolet spectra of flavones and flavonols, isoflavones, dihydroxyflavonols. In The Systematics Identification of Flavonoids; Springer–Verlag: New York, NY, USA, 1970. [Google Scholar]
- Cuyckens, F.; Cleays, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A. Flavone and flavonol O-glycosides. In Flavonoids: Chemistry, Biochemistry and Applications; Anderson, Ø.M., Markham, K.R., Eds.; Taylor & Francis/CRC: Boca Raton, FL, USA, 2006; pp. 749–856. [Google Scholar]
- Becchi, M.; Fraisse, D. Fast atom bombardment and fast atom bombardment collision-activated dissociation/mass-analysed ion kinetic energy analysis of C-glycosidic flavonoids. Biomed. Environ. Mass Spectrom. 1989, 18, 122–130. [Google Scholar] [CrossRef]
- Cuyckens, F.; Rozenberg, R.; de Hoffmann, E.; Claeys, M. Structure characterization of flavonoid O-diglycosides by positive and negative nano-electrospray ionization ion trap mass spectrometry. J. Mass Spectrom. 2001, 36, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Ablajan, K.; Abliz, Z.; Shang, X.-Y.; He, J.-M.; Zhang, R.-P.; Shi, J.-G. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Gil-Izquierdo, A.; Andrade, P.B.; Valentão, P.; Tomás-Barberán, F.A. Characterization of C-glycosyl flavones O-glycosylated by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1161, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Cuyckens, F.; Ma, Y.L.; Pocsfalvi, G.; Cleays, M. Tandem mass spectral strategies for the structural characterisation of flavonoid glycosides. Analusis 2000, 28, 888–895. [Google Scholar] [CrossRef]
- Ma, Y.L.; Li, Q.M.; van den Heuvel, H.; Claeys, M. Characterization of flavone and flavonol aglycones by collision-induced dissociation tandem mass spectrometry. Rapid Com. Mass Spectrom. 1997, 11, 1357–1364. [Google Scholar] [CrossRef]
- Ferreres, F.; Silva, B.M.; Andrate, P.B.; Seabra, R.M.; Ferreira, M.A. Approach to the study of C-glycosyl flavones by ion trap HPLC–PAD–ESI/MS/MS: Application to seeds of quince (Cydonia oblonga). Phytochem. Anal. 2003, 14, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Distribution of C- and O-glycosyl flavonoids, (3-hydroxy-3-methylglutaryl) glycosyl flavanones and furocoumarins in Citrus aurantium L. juice. Food Chem. 2011, 124, 576–582. [Google Scholar] [CrossRef]
- Ferreres, F.; Gil-Izquierdo, A.; Vinholes, J.; Grosso, C.; Valentão, P.; Andrade, P.B. Approach to the study of C-glycosyl flavones acylated with aliphatic and aromatic acids from Spergularia rubra by high-performance liquid chromatography-photodiode array detection/electrospray ionization multi-stage mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Andrade, P.B.; Valentão, P.; Gil-Izquierdo, A. Further knowledge on barley (Hordeum vulgare L.) leaves O-glycosyl-C-glycosyl flavones by liquid chromatography–UV diode-array detection-electrospray ionization mass spectrometry. J. Chromatogr. A 2008, 1182, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, S.C.; Castilho, P.C. Characterization of phenolic compounds in Helichrysum melaleucum by high-performance liquid chromatography with on-line ultraviolet and mass spectrometry detection. Rapid Commun. Mass Spectrom. 2010, 24, 1851–1868. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. The analysis and characterization of chlorogenic foods and other cinnamates. In Methods in Polyphenol Analysis; Santos-Buelga, C.W.G., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2003; pp. 314–333. [Google Scholar]
- Romani, A.; Vignolini, P.; Isolani, L.; Ieri, F.; Heimler, D. HPLC–DAD/MS characterization of flavonoids and hydroxycinnamic derivatives in turnip tops (Brassica rapa L. Subsp. sylvestris L.). J. Agric. Food Chem. 2006, 54, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Method 3545A (SW-846): Pressurized Fluid Extraction (PFE) (Revision 1). Available online: http://www.epa.gov/sam/pdfs/EPA-3545a.pdf (accessed on 6 July 2013).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Characterization of Flavonoid Glycosides from Fenugreek (Trigonella foenum-graecum) Crude Seeds by HPLC–DAD–ESI/MS Analysis. Int. J. Mol. Sci. 2014, 15, 20668-20685. https://doi.org/10.3390/ijms151120668
Benayad Z, Gómez-Cordovés C, Es-Safi NE. Characterization of Flavonoid Glycosides from Fenugreek (Trigonella foenum-graecum) Crude Seeds by HPLC–DAD–ESI/MS Analysis. International Journal of Molecular Sciences. 2014; 15(11):20668-20685. https://doi.org/10.3390/ijms151120668
Chicago/Turabian StyleBenayad, Zakia, Carmen Gómez-Cordovés, and Nour Eddine Es-Safi. 2014. "Characterization of Flavonoid Glycosides from Fenugreek (Trigonella foenum-graecum) Crude Seeds by HPLC–DAD–ESI/MS Analysis" International Journal of Molecular Sciences 15, no. 11: 20668-20685. https://doi.org/10.3390/ijms151120668